Abstract
The objective of this study was to rapidly develop osteoporotic model animals by combining ovariectomy with a low calcium diet in rats. Thirty, eight-week-old, female, Sprague-Dawley rats were either sham-operated (Sham) or ovariectomized (Ovx) and divided into three groups: Sham, Ovx, and Ovx + low calcium diet. Rats in the Sham and Ovx groups were fed a standard diet containing 1.1% w/w calcium while rats in the Ovx + low calcium diet group were fed a diet containing 0.1% w/w calcium. Serum osteocalcin and bone mineral density (BMD) of the lumbar vertebrae were measured 4 and 8 weeks after surgery. The rats were euthanized 12 weeks after surgery, and the BMD of the right femur and histomorphometry of the femoral neck were assessed at that time. The Ovx + low-calcium diet group had a significantly lower mean BMD of the lumbar vertebra and higher mean serum osteocalcin concentration than the Sham and Ovx groups. Twelve weeks after surgery, rats in the Ovx + low calcium diet group had a significantly lower BMD, smaller Tb.Th and Tb.N, and larger Tb.Sp of the right femoral neck than did rats in the Sham and Ovx groups. These data indicate that a low calcium diet can significantly accelerate bone loss in ovariectomized rats. Combining ovariectomy and a low calcium diet can save considerable time in the creation of osteoporotic model animals.
Keywords: Animal model, osteoporosis, ovariectomy, low calcium diet
Introduction
With the coming acceleration of global population aging, osteoporosis has become a global public health problem [1]. Osteoporosis is a disease characterized by low bone mineral density and structural deterioration of bone tissue, leading to an increased risk of fracture [2]. Many therapeutic advances in the management of osteoporosis were first developed in animal models, and then subsequently entered clinical practice [3].
The ovariectomized rat model is commonly used in research on postmenopausal osteoporosis because the bone loss in these animals is considered to mimic that of postmenopausal women [4]. During the initial period after ovariectomy, bone resorption exceeds bone formation resulting in a net loss of bone [5]. Statistically significant bone loss is not detectable in the lumbar vertebral bodies until 60 d after ovariectomy [6]. Since animal models of bone loss are vital for screening new osteoporosis treatments, developing an animal model that becomes osteoporotic more rapidly would allow faster testing of potential therapies. In this study, we tried novel methods to shorten the time until development of osteoporosis in an established animal model.
Calcium is required for normal skeletal growth and mineralization, and plays an important role in regulating bone remodeling and bone mass [7]. Some studies have described the structural changes induced by a low calcium diet in immature and mature rats [8]; however, little is known about the effect of a low calcium diet in ovariectomized rats. In the present study, we investigated the dynamic effects of a low calcium diet on serum osteocalcin, bone mineral density (BMD), and histomorphometry of the femoral neck in a rat model of ovariectomy-induced osteoporosis. Our objective was to determine whether feeding a low calcium diet would shorten the time until development of osteopenia after ovariectomy in rats.
Materials and methods
Animals
Thirty, eight-week-old, female, Sprague Dawley rats were obtained from the Beijing Animal Center (Beijing, China). The animals were acclimated to a colony room with an ambient temperature of 22 ± 1°C, humidity of 50 ± 10%, and a 12 h light/dark cycle for at least 5 d before the start of the experiment. Food and water were available ad libitum. All animal treatments were strictly in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. The experimental procedures were approved by the Committee on Animal Care and Use of the Beijing Institute of Pharmacology & Toxicology.
Surgery and treatment
The animals were divided into the three groups of 10-12 rats each: the Sham group was subjected to sham surgery and fed a standard diet (1.1% w/w calcium), the Ovx group was bilaterally ovariectomized and fed a standard diet, and the Ovx + low calcium diet group was bilaterally ovariectomized and fed a low calcium diet (0.1% w/w calcium). Ovariectomy was performed via a dorsal approach with the rats under chloral hydrate anesthesia (400 mg/kg, i.p.). The ovaries of the rats in the Sham group were exteriorized but not removed. After surgery, all three groups of rats were fed their respective diets for 12 weeks.
Determination of serum osteocalcin concentration
Four and 8 weeks after treatment, blood samples were obtained from the orbital sinuses of the rats and serum was immediately separated and stored at -20°C. Serum osteocalcin was measured using a commercial radioimmunoassay kit (Purevalley Biotech, Beijing, China) according to the manufacturer’s instructions.
Measurement of bone mineral density (BMD)
Four and 8 weeks after treatment, the BMD of the lumbar vertebrae (L4-5) was measured by dual-energy X-ray absorptiometry (GE Lunar DPX-L, Madison, WI, USA) and data were analyzed with the accompanying software. The rats were sacrificed 12 weeks after ovariectomy and the right femur was removed and its BMD measured.
Histomorphometry of the femoral neck
Histomorphometry of the right femoral neck was performed on 5 animals/group. After measurement of BMD, the right femurs were dehydrated in graded, ascending concentrations of ethanol (70, 95 and 100%) and xylene and then embedded without decalcification in methyl methacrylate. Serial transverse sections of the femoral neck were cut to a thickness of 230μm using a microtome (Leica Inc., Nussloch, Germany) and the mid-cross sections were selected and ground to 30 μm thickness for histomorphometric analysis. Histomorphometry was performed using a digitizing morphometry system (Axio Imager A2, ZEISS, Jena, Germany). Cancellous bone measurements within the femur included trabecular thickness (Tb.Th; μm), trabecular number (Tb.N; #/μm), and trabecular separation (Tb.Sp; μm).
Statistical analyses
Data were analyzed by one-way analysis of variance and unpaired t tests using GraphPad Prism 5 (GraphPad Software Inc, San Diego, CA, USA). P < 0.05 was considered to be statistically significant. Bonferroni’s Multiple Comparison Test was used to assess the significance of between-group differences. Data are expre-ssed as means ± S.M.E.
Results
Effects of a low calcium diet on serum osteocalcin concentrations in Ovx rats
Figure 1A and 1B show the serum concentrations of osteocalcin, which is a sensitive biochemical marker of bone resorption after ovariectomy. At 4 weeks after surgery, the serum osteocalcin concentrations in the Ovx + low calcium diet group were significantly higher than in the Sham group (P < 0.05). Compared with the Sham group, the Ovx group also had slightly higher osteocalcin concentrations, but this difference was not statistically significant (P > 0.05). However, at 8 weeks after surgery there were no significant between-group differences in serum osteocalcin.
Figure 1.
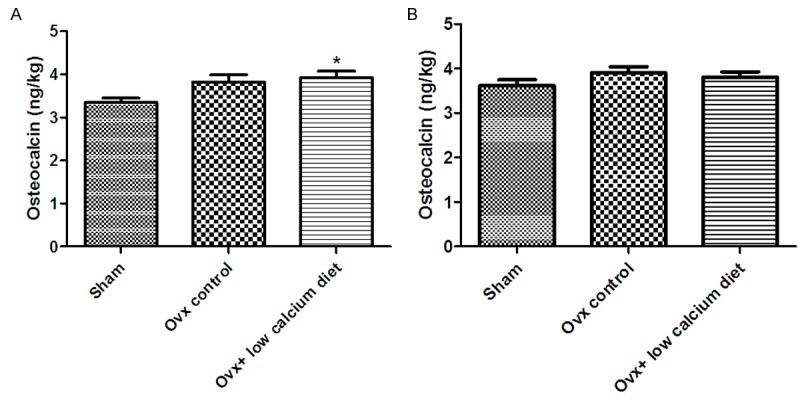
Effects of a low calcium diet on serum osteocalcin concentrations in Ovx rats. A. Serum osteocalcin concentrations at 4 weeks after ovariectomy. B. Serum osteocalcin concentrations at 8 weeks after ovariectomy. *Significantly different from Sham group (P < 0.05).
Effects of low calcium diet on BMD in Ovx rats
Figure 2A-C show the mean BMDs of the lumbar vertebrae and femurs of all groups. At 4 weeks after surgery, the BMD of the lumbar vertebrae of rats in the Ovx + low calcium diet group had declined more than in the other groups (P < 0.05), while there were no significant differences between the Sham and Ovx groups. At 8 weeks after surgery, both the Ovx and Ovx + low calcium diet groups had experienced a significant reduction in BMD of the lumbar vertebrae (P < 0.05), and BMD was not significantly different between these two groups. Twelve weeks after ovariectomy, the Ovx + low calcium diet group rats had significantly lower BMD of the right femur than the rats in the Sham and Ovx groups (P < 0.05). The femoral BMD of rats in the Ovx group was also significantly lower than that of rats in the Sham group at 12 weeks after surgery (P < 0.05).
Figure 2.
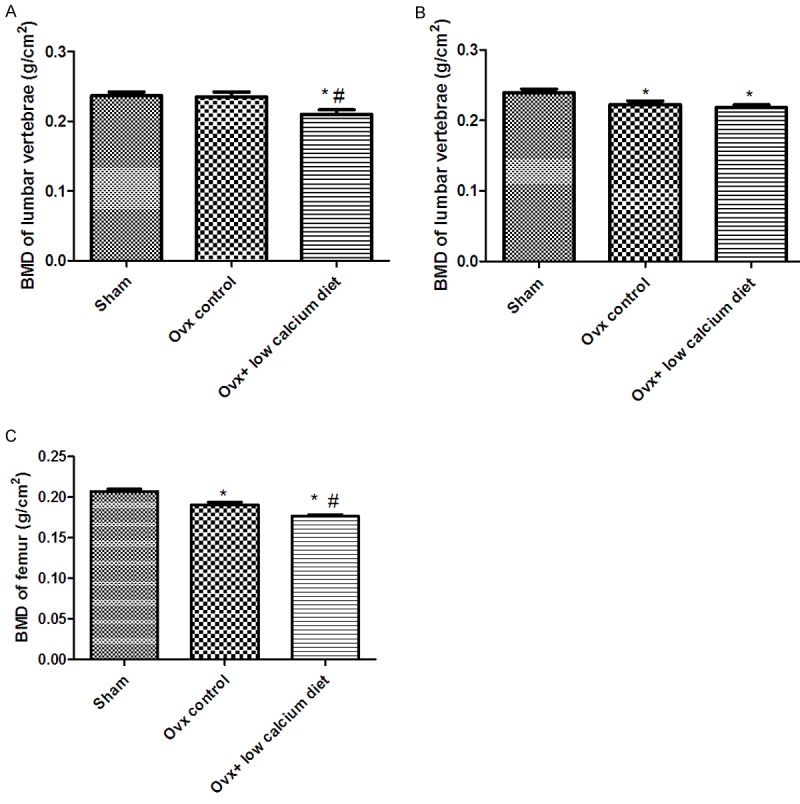
Effects of a low calcium diet on BMD in Ovx rats. A. BMD of the lumbar vertebrae 4 weeks after ovariectomy. B. BMD of the lumbar vertebrae 8 weeks after ovariectomy. C. BMD of the right femur 12 weeks after ovariectomy. *Significantly different from Sham group (P < 0.05). #Significantly different from Ovx group (P < 0.05).
Effects of a low calcium diet on trabecular bone microarchitecture in Ovx rats
As shown in Table 1, the Ovx and Ovx + low calcium diet groups had significantly smaller Tb.Th and Tb.N, and larger Tb.Sp, than the Sham group at 12 weeks after ovariectomy (P < 0.05). There were also significant differences in Tb.Th, Tb.N, and Tb.Sp between the Ovx group and the Ovx + low calcium diet group (P < 0.05).
Table 1.
Effects of a low calcium diet on the static parameters of the femoral neck in OVX rats
| Group | Tb.Th (μm) | Tb.N (#/μm) | Tb.Sp (μm) |
|---|---|---|---|
| Sham | 137.43 ± 19.38 | 4.27 + 0.21 | 139.23 ± 29.48 |
| Ovx | 120.45 ± 27.23* | 3.26 + 0.30* | 205.32 ± 34.23* |
| Ovx + low calcium diet | 106.38 ± 19.47*,# | 2.67 ± 0.45*,# | 257.46 ± 37.56*,# |
Data are the mean±SE of 5 rats/group. Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation.
Significantly different from Sham group (P < 0.05);
Significantly different from Ovx group (P < 0.05).
Discussion
In this study, we determined the dynamic effects of the combination of ovariectomy and a low-calcium diet on serum osteocalcin, BMD, and femoral neck histomorphometry in rats. At 4 weeks after ovariectomy, a statistically significant reduction in lumbar vertebral BMD was seen only in ovariectomized rats fed a low calcium diet. This suggests that the development of post-ovariectomy osteopenia can be accelerated by feeding a diet low in calcium.
The ovariectomized rat is considered the gold standard model for evaluation of drugs used in the prevention and treatment of osteoporosis; it has been well-validated and shares many clinical similarities with estrogen deficiency-induced (or postmenopausal) bone loss in adult humans [9]. These similarities include: an increased rate of bone turnover; an initial phase of rapid bone loss followed by a much slower decline in bone loss; and a greater loss of cancellous than cortical bone [10]. After ovariectomy in rats, or menopause in women, the loss of estrogen creates an imbalance in osteoblast and osteoclast activity such that bone resorption outpaces bone formation leading to high turnover bone loss [11]. As a biochemical marker of bone formation, changes in serum osteocalcin concentrations represent changes in whole skeleton bone metabolism [12]. In the present study, at 4 weeks after ovariectomy the rats fed a low-calcium diet had higher serum osteocalcin concentrations than rats in the Ovx or Sham groups. These data suggest that a low-calcium diet can significantly accelerate bone turnover after ovariectomy. However, at 8 weeks after surgery there were no between-group differences in serum osteocalcin concentrations; this may be due to all animals being in the relatively slower phase of bone loss that follows the initial rapid decline in bone loss after ovariectomy [13].
Ovariectomy results in decreases in BMD of the lumbar vertebrae and femur, and this bone loss can be significantly accelerated by a low-calcium diet. In this study, the BMD of the lumbar vertebrae of rats in the Ovx + plus low-calcium diet group consistently differed from that of the Sham rats, while the lumbar vertebral and femoral BMD of the Ovx group rats differed from that of the Sham rats at only 8 and 12 weeks, respectively. Rats in the Ovx + low calcium diet group had a lower femoral BMD than Ovx rats at 12 weeks after surgery. Histomorphometry revealed similar results; there were significant differences in the Tb.Th, Tb.N, and Tb.Sp of the femoral neck between the Ovx and Ovx + low calcium diet groups at 12 weeks after surgery. These data indicate that a low calcium diet increased the loss of BMD and facilitated the development of osteopenia after ovariectomy.
Calcium is one of the most commonly used nutritional supplements because it is the mineral that is lost during bone resorption [14]. Bone itself undergoes continuous remodeling, with constant resorption and deposition of calcium into new bone [15]. Low dietary calcium intake produces no obvious symptoms in the short-term; however, long-term calcium deficiency can lead to osteoporosis [8]. In aging, postmenopausal women, decreases in estrogen production result in bone breakdown exceeding formation, which leads to bone loss and increases the risk of osteoporosis over time [16]. Similar changes occur after ovariectomy in the rat, and our results indicate that the time until development of osteopenia after ovariectomy in rats can be halved by feeding a low calcium diet.
In a conclusion, this work confirms that rapid bone loss occurs in ovariectomized rats fed a low calcium diet. Combining ovariectomy plus a low-calcium diet can save considerable time in the creation of osteoporotic model animals.
Acknowledgements
Funding for this study was provided by the Integrated Drug Discovery Technology Platform (2012ZX09301003-001) of National Science and Technology Major Projects for “Major New Drugs Innovation and Development” in China.
Disclosure of conflict of interest
All authors declare that there is no conflict of interest.
References
- 1.Boonen S, Singer AJ. Osteoporosis management: impact of fracture type on cost and quality of life in patients at risk for fracture I. Curr Med Res Opin. 2008;24:1781–1788. doi: 10.1185/03007990802115796. [DOI] [PubMed] [Google Scholar]
- 2.Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010;6:99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 3.Turner RT, Lotinun S, Hefferan T, Evans GL, Zhang M, Sibonga JD. Animal models for osteoporosis. Rev Endocr Metab Disord. 2001;2:117–127. doi: 10.1023/a:1010067326811. [DOI] [PubMed] [Google Scholar]
- 4.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–430. [PMC free article] [PubMed] [Google Scholar]
- 5.Wronski TJ, Cintron M, Dann LM. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int. 1988;43:179–183. doi: 10.1007/BF02571317. [DOI] [PubMed] [Google Scholar]
- 6.Wronski TJ, Dann LM, Horner SL. Time course of vertebral osteopenia in ovariectomized rats. Bone. 1990;10:295–301. doi: 10.1016/8756-3282(89)90067-7. [DOI] [PubMed] [Google Scholar]
- 7.Creedon A, Cashman KD. The effect of calcium intake on bone composition and bone resorption in the young growing rat. Br J Nutr. 2001;86:453–459. doi: 10.1079/bjn2001419. [DOI] [PubMed] [Google Scholar]
- 8.Seto H, Aoki K, Kasugai S, Ohya K. Trabecular bone turnover, bone marrow cell development, and gene expression of bone matrix proteins after low calcium feeding in rats. Bone. 1999;25:687–695. doi: 10.1016/s8756-3282(99)00229-x. [DOI] [PubMed] [Google Scholar]
- 9.Kharode YP, Sharp MC, Bodine PV. Utility of the ovariectomized rat as a model for human osteoporosis in drug discovery. Methods Mol Biol. 2008;455:111–124. doi: 10.1007/978-1-59745-104-8_8. [DOI] [PubMed] [Google Scholar]
- 10.Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–191. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 12.Seibel MJ. Molecular markers of bone turnover: biochemical, technical, and analytical aspects. Osteoporos Int. 2000;11:S18–S29. doi: 10.1007/s001980070003. [DOI] [PubMed] [Google Scholar]
- 13.Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA Guidelines and animal models for osteoporosis. Bone. 1995;17:125S–133S. doi: 10.1016/8756-3282(95)00285-l. [DOI] [PubMed] [Google Scholar]
- 14.Blair HC, Robinson LJ, Huang CL, Sun L, Friedman PA, Schlesinger PH, Zaidi M. Calcium and bone disease. Biofactors. 2011;37:159–167. doi: 10.1002/biof.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elders PJ, Lips P, Netelenbos JC, van Ginkel FC, Khoe E, van der Vijgh WJ, van der Stelt PF. Long-term effect of calcium supplementation on bone loss in perimenopausal women. J Bone Miner Res. 1994;9:963–970. doi: 10.1002/jbmr.5650090702. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher JC. Role of estrogens in the management of postmenopausal bone loss. Rheum Dis Clin North Am. 2001;27:143–162. doi: 10.1016/s0889-857x(05)70191-5. [DOI] [PubMed] [Google Scholar]


