Abstract
Background: Preeclampsia (PE) and eclampsia remain leading causes of maternal and fetal mortality worldwide. The kidney is considered the first and most severely affected organ in women with PE/eclampsia. In this study, we analyzed new morphologic features of kidney biopsies and clinical findings in patients with PE or eclampsia at our hospital. Methods: Eight patients with PE/eclampsia underwent renal biopsies during the antepartum (3/8) or postpartum (5/8) period. Maternal clinical findings, major serological indices, neonatal outcomes, and renal histopathologic and immunofluorescent characteristics were reviewed for each case. Results: Most patients had abnormal serum cholesterol (8/8), triglyceride (6/8), albumin (7/8), and uric acid (5/8). The ratio of blood urea nitrogen (BUN) to serum creatinine (SCr) was elevated in all patients. Five of eight newborns survived. Various degrees of morphologic change were present in the renal glomeruli, and were associated with proteinuria. All patients had deposition of complement factor 4 (C4) in the renal glomeruli and seven had deposition of immunoglobulin M (IgM). Conclusion: Endotheliosis, vacuolation of podocytes, proliferation of mesangial cells, and protein casts in the tubule lumens were found in the kidneys of women with PE/eclampsia. Immune depositions of C4 and IgM are major contributors to renal lesions in preeclamptic patients, whose neonates can generally survive. Eclampsia can occur without increased blood pressure.
Keywords: Renal biopsy, preeclampsia, eclampsia, morphologic finding, clinical finding
Introduction
Scientists and clinicians have studied preeclampsia (PE) and eclampsia for several decades. The conditions remain life-threatening complications of pregnancy and leading causes of maternal and fetal morbidity and mortality around the world, especially in developing countries. PE is defined as de novo hypertension (≥ 140/90 mmHg) with substantial proteinuria (≥ 0.3 g/24 h) at or after 20 weeks’ gestation [1,2]. The worldwide incidence of PE is 2-8% [3]. It has been reported that nearly a half of maternal deaths and more than a half of fetal deaths can be attributed to PE worldwide [4], and that PE increases the risk of maternal cardiovascular disease later in life [5]. Eclampsia is defined as seizures occurring as a result of PE without any other explicit causes, and is also an important cause of both perinatal fetal and peripartum maternal death [6].
The pathogenesis of PE/eclampsia remains unclear because it is a multifactorial disease with no single causative factor [7]. A multi-stage hypothesis can explain many of the multisystemic pathological alterations [8], including initial abnormal immune tolerance, followed by abnormal placentation and spiral artery remodeling, and subsequent placental hypoxia-ischemia, which induces the release of placenta-derived adverse factors into the maternal circulation. These factors can cause systemic inflammation and have extensive effects on the maternal body, and the kidney is usually the first and most severely affected organ. Thus, the kidney deserves particular attention because of its significant physiological and pathologic changes during pregnancy.
The main purpose of renal biopsy in preeclamptic patients is to examine the intrinsic etiology and histopathology of renal injuries, and to determine definitively the best therapeutic strategies for patients. For example, the diagnosis of latent or chronic renal disease during pregnancy with high blood pressure would dictate further treatment and maintenance of pregnancy, whereas the diagnosis of PE would lead to immediate termination of pregnancy. Percutaneous renal biopsy by ultrasound-guided fine needle aspiration is a frequently used method to distinguish the cause of renal damage, despite its debated safety in pregnancy [9]. Because of the risk of fetal or maternal complications, renal biopsy should be delayed until the postpartum period in patients with definitively diagnosed renal disease or with no features of systemic disease [10].
There are few immunofluorescent studies on the deposition of crucial antibody or complement within the renal glomeruli of PE patients [11]. In this study, we focused primarily on the histopathologic and immunofluorescent characteristics of kidney biopsies taken antepartum or immediately postpartum from PE patients. Maternal and neonatal clinical findings and major serological indices were also analyzed. This study provides new morphologic findings, new clinical findings, and new serological predictors of PE/eclampsia for a better understanding of this challenging disease.
Methods
Subjects
From January 2007 to January 2012, eight renal biopsies were performed on patients during pregnancy or immediately after delivery at our hospital. Renal biopsies were performed for clinical purposes during pregnancy to help differentiate renal disease secondary to severe PE and eclampsia from other renal diseases, and in the postpartum period to confirm the degree of renal injury as a guide to treatment [12]. Patients were diagnosed with either severe PE or eclampsia according to the criteria of Williams Obstetrics (23rd edition). PE was defined as blood pressure ≥ 140/90 mmHg on two or more readings after 20 weeks’ gestation and without previous hypertension history, combined with proteinuria ≥ 0.3 g/24 h or ≥ 1+ in random clean-catch midstream urine collected for dipstick testing. Severe PE was defined as systolic blood pressure ≥ 160 mmHg (with or without diastolic blood pressure ≥ 110 mmHg) and proteinuria (≥ 2 g/24 h urine or ≥ 2+), as we have previously described [13,14]. Eclampsia was defined as seizures in a woman with PE that could not be attributed to other causes. None of the patients had a history of diabetes mellitus, systemic lupus erythematosus, chronic hypertension, nephropathy, or recent urinary tract infection.
The study protocol was reviewed and approved by the Research Ethics Committee of Daping Hospital, and informed consent was received from all participating patients and their families. The patients were thoroughly informed about the renal biopsy procedure and the risk of complications, including renal hematoma or hematuria, and they agreed that their renal tissue could be used for additional research.
Preparation for b
All pregnancies were dated according to ultrasonographic measurement of the fetal biparietal diameter and crown-rump length at the 10th to 12th week of gestation, and renal biopsies were obtained during the 23th to 38th gestational week. Electrocardiogram, complete blood cell counts, blood typing, and blood coagulation testing (including plasma-activated partial thromboplastin time and plasma prothrombin complex activity) were checked for normality the day before biopsy. Serum creatinine, urea nitrogen, and urine protein were also tested to further clarify the state of illness. To determine kidney size, morphology, and cortical thickness, an ultrasound exam was performed prior to biopsy. The puncture process was explained to patients to ensure their close cooperation. Patients were trained in advance to hold their breath and to urinate in bed so as to adapt themselves to the conditions during and after the biospy. Patient blood pressure was maintained close to 140/90 mmHg by continuous intravenous infusion of nicardipine hydrochloride at a rate of 0.5-3 μg/(kg·min).
Fine needle aspiration
The eight renal biopsies were performed by the same highly experienced nephrologist according to a standardized procedure. The right kidney was preferred unless there was pronounced hydronephrosis. The patient lay in a prone position, with a firm pillow positioned below the costal margin to fix the bilateral kidneys. After routine sterilization with iodophors, the skin at the insertion position was covered with a sterile drape. Local anesthesia was achieved with 8-10 mL of a 2 mg/mL solution of lidocaine hydrochloride deposited subcutaneously and towards the kidney. A 3- to 5-mm skin incision was made, and renal tissue was obtained using an automated biopsy device (Bard Biopsy Systems, Tempe, AZ, USA) and an 18-gauge disposable cutting needle. The biopsy device was fitted with an ultrasonographic transducer, which allowed real-time ultrasound evaluation. The scanning profile was adjusted according to the biopsy guidelines on the ultrasonography platform to facilitate the choice of biopsy sites within the kidney [15]. When the needle tip reached the renal capsule, the physician instructed the patient to hold her breath. The needle promptly penetrated the renal capsule and retrieved a small biopsy sample, approximately 20 mm in length. As a rule, two samples from two specific locations were taken from each patient, including the caudal pole and a renal column (renal cortex between two pyramids). The biopsy samples were immediately immersed in sodium chloride solution.
All patients remained in a decubitus position for 24 h, and were monitored for complications after biopsy. Urine color was continuously observed. Vital signs including blood pressure, heart rate, and respiratory frequency were measured every 30 min for 6 h, and then hourly for an additional 6 h. Fetal heart rate was monitored for 1 h following the biopsy procedure. The punctured kidney was re-examined by ultrasonography. Patients were encouraged to drink extra water to induce mild diuresis to avoid urinary obstruction by blood clot after renal hemorrhage. To prevent infection and hemorrhage, antibiotics and hemostatics were administered intravenously for 2-3 days.
Light microscope and immunofluorescence analysis
Freshly biopsied tissues were fixed with 10% polyoxymethylene in phosphate-buffered solution (PBS) for 24 h at 4°C, and then dehydrated in 30% sucrose in PBS for another 24 h. The specimens were then embedded in paraffin using standard techniques. Histological sections 6 μm thick were applied to polylysine-treated microscope slides and air dried overnight at room temperature [16]. The sections were divided into two groups for either light microscope or immunofluorescence analysis. The sections examined by light microscope were stained with hematoxylin-eosin, periodic acid-Schiff (PAS), and Masson trichrome. Immunofluorescent analysis of the sections was performed by direct staining with fluorescent-labeled rabbit anti-human immunoglobulins (Ig) IgM, IgA, IgG, and complement components C1q, C3, and C4 [12]. Only those light microscope sections containing ≥ 10 renal glomeruli were considered acceptable specimens for diagnosis. Histological and pathological features were evaluated by two experienced pathologists with no knowledge of the patient’s clinical condition (single blind). Finally, all sections were evaluated by a third senior pathologist to minimize the possibility of observational errors.
Endotheliosis was evaluated and semiquantitatively graded as none, mild, moderate, or severe. In light microscopy, the grades were defined as follows: none, no endotheliosis; mild, less than 20% obliteration of the glomerular capillary lumen; moderate, 20-80% obliteration of the lumen; severe, more than 80% obliteration of the lumen [15].
Results
Clinical manifestations and laboratory findings
In this study, eight women, aged 24-37 years (mean, 30.5 ± 4.1 years), underwent renal biopsies, including three antepartum and five in the first 2 weeks postpartum (Table 1). All patients were given antihypertensive medication at the time of biopsy. The patients all had pitting edema of both lower extremities. Five women were nulliparous and three were multiparous. Their maximal blood pressures met the standard of systolic pressure ≥ 140 mmHg and/or diastolic pressure ≥ 90 mmHg. Although the 24 h urinary protein of one patient (No. 2) did not meet or exceed 2 g (Table 1), her random urine protein was +++ (Table 2), meeting the criterion for diagnosis of severe PE. Therefore, all patients were diagnosed with severe PE except one patient with eclampsia (Table 1). One patient (No. 5) had a generalized seizure after admission for treatment of PE complicated by edema, hypertension, and proteinuria. The seizure could not be attributed to other causes, so the patient was diagnosed with eclampsia. The blood pressures of all patients decreased to normal levels within 1 week after placental expulsion. All eight women had singleton pregnancies.
Table 1.
Maternal characteristics and clinical features
| Patient (No.) | Age (yr) | Gestation at delivery | Gestation at biopsy | Gravidity | Parity | Systolic pressure (mmHg) | Diastolic pressure (mmHg) | 24 h urinary protein (g/L) | Principal diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 33 wk 5 d | Postpartum d 3 | 2 | 0-1-1-1 | 180 | 100 | 2.47 | sPE |
| 2 | 37 | 23 wk 4 d | Postpartum d 1 | 4 | 1-0-2-1 | 180 | 100 | 0.92 | sPE |
| 3 | 30 | 34 wk 4 d | 28 wk 1 d | 2 | 0-1-1-1 | 150 | 110 | 4.02 | sPE |
| 4 | 33 | 33 wk 6 d | 30 wk 5 d | 5 | 1-1-3-2 | 150 | 105 | 3.41 | sPE |
| 5 | 24 | 38 wk 4 d | Postpartum d 5 | 1 | 1-0-0-1 | 135 | 93 | 8.91 | Eclampsia |
| 6 | 34 | 34 wk 2 d | 28 wk 3 d | 1 | 0-1-0-1 | 160 | 110 | 17.16 | sPE |
| 7 | 28 | 32 wk 0 d | Postpartum d 5 | 1 | 0-1-0-0 | 180 | 110 | 4.8 | sPE |
| 8 | 26 | 33 wk 6 d | Postpartum d 7 | 2 | 1-1-0-0 | 160 | 120 | 3.58 | sPE |
sPE: severe preeclampsia.
Table 2.
Serologic testing results
| Patient (No.) | Urinalysis | Hepatic function tests | Renal function tests | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Urinary protein | Urinary occult blood | Total cholesterol (mmol/L) | Total bilirubin (µmol/L) | Bile acids (µmol/L) | Triglyceride (mmol/L) | Plasma albumin (g/L) | BUN (mmol/L) | SCr (μmol/L) | BUN/SCr ratio | Serum uric acid (μmol/L) | |
| 1 | ++++ | +++ | 9.08 | 9.6 | 8.1 | 3.75 | 13.3 | 3.87 | 160.0 | 24.2 | 266.8 |
| 2 | +++ | ++++ | 6.59 | 13.4 | 14.2 | 2.26 | 26.4 | 4.90 | 168.9 | 29.0 | 201.6 |
| 3 | +++ | + | 6.43 | 6 | 52.7 | 2.24 | 23.7 | 1.96 | 65.3 | 30.0 | 388 |
| 4 | +++++ | +++++ | 6.22 | 15.7 | 26.5 | 1.43 | 26.6 | 3.84 | 110.0 | 35.0 | 584.6 |
| 5 | ++++ | + | 6.17 | 12.9 | 13.3 | 1.22 | 16.3 | 2.88 | 90.0 | 32.0 | 631.5 |
| 6 | ++++ | +/- | 8.2 | 12.1 | 9.1 | 3.41 | 23.2 | 3.64 | 52.4 | 69.5 | 481.5 |
| 7 | +++ | + | 6.26 | 11.9 | 3.2 | 2.26 | 43.4 | 1.54 | 52.5 | 29.3 | 377.9 |
| 8 | +++ | ++ | 6.0 | 7.2 | 14.6 | 1.73 | 18.3 | 1.97 | 44.6 | 44.2 | 184.0 |
All patients presented with greater than +++ random urine protein and with microscopic hematuria of different degrees (Table 2). In addition to those laboratory tests conducted to prepare for biopsy, blood tests included cholesterol, plasma albumin, bile acids, triglyceride (TG), total bilirubin, blood urea nitrogen (BUN), and serum creatinine (SCr) (Table 2). All patients had high cholesterol (≥ 6 mmol/L), and most also had elevated TG (≥ 2.00 mmol/L). Although the total bilirubin of all patients was within the normal range, more than a half had elevated bile acids, with one reaching 52.7 μmol/L (No. 3). All patients had very low plasma albumin levels, with a mean of 23.9 ± 4.1 g/L. The lowest plasma albumin was 13.3 g/L (Table 2). Five patients had markedly elevated BUN; however, only patient No. 6 had an especially high BUN/SCr ratio, because the level of SCr generally elevated correspondingly. Another important marker of renal function, serum uric acid (SUA), rose significantly in five patients, with a mean of 23.9 ± 4.1 g/L (Table 3).
Table 3.
Mode of delivery and neonatal characteristics
| Maternal | Neonatal | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Subject (No.) | Delivery Mode | Apgar Score | Fetal weight (g) | Morbidity | Mortality |
| 1 | Caesarian Section | 9-9-9 | 2120 | Pathologic jaundice, low birth weight | Survived |
| 2 | Induced Labor | - | Unknown | - | Neonatal death |
| 3 | Caesarian Section | 10-10-10 | 1380 | Acute respiratory distress syndrome; pathologic jaundice; intrauterine growth restriction; very low birth weight | Survived |
| 4 | Caesarian Section | 9-9-9 | 2150 | Pathologic jaundice, anemia, low birth weight | Survived |
| 5 | Caesarian Section | 10-10-10 | 2700 | None | Survived |
| 6 | Caesarian Section | 10-10-10 | 2150 | None | Survived |
| 7 | Induced Labor | - | Unknown | - | Intrauterine fetal death |
| 8 | Induced Labor | - | Unknown | - | Intrauterine fetal death |
For two cases of intrauterine fetal death the fetal body weights were not measured because of the guardians’ refusal (Nos. 7 and 8). In the case of one neonatal death at another hospital, the neonate’s body weight was also unknown (No. 2). The other neonates all survived, according to our follow-up. The Apgar scores of the surviving neonates 1 min-5 min-10 min after birth were all ≥ 9-9-9 (Table 3). One neonate with a body weight of 1380 g was diagnosed with pathologic jaundice, intrauterine growth restriction, and very low birth weight (No. 3). This newborn and two others (Nos. 1 and 4) who were diagnosed with pathologic jaundice were sent to the neonatal intensive care unit at Daping Hospital immediately after birth for treatment. Detailed data are shown in Tables 1, 2, and 3.
Pathological characteristics of renal tissues
At least two bands of renal cortex and medulla tissues were taken for full examination from each biopsy sample. Each sample contained more than 10 glomeruli, with a mean of 25.6 ± 12.1, to ensure the diagnostic quality of each pathological section. The sample from patient No. 1 had three segmental sclerotic, four completely superseded, and 11 intact glomeruli. The sample from patient No. 3 had five global sclerotic and 28 intact glomeruli. Other patients had only three superseded glomeruli (No. 2) or two segmental sclerotic glomeruli (No. 7), or no injured glomeruli observed under the light microscope. Each intact glomerulus contained approximately 100-110 ± 10 cells. The glomerular volume was slightly high in most samples, but one sample had an increased volume with inflammatory cell infiltration surrounding the glomerulus (No. 5). Four patients’ sections displayed one or two thickened and laminated capsule walls, as well as adhesions between glomeruli and capsule to different degrees (Nos. 1, 3, 5, and 7). The other four patients showed no thickening, lamination, or adhesions of Bowman’s capsules to glomeruli (Nos. 2, 4, 6, and 8). Vacuolation appeared in the endothelial cells of three biopsy samples (Nos. 1, 2, and 3), while in the other five samples the endothelial cells displayed partial vacuolation or swelling. Patient No. 6, whose six renal pathological sections are shown in Figure 1, was diagnosed with severe PE based on a 24 h urinary protein of 17.16 g/L. Most of the endothelial cells appeared in pairs in one glomerulus (Figure 1A), and displayed diffuse cytoplasmic vacuolation in another glomerulus (Figure 1B).
Figure 1.
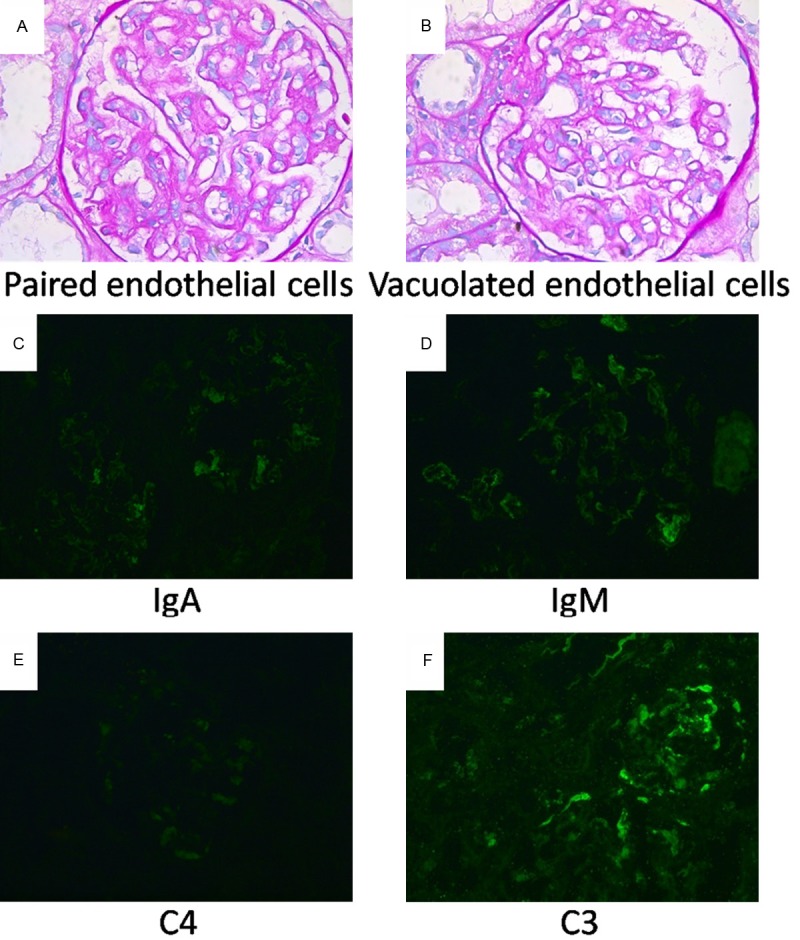
Typical pathological and immunofluorescent features of renal glomeruli in a patient (No. 6) with severe preeclampsia. (A, B) PAS staining of renal glomeruli. Mesangial cells and mesangial matrix of the glomeruli are increased slightly. Most endothelial cells are swollen, and some present in pairs (A) while some show vacuolation (B). The glomerular basement membrane shows no thickening, no spikes, and no track formation. A small amount of protein is seen in the renal tubular lumen. No significant tubular atrophy, interstitial fibrosis, or mononuclear cell infiltration are present in the renal glomeruli. The arch arteries and interlobular arteries also have no obvious abnormalities. (C-F) Immunofluorescent staining of renal glomeruli. Both IgM (D) and C3 (F) are deposited along the mesangial area with strong fluorescence intensity (++). The depositions of IgA (C) and C4 (E) are observed, but the fluorescence intensity is weak (±). The fluorescence of IgG and C1q were not observed (not shown). Original magnification ×200.
The renal glomeruli in most biopsy samples had endotheliosis of different grades on semiquantitative microscopic examination. The glomerular endotheliosis contributed directly to occlusion of the capillary loops. Details of the endothelial manifestations and degrees of capillary loop occlusion are shown in Table 4. The glomerular podocytes were vacuolated in all patients. There was mild proliferation of mesangial cells in all subjects, and moderate proliferation in one (No. 1). The extent of mesangial matrix increase varied among patients, and included segmental increase, slight increase, slight segmental increase, and no obvious increase. The specific results from each patient are shown in Table 4.
Table 4.
Light microscope findings with HE staining
| Subject (No.) | Number of injured glomeruli in different degrees | Cells per glomerulus | Characteristics of glomeruli | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Intact | Segmental sclerosing | Global sclerosing | Superseded | Volume | Bowman’s capsule | Endothelial cells | Endotheliosis | Podocytes | Mesangial cells | Mesangial matrix | Patency of capillary loops | ||
| 1 | 11 | 3 | 0 | 4 | 110 ± 10 | Slightly enlarged | One capsule wall thickened and laminated, two adhesions of glomerulus and capsule | Vacuolation | None | Vacuolation | Proliferation | Segmental increase | Poor |
| 2 | 21 | 0 | 0 | 3 | 105 ± 10 | Slightly enlarged | No thickening lamination, or adhesions | Vacuolation | Moderate | Vacuolation | Mild proliferation | Slight segmental increase | Good |
| 3 | 28 | 0 | 5 | 0 | 105 ± 10 | Slightly enlarged | One capsule wall thickened and laminated, two adhesions of glomerulus and capsule, one parvicellular crescent and two cellular fibrinous crescents | Vacuolation | Moderate | Vacuolation | Mild proliferation | Slight segmental increase | Good |
| 4 | 38 | 0 | 0 | 0 | 100 ± 10 | Slightly enlarged | No thickening, lamination, or adhesions | In pairs and partial vacuolation | Mild | Vacuolation | Mild proliferation | No increase | Fair |
| 5 | 11 | 0 | 0 | 0 | 110 ± 10 | Enlarged | Two capsule walls thickened and laminated, no adhesions, inflammatory cell infiltration around glomerulus | In pairs and partial vacuolation | Mild | Vacuolation | Mild proliferation | Slight increase | Fair |
| 6 | 24 | 0 | 0 | 0 | 100 ± 10 | Slightly enlarged | No thickening, lamination, or adhesions | In pairs and swollen | Severe | Vacuolation | Mild proliferation | Slight increase | Fair |
| 7 | 26 | 2 | 0 | 0 | 105 ± 10 | Slightly enlarged | Two capsule walls thickened and laminated, one adhesion of glomerulus and capsule | In pairs and partial vacuolation | Mild | Vacuolation | Mild proliferation | No increase | Fair |
| 8 | 46 | 0 | 0 | 0 | 100 ± 10 | Slightly enlarged | No thickening, lamination, or adhesions | In pairs and partial vacuolation | Mild | Vacuolation | Mild proliferation | No increase | Fair |
Changes in glomerular basement membrane and kidney tubules
Pathological changes in the glomerular basement membrane (GBM) and kidney tubules were analyzed using PAS staining (Table 5). Various pathological changes were present in the GBM in these patients. None of the GBM showed rete pegs. The GBM of five patients were thickened, with segmental, accidental, or no double track, or even false double track sign (Nos. 2, 3, 4, 7, and 8). Another two patients had vacuolated GBM (Nos. 1 and 5), and the final patient showed no thickening or double track sign in the GBM (No. 6). There were subepithelial fuchsinophilic deposits in the GBM of two subjects (Nos. 5 and 8). The outer capillary loops in the GBM of one patient were delaminated. Seven patients had protein casts in the tubule lumens. Red blood cell casts were also found in the lumen of one patient’s renal tubules (No. 4). Focal atrophy, interstitial fibrosis, and inflammatory cell infiltration were found in the renal tubules of two patients (NOs. 3 and 5).
Table 5.
Microscopic findings with PAS and immunofluorescent staining
| Subject (No.) | PAS staining | Immunofluorescent staining | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Glomerular basement membrane | Renal tubule | IgM | C4 | C3 | IgG | IgA | C1q | Deposit area | |
| 1 | Vacuolation | Protein cast in lumen | ++ | + | + | - | - | - | Deposits in capillary loops |
| 2 | Thickening, no prominent spike or double track sign | Protein cast in lumen | - | + | - | - | - | - | Deposits in capillary loops |
| 3 | Thickening and segmental double track, but no spike | Protein cast in lumen, focal atrophy, interstitial fibrosis, and inflammatory cell infiltration | ++ | ++ | - | - | - | - | Deposits in capillary loops |
| 4 | Thickening, accidental double track, but no spike | Protein and red blood cell casts in lumen | + | + | - | - | + | - | Granular deposits along capillary loops |
| 5 | Vacuolation, false double track sign, subepithelial fuchsinophilic deposits | Small necrotic focus, interstitial fibrosis and inflammatory cell infiltration | ++ | + | - | - | - | + | Deposits in capillary loops |
| 6 | No thickening, prominent spike, or double track | Small protein cast in lumen | ++ | + | ++ | - | ± | - | Deposits along mesangial area |
| 7 | Thickening, but no prominent spike or double track | Small protein cast in lumen | ++ | + | ++ | - | ± | - | Granular deposits along capillary loops |
| 8 | Thickening, delamination in outer capillary loop, segmental double track, slight subepithelial fuchsinophilic deposits | Small protein cast in lumen | ++ | + | - | ++ | ++ | - | Granular deposits along capillary loops |
The immunopathologic characteristics of the glomeruli and adjacent tissues were revealed by immunofluorescent staining (Table 5). Complement component 4 (C4) was positive or strongly positive (+/++) in the glomeruli of all patients. However, only two displayed strongly positive complement component 3 (C3) and only one had positive complement component 1q (C1q). IgM was positive or strongly positive (+/++) in all but one patient, while IgG was negative (-) in all patients except No. 5. IgA varied from ++, +, ±, to - among patients. The immunofluorescent staining results from the renal glomeruli of patient No. 6 are shown in Figure 1. The fluorescence intensity of IgM and C3 were both strongly positive (++), with green fluorescence mainly distributed on the surface of endothelial cells (Figure 1D and 1F). The fluorescence intensity of IgA and C4 were mild and positive (± and +), respectively (Figure 1C and 1E). The immune deposits in most renal glomeruli, both granular and linear, were positioned in or along the capillary loops. In one patient, the immune complex depositions were distributed along the mesangial area.
Discussion
Maternal characteristics and outcomes of biopsy cases
PE is a serious gestational complication worldwide that causes short- and long-term morbidity, and eclampsia is potentially lethal for both pregnant women and newborns. The patient in our study who suffered from eclampsia (No. 5) did not show any signs or complain of discomfort in advance. Her blood pressure was only 135/93 mmHg. This finding reminds us of the importance of vigilance to prevent the occurrence of eclampsia in patients without symptoms and without markedly elevated blood pressure. This patient’s urinalysis results and renal and hepatic function tests were not extremely abnormal, except for SUA. Although SUA has not been considered a good predictor of PE in the past [17,18], an increasing number of studies have found that high SUA levels in women with gestational hypertension indicate a high risk of developing adverse pregnancy outcomes, including HELLP syndrome and eclampsia [19-21]. All but one patient in this report was under 35 years of age, although maternal age over 35 years is an independent risk factor for PE [22]. This finding indicates that younger mothers are also susceptible to severe PE and eclampsia. Severe PE and eclampsia can occur in both primiparae and multiparae.
A variety of complex changes in hepatic function and lipid profile metabolism occur in pregnant women, and some serological markers reach very high levels in patients with PE or eclampsia [23]. There is evidence that a significant change in the absolute magnitude of liver function tests is related to bad perinatal outcomes [24]. Cholesterol acts as an atherogenic lipid, which may contribute to endothelial cell dysfunction and different pathologic processes, inducing hypertension [25]. The total cholesterol levels of all eight patients in this study were elevated. The patients’ normal total bilirubin levels indicated no severe liver disease or biliary obstruction, a finding in agreement with previous studies [26]. The bile acids of five patients were elevated to ≥ 10 μmol/L. Serum bile acids are synthesized in the liver from cholesterol and may be a potential predictive biomarker for PE [27]. Five patients in our study showed a high TG level, a common feature of metabolic syndrome. There is a consistent positive association between increased serum TG and the occurrence of PE, but further research is needed [28]. In recent years, TG assessment has been considered a good predictor of PE during the third trimester of pregnancy [29].
Most pregnant women have low plasma albumin levels because of maternal metabolism and fetal absorption. In preeclamptic women, hepatic blood flow is reduced because of the high filtration pressure in the capillaries, leading to a decline in plasma albumin production. At the same time, various renal injuries, especially glomerular endothelial injury, can result in albuminuria (see below). Thus, all but one patient in our study has decreased plasma albumin levels. Plasma albumin is not an independent marker for the severity of PE or for other probable adverse complications and conditions [30]. However, previous investigations have found that hypoalbuminemia is an early sign of developing PE [31]. More stringent trials are necessary to resolve this controversy.
BUN is produced in the liver as a by-product of protein metabolism, and is excreted through the kidneys, and thus is an important indicator of renal and liver function [32]. As pregnancy continues, BUN decreases because of increased glomerular filtration rate (GFR) and blood volume as well as decreased maternal protein metabolism [33]. SCr is commonly used to assess renal function, but is influenced by many factors, including age, body size, diet, drugs, gender, and race. In our study, the BUN/SCr ratio of all patients was higher than 20:1, indicating severe impairment of renal function [32,34,35]. Some studies have suggested that the BUN/SCr ratio is not an adequate indicator of renal lesions in PE patients [36,37], although some researchers have considered it a substitute for 24 h urine protein testing [38,39]. Based on our findings, we recommend the BUN/SCr ratio as a new predictive factor for severe PE.
Fetal outcomes of biopsy cases
Three patients (Nos. 2, 7, and 8) received initial treatment at other primary hospitals, and chose labor induction to terminate pregnancy. One premature infant did not survive because of early gestational age (23 weeks, 4 days). The other five patients gave birth by caesarean section, and four of these neonates with low birth weight survived. They were sent to the neonatal intensive care unit at our hospital immediately after birth. With spreading improvements in the clinical management of neonates, the mortality of very low birth weight newborns of preeclamptic pregnancies in developing countries has decreased drastically in recent decades [6]. The Apgar scores of the neonates in this study were favorable, although most were at only 32-34 weeks’ gestation. The placenta in patients with PE is chronically ischemic and hypoxic [40], so that the fetus grows in an environment lacking nutrition and oxygen. These conditions may lead to increased ability of premature newborns to survive the extrauterine environment and obtain high Apgar scores, even when the birth weight is very low.
Complications and significance of renal biopsy
Pregnancy is regarded by many clinicians as a relative contraindication for renal biopsy because of the underlying risk of complications. It is recommended that renal biopsy be considered if a pregnant woman shows sudden deterioration for unknown reason before 32 weeks’ gestation [15,41]. In our hospital, the outcome of neonates after week 30 is generally good, regardless of the underlying condition. This information provides strong support for a wide time window, usually before gestational week 30, for performing renal biopsy. In fact, our treatment level compares favorably with neonatal intensive care in developed countries [9]. Close maternal and fetal assessments are undoubtedly indispensable after renal biopsy. Previous studies have reported that the major complications of renal biopsy include post-biopsy hemorrhage, perirenal hematoma, infection, perirenal abscess, and even sepsis [42]. The incidence of complications is controversial, varying from 2% to 6.7% [10]. In our study, most participants merely underwent minor discomfort, and two patients complained of loin pain, while others had no complications. A low dose of an analgesic such as ibuprofen effectively alleviated the loin pain. All patients had fully recovered within 2 days. Our obstetricians and nephrologists agree that renal biopsy should not be considered if the patient’s blood pressure is poorly controlled, or if the patient has a severe hemorrhagic tendency. Renal biopsy should be recommended if the clinical evidence for a diagnosis of PE is insufficient or when clarification of the diagnosis will lead to significant treatment decisions, for example, the decision to terminate pregnancy. Our data support the view that renal biopsy in pregnancy should not be performed liberally, but is useful to help diagnose disease and to guide therapeutic decisions.
Glomerular lesions were present in all of our patients. Generally, the magnitude of the glomerular lesions increased with worsening clinical condition, a finding in accordance with a previous study [43]. Glomeruloendotheliosis in different degrees was noted in seven patients. Four patients (Nos. 1, 3, 4, and 5) were advised to prolong their pregnancies, which may have contributed to the survival of their babies. Although there is some evidence that glucocorticoid therapy may promote the recovery from glomeruloendotheliosis [44], we did not use glucocorticoids antenatally in our patients because of their potential side effects, including fetal malformation and premature delivery.
Normal pregnancy and preeclampsia contribute to renal lesions
Normal pregnancy can cause a series of renal changes. The length of the kidney increases by 1 cm, and renal volume increases by at least 30% because the renal vasculature and interstitium enlarge. Renal blood flow increases by 80% during pregnancy. The renal collecting system of many gravidae is mildly expanded in middle pregnancy. These physiological changes in the kidney during pregnancy, which can be present even without complications, disappear soon after delivery.
The glomeruli, renal tubules, and arterioles all undergo varying degrees of structural change in preeclamptic pregnancies. The most outstanding feature is glomerular lesions, including slightly increased volume and decreased capillary lumen diameter. In our findings, the increase in glomerular volume correlated with the severity of the patient’s clinical condition. Glomerular changes result from endothelial swelling, vacuolization of endothelial fenestrae, and encroachment in the capillary lumen, a combination of changes known as endotheliosis, which can lead to occlusion of capillary lumens and ischemia. Although endotheliosis occurs in normal pregnancy, diffuse glomerular endotheliosis is a specific and pathognomonic feature of glomerular lesions of PE patients [45,46].
The GFR increases from the 6th gestational week until delivery in normal pregnancy, but SCr, BUN, and SUA decrease. Renal ultrafiltration in pregnant women is caused by decreased wall pressure of blood flow across the glomerular capillaries. A study of renal physiology reported that PE patients suffer hypoperfusion of renal plasma flow to different degrees [47]. The low filtration in PE patients is secondary to the structural glomerular changes. As previously reported and confirmed by our findings, the effective filtration area of preeclamptic kidneys decreases significantly because of fibrinoid deposition and mesangial cell proliferation. The glomerular endothelial fenestrae are large enough to allow passage of albumin and immunoglobulin. The reduction in the density and aperture size of the endothelial fenestrae, together with the accumulation of subendothelial fibrinoid deposits, depress the glomerular hydraulic permeability by 25-30% [47]. In fact, it has been reported that plasma albumin does not penetrate significantly into endothelial fenestrae during normal blood flow [48]. However, when blood flow stops, albumin moves into the subendothelial space, which may be the source of subepithelial fuchsinophilic deposits in patients with PE. The thickening and vacuolization of the GBM may result in part from the low renal filtration in PE patients, although this possibility has not been seriously considered to date.
The 24 h urinary protein excretion test has been considered the “gold standard” for quantitative analysis of proteinuria during pregnancy worldwide [17,49]. We employed this classic method to assess and diagnose the patients in our biopsy study. A small amount of proteinuria is regarded as a normal result of renal ultrafiltration in pregnant women. The selectivity of solute molecules passing through renal glomeruli depends not only on molecular size but also on electric charge. The renal podocytes, adhering to the GBM with many digitations, play a crucial role in maintaining the integrity of the glomerular slit diaphragm, which is considered the primary filtration barrier for macromolecular proteins [50]. If plasma proteins cannot pass through the endothelial fenestrae, the glomerular capillary wall is blocked by the slit diaphragm, and filtration ceases [51]. The podocytes in all eight of our PE patients were injured with vacuolation, which can lead to podocyte apoptosis, podocyte loss, and even detachment from the GBM. In addition, some lesions of the glomerulus mechanical barrier were present, such as the double track sign and subepithelial fuchsinophilic deposits. These changes may partially explain the increased protein in the urine of PE patients. At the same time, the electric charge of protein molecules directly affects their filtration across the glomerulus and their reabsorption from the kidney tubules. It has been shown that a thick (> 200 nm) layer of highly glycosylated and negatively charged proteins anchored in the glomerular endothelial plasma membrane covers the endothelial cells and endothelial fenestrae to form the end othelial surface layer [52]. This layer may establish a charge difference across the glomerular capillary that further enhances its selectivity [53]. Disruption of the endothelial surface layer may disturb its charge selectivity and alter its permeability to macromolecules [54,55]. Thus, lesions of the glomerular charge barrier may also contribute to massive proteinuria and protein casts in the lumen of renal tubules. Conversely, casts of different types, focal necrosis, interstitial fibrosis, and inflammatory cell infiltration can alter the electrical charge microenvironment around the GBM and induce a vicious circle of altered renal filtration.
Complement components and immunoglobulin deposits in renal glomerulus
Pregnancy can be considered a successful allotransplantation. Maternal kidneys can develop immune reactions to proteins or other substances released from the fetus or placenta, leading to renal lesions of different degrees, though this is a rare occurrence. The complement system plays an important role in both innate and adaptive immunity, engaging in immunological attacks in seconds to opsonize and induce the immediate release of proinflammatory and chemotactic peptides [56]. The complement system can generally be activated in three ways: the classical pathway, the alternative pathway, and the lectin pathway. C3 occupies a pivotal position in these complement activation pathways, and the initial recognition event leads to cleavage of C3 molecules into C3a and C3b [57]. In PE, the serum C3 concentration usually correlates with the patient’s clinical outcome. C3b is involved in the production of C5b-9 (membrane attack complex, MAC) via the cleavage of C5 in the classical and lectin pathways [58], and C5b-9 is associated with cell injury [59]. C3a and C5a are the cleavage products of C3 and C5, respectively. They can bind to their corresponding receptors on various cells (including epithelial, endothelial, and immune cells) to induce inflammatory responses. C3 depositions in the glomerulus indicate severe glomerulopathy [60], which mainly manifested in our study as endothelial cell swelling, expansion of the subendothelial zone, duplication of GBM, and mesangial cell interposition. C3 and MAC deposits along the basement membrane may also partly result from renal ischemia-reperfusion injury, which is caused by inflammatory cascades inducing transient interruption of renal blood flow and the subsequent restoration of renal blood supply [61]. C4 acts as a promoting and regulating protein, especially in the production of MAC. C4 depositions were present in all renal glomeruli samples in this study. C1q protein, known as a complex complement, is responsible for activation of the classical pathway. It has several binding sites for immunological molecules (including IgG and IgM) to form immune complexes [62]. C1q deposition in the meruli of preeclamptic patients may imply renal lesions, including focal segmental glomerulosclerosis.
Immunoglobulin of different isotypes can deposit in the capillary loops and mesangial areas. Granular IgG depositions along glomerular capillary loops can directly lead to delamination of the outer capillary loop, and to subsequent mesangial proliferation and segmental double track. However, the patient’s condition and serum IgG levels may not correlate with the density of IgG deposits [63]. The morbidity caused by glomerular IgG depositions is lower than that caused by IgM or IgA. IgM is a large molecule with a polymeric structure and a high avidity for antigen. Thus, IgM contributes greatly to activation of the complement system and is particularly effective in causing C3b to bind to antigen [64]. Granular IgM deposits in the glomerular mesangium are distributed in regions of focal segmental glomerulosclerosis. Diffuse and global IgM deposits were found in the renal glomeruli of nearly all of our patients. IgA deposits distribute predominantly in the mesangium and paramesangial regions of glomeruli. They may combine with various antigens to form immune complexes on renal cells and they increase the production of various cytokines and growth factors [65,66]. These effects can lead to the common glomerular histological features of mesangial cell proliferation and mesangial matrix expansion. Ultimately, protein and erythrocyte casts form in the renal tubules because of glomerular injury, leading to further tubule damage and proteinuria, hematuria, and even end-stage renal failure [67,68].
In conclusion, endotheliosis, vacuolation of podocytes, proliferation of mesangial cells, and protein casts in the renal tubule lumens were the most common pathologic characteristics of renal biopsies from these eight pregnant Chinese women with PE/eclampsia. As indicated by previous studies, deposition of complement components and immunoglobulin, especially C4 and IgM, may contribute to serious renal lesions. The BUN/SCr ratio may be a new marker for severe PE, based on analysis of serological indices from these biopsied patients, but this hypothesis needs further experimental and clinical support. Eclampsia occurred in one of our patients with a blood pressure of 135/93 mmHg, which challenges our previous experience and understanding. Severe PE and eclampsia may also occur in patients with a high SUA level, even those aged older than 35 years and multiparous. Because of advances in neonatal treatment technology, most neonates of preeclamptic patients can survive. To minimize complications, renal biopsy in pregnancy should only be recommended for definitive diagnosis and improvement of therapeutic strategy, and should be performed based on the general conditions of the patient, including blood pressure and coagulation function.
Acknowledgements
This work was supported by grant of the National Natural Foundation of China (81170576).
Disclosure of conflict of interest
None.
References
- 1.ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77:67–75. [PubMed] [Google Scholar]
- 2.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 3.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 4.WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. Geneva: World Health Organization; 2011. WHO Guidelines Approved by the Guidelines Review Committee. [PubMed] [Google Scholar]
- 5.Milne F, Redman C, Walker J, Baker P, Bradley J, Cooper C, de Swiet M, Fletcher G, Jokinen M, Murphy D, Nelson-Piercy C, Osgood V, Robson S, Shennan A, Tuffnell A, Twaddle S, Waugh J. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ. 2005;330:576–580. doi: 10.1136/bmj.330.7491.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jido TA, Yakasai IA. Preeclampsia: a review of the evidence. Ann Afr Med. 2013;12:75–85. doi: 10.4103/1596-3519.112395. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela FJ, Perez-Sepulveda A, Torres MJ, Correa P, Repetto GM, Illanes SE. Pathogenesis of preeclampsia: the genetic component. J Pregnancy. 2012;2012:632732. doi: 10.1155/2012/632732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, Charnock-Jones DS, Redman CW. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013;61:932–942. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 9.Kuller JA, D'Andrea NM, McMahon MJ. Renal biopsy and pregnancy. Am J Obstet Gynecol. 2001;184:1093–1096. doi: 10.1067/mob.2001.114917. [DOI] [PubMed] [Google Scholar]
- 10.Chen HH, Lin HC, Yeh JC, Chen CP. Renal biopsy in pregnancies complicated by undetermined renal disease. Acta Obstet Gynecol Scand. 2001;80:888–893. doi: 10.1034/j.1600-0412.2001.801004.x. [DOI] [PubMed] [Google Scholar]
- 11.Petrucco OM, Thomson NM, Lawrence JR, Weldon MW. Immunofluorescent studies in renal biopsies in pre-eclampsia. Br Med J. 1974;1:473–476. doi: 10.1136/bmj.1.5906.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Z, Ren H, Yang G, Chen H, Zhou H, Zeng C, Liu Z, Li L. Significance of vascular endothelial growth factor expression in renal tissue of patients with preeclamptic nephropathy. Am J Nephrol. 2005;25:579–585. doi: 10.1159/000089265. [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Chen M, Zhao D, Yi P, Lu L, Han J, Zheng X, Zhou Y, Li L. The H19 gene imprinting in normal pregnancy and pre-eclampsia. Placenta. 2009;30:443–447. doi: 10.1016/j.placenta.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Han J, Li L, Hu J, Yu L, Zheng Y, Guo J, Zheng X, Yi P, Zhou Y. Epidermal growth factor stimulates human trophoblast cell migration through Rho A and Rho C activation. Endocrinology. 2010;151:1732–1742. doi: 10.1210/en.2009-0845. [DOI] [PubMed] [Google Scholar]
- 15.Wide-Swensson D, Strevens H, Willner J. Antepartum percutaneous renal biopsy. Int J Gynaecol Obstet. 2007;98:88–92. doi: 10.1016/j.ijgo.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 16.Lafayette RA, Druzin M, Sibley R, Derby G, Malik T, Huie P, Polhemus C, Deen WM, Myers BD. Nature of glomerular dysfunction in pre-eclampsia. Kidney Int. 1998;54:1240–1249. doi: 10.1046/j.1523-1755.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- 17.Thangaratinam S, Ismail KM, Sharp S, Coomarasamy A, Khan KS. Accuracy of serum uric acid in predicting complications of pre-eclampsia: a systematic review. BJOG. 2006;113:369–378. doi: 10.1111/j.1471-0528.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 18.Cnossen JS, de Ruyter-Hanhijarvi H, van der Post JA, Mol BW, Khan KS, ter Riet G. Accuracy of serum uric acid determination in predicting pre-eclampsia: a systematic review. Acta Obstet Gynecol Scand. 2006;85:519–525. doi: 10.1080/00016340500342037. [DOI] [PubMed] [Google Scholar]
- 19.Koopmans CM, van Pampus MG, Groen H, Aarnoudse JG, van den Berg PP, Mol BW. Accuracy of serum uric acid as a predictive test for maternal complications in pre-eclampsia: bivariate meta-analysis and decision analysis. Eur J Obstet Gynecol Reprod Biol. 2009;146:8–14. doi: 10.1016/j.ejogrb.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Xiong X, Fraser WD, Luo ZC. Association of uric acid with progression to preeclampsia and development of adverse conditions in gestational hypertensive pregnancies. Am J Hypertens. 2012;25:711–717. doi: 10.1038/ajh.2012.18. [DOI] [PubMed] [Google Scholar]
- 21.Kaypour F, Masomi Rad H, Ranjbar Novin N. The predictive value of serum uric acid, roll-over test, and body mass index in pre-eclampsia. Int J Gynaecol Obstet. 2006;92:133–134. doi: 10.1016/j.ijgo.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Lamminpaa R, Vehvilainen-Julkunen K, Gissler M, Heinonen S. Preeclampsia complicated by advanced maternal age: a registry-based study on primiparous women in Finland 1997-2008. BMC Pregnancy Childbirth. 2012;12:47. doi: 10.1186/1471-2393-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohil JT, Patel PK, Gupta P. Estimation of lipid profile in subjects of preeclampsia. J Obstet Gynaecol India. 2011;61:399–403. doi: 10.1007/s13224-011-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozic JR, Benton SJ, Hutcheon JA, Payne BA, Magee LA, von Dadelszen P. Abnormal liver function tests as predictors of adverse maternal outcomes in women with preeclampsia. J Obstet Gynaecol Can. 2011;33:995–1004. doi: 10.1016/S1701-2163(16)35048-4. [DOI] [PubMed] [Google Scholar]
- 25.Belo L, Caslake M, Gaffney D, Santos-Silva A, Pereira-Leite L, Quintanilha A, Rebelo I. Changes in LDL size and HDL concentration in normal and preeclamptic pregnancies. Atherosclerosis. 2002;162:425–432. doi: 10.1016/s0021-9150(01)00734-1. [DOI] [PubMed] [Google Scholar]
- 26.Adiga U, D’Souza V, Kamath A, Mangalore N. Antioxidant activity and lipid peroxidation in preeclampsia. J Chin Med Assoc. 2007;70:435–438. doi: 10.1016/S1726-4901(08)70034-0. [DOI] [PubMed] [Google Scholar]
- 27.Jamjute P, Ahmad A, Ghosh T, Banfield P. Liver function test and pregnancy. J Matern Fetal Neonatal Med. 2009;22:274–283. doi: 10.1080/14767050802211929. [DOI] [PubMed] [Google Scholar]
- 28.Ray JG, Diamond P, Singh G, Bell CM. Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. BJOG. 2006;113:379–386. doi: 10.1111/j.1471-0528.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 29.Ziaei S, Bonab KM, Kazemnejad A. Serum lipid levels at 28-32 weeks gestation and hypertensive disorders. Hypertens Pregnancy. 2006;25:3–10. doi: 10.1080/10641950500543756. [DOI] [PubMed] [Google Scholar]
- 30.Benoit J, Rey E. Preeclampsia: should plasma albumin level be a criterion for severity? J Obstet Gynaecol Can. 2011;33:922–926. doi: 10.1016/s1701-2163(16)35017-4. [DOI] [PubMed] [Google Scholar]
- 31.Gojnic M, Petkovic S, Papic M, Mostic T, Jeremic K, Vilendecic Z, Djordjevic S. Plasma albumin level as an indicator of severity of preeclampsia. Clin Exp Obstet Gynecol. 2004;31:209–210. [PubMed] [Google Scholar]
- 32.Brisco MA, Coca SG, Chen J, Owens AT, McCauley BD, Kimmel SE, Testani JM. Blood urea nitrogen/creatinine ratio identifies a high-risk but potentially reversible form of renal dysfunction in patients with decompensated heart failure. Circ Heart Fail. 2013;6:233–239. doi: 10.1161/CIRCHEARTFAILURE.112.968230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia RZ, Qian YJ, Zhang X, Ding HJ, Wu HQ, Shao KM. Contribution of Dysfunction of Maternal Hemodynamics to Renal Impairment in Preeclampsia. Gynecol Obstet Invest. 2013;76:95–9. doi: 10.1159/000353275. [DOI] [PubMed] [Google Scholar]
- 34.Kouki T, Komiya I, Masuzaki H. The ratio of the blood urea nitrogen/creatinine index in patients with acute renal failure is decreased due to dextran or mannitol. Intern Med. 2010;49:223–226. doi: 10.2169/internalmedicine.49.2681. [DOI] [PubMed] [Google Scholar]
- 35.Seow KM, Tang MH, Chuang J, Wang YY, Chen DC. The correlation between renal function and systolic or diastolic blood pressure in severe preeclamptic women. Hypertens Pregnancy. 2005;24:247–257. doi: 10.1080/10641950500281126. [DOI] [PubMed] [Google Scholar]
- 36.Durnwald C, Mercer B. A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia. Am J Obstet Gynecol. 2003;189:848–852. doi: 10.1067/s0002-9378(03)00849-4. [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal N, Suri V, Soni S, Chopra V, Kohli HS. A prospective comparison of random urine protein-creatinine ratio vs 24-hour urine protein in women with preeclampsia. Medscape J Med. 2008;10:98. [PMC free article] [PubMed] [Google Scholar]
- 38.Wheeler TL 2nd, Blackhurst DW, Dellinger EH, Ramsey PS. Usage of spot urine protein to creatinine ratios in the evaluation of preeclampsia. Am J Obstet Gynecol. 2007;196:465, e461–464. doi: 10.1016/j.ajog.2006.10.892. [DOI] [PubMed] [Google Scholar]
- 39.Roudsari FV, Ayati S, Ayatollahi H, Shakeri MT. Protein/creatinine ratio on random urine samples for prediction of proteinuria in preeclampsia. Hypertens Pregnancy. 2012;31:240–242. doi: 10.3109/10641955.2010.507838. [DOI] [PubMed] [Google Scholar]
- 40.Vitoratos N, Hassiakos D, Iavazzo C. Molecular mechanisms of preeclampsia. J Pregnancy. 2012;2012:298343. doi: 10.1155/2012/298343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nettles JB, Brown WE. Renal biopsy in normal and toxemic pregnancy. Clin Obstet Gynecol. 1961;4:757–766. doi: 10.1097/00003081-196109000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Day C, Hewins P, Hildebrand S, Sheikh L, Taylor G, Kilby M, Lipkin G. The role of renal biopsy in women with kidney disease identified in pregnancy. Nephrol Dial Transplant. 2008;23:201–206. doi: 10.1093/ndt/gfm572. [DOI] [PubMed] [Google Scholar]
- 43.Hill PA, Fairley KF, Kincaid-Smith P, Zimmerman M, Ryan GB. Morphologic changes in the renal glomerulus and the juxtaglomerular apparatus in human preeclampsia. J Pathol. 1988;156:291–303. doi: 10.1002/path.1711560404. [DOI] [PubMed] [Google Scholar]
- 44.Nochy D, Birembaut P, Hinglais N, Freund M, Idatte JM, Jacquot C, Chartier M, Bariety J. Renal lesions in the hypertensive syndromes of pregnancy: immunomorphological and ultrastructural studies in 114 cases. Clin Nephrol. 1980;13:155–162. [PubMed] [Google Scholar]
- 45.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18:2281–2284. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 46.Strevens H, Wide-Swensson D, Hansen A, Horn T, Ingemarsson I, Larsen S, Willner J, Olsen S. Glomerular endotheliosis in normal pregnancy and pre-eclampsia. BJOG. 2003;110:831–836. [PubMed] [Google Scholar]
- 47.Jeyabalan A, Conrad KP. Renal function during normal pregnancy and preeclampsia. Front Biosci. 2007;12:2425–2437. doi: 10.2741/2244. [DOI] [PubMed] [Google Scholar]
- 48.Russo PA, Bendayan M. Distribution of endogenous albumin in the glomerular wall of proteinuric patients. Am J Pathol. 1990;137:1481–1490. [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Q, Gao Y, Yu Y, Wang W, Wang S, Zhong M. Urinary spot albumin:creatinine ratio for documenting proteinuria in women with preeclampsia. Rev Obstet Gynecol. 2012;5:9–15. [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner SJ, Craici IM, Grande JP, Garovic VD. From placenta to podocyte: vascular and podocyte pathophysiology in preeclampsia. Clin Nephrol. 2012;78:241–249. doi: 10.5414/cn107321. [DOI] [PubMed] [Google Scholar]
- 51.Smithies O. Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci U S A. 2003;100:4108–4113. doi: 10.1073/pnas.0730776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hjalmarsson C, Johansson BR, Haraldsson B. Electron microscopic evaluation of the endothelial surface layer of glomerular capillaries. Microvasc Res. 2004;67:9–17. doi: 10.1016/j.mvr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Menzel S, Moeller MJ. Role of the podocyte in proteinuria. Pediatr Nephrol. 2011;26:1775–1780. doi: 10.1007/s00467-010-1725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuwabara A, Satoh M, Tomita N, Sasaki T, Kashihara N. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia. 2010;53:2056–2065. doi: 10.1007/s00125-010-1810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friden V, Oveland E, Tenstad O, Ebefors K, Nystrom J, Nilsson UA, Haraldsson B. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 2011;79:1322–1330. doi: 10.1038/ki.2011.58. [DOI] [PubMed] [Google Scholar]
- 56.Java A, Atkinson J, Salmon J. Defective complement inhibitory function predisposes to renal disease. Annu Rev Med. 2013;64:307–324. doi: 10.1146/annurev-med-072211-110606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denny KJ, Woodruff TM, Taylor SM, Callaway LK. Complement in pregnancy: a delicate balance. Am J Reprod Immunol. 2013;69:3–11. doi: 10.1111/aji.12000. [DOI] [PubMed] [Google Scholar]
- 58.Kusters DM, Lahsinoui HH, van de Post JA, Wiegman A, Wijburg FA, Kastelein JJ, Hutten BA. Statin use during pregnancy: a systematic review and meta-analysis. Expert Rev Cardiovasc Ther. 2012;10:363–378. doi: 10.1586/erc.11.196. [DOI] [PubMed] [Google Scholar]
- 59.Rampersad R, Barton A, Sadovsky Y, Nelson DM. The C5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta. 2008;29:855–861. doi: 10.1016/j.placenta.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Couser WG. Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol. 2012;23:381–399. doi: 10.1681/ASN.2011030304. [DOI] [PubMed] [Google Scholar]
- 61.Renner B, Ferreira VP, Cortes C, Goldberg R, Ljubanovic D, Pangburn MK, Pickering MC, Tomlinson S, Holland-Neidermyer A, Strassheim D, Holers VM, Thurman JM. Binding of factor H to tubular epithelial cells limits interstitial complement activation in ischemic injury. Kidney Int. 2011;80:165–173. doi: 10.1038/ki.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vizjak A, Ferluga D, Rozic M, Hvala A, Lindic J, Levart TK, Jurcic V, Jennette JC. Pathology, clinical presentations, and outcomes of C1q nephropathy. J Am Soc Nephrol. 2008;19:2237–2244. doi: 10.1681/ASN.2007080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fakhouri F, Darre S, Droz D, Lemaire M, Nabarra B, Machet MC, Chauveau D, Lesavre P, Grunfeld JP, Noel LH, Knebelmann B. Mesangial IgG glomerulonephritis: a distinct type of primary glomerulonephritis. J Am Soc Nephrol. 2002;13:379–387. doi: 10.1681/ASN.V132379. [DOI] [PubMed] [Google Scholar]
- 64.McAlister CC, Gao ZH, McAlister VC, Gupta R, Wright JR Jr, MacDonald AS, Peltekian K. Protective anti-donor IgM production after crossmatch positive liver-kidney transplantation. Liver Transpl. 2004;10:315–319. doi: 10.1002/lt.20062. [DOI] [PubMed] [Google Scholar]
- 65.Wada J, Sugiyama H, Makino H. Pathogenesis of IgA nephropathy. Semin Nephrol. 2003;23:556–563. doi: 10.1053/s0270-9295(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 66.Julian BA, Novak J. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004;13:171–179. doi: 10.1097/00041552-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Lai AS, Lai KN. Molecular basis of IgA nephropathy. Curr Mol Med. 2005;5:475–487. doi: 10.2174/1566524054553450. [DOI] [PubMed] [Google Scholar]
- 68.Woo KT, Lau YK, Wong KS, Zhao Y, Chan CM. Parallel genotyping of 10,204 single nucleotide polymorphisms to screen for susceptible genes for IgA nephropathy. Ann Acad Med Singapore. 2009;38:894–899. [PubMed] [Google Scholar]


