Abstract
Objectives: This study investigated the expression of Sonic Hedgehog (Shh) protein in gastric cancer, and correlated it with clinicopathological parameters. The prognostic significance of Shh protein was analyzed. Methods: Shh protein expression was evaluated in 113 cases of gastric cancer and 60 cases of normal gastric mucosa. The immunoreactivity was scored semi quantitatively as: 0 = absent; 1 = weak; 2 = moderate; and 3 = strong. All cases were further classified into two groups, namely non-overexpression group with score 0 or 1, and overexpression group with score 2 or 3. The overexpression of Shh protein was correlated with clinicopathological parameters. Survival analysis was then performed to determine the Shh protein prognostic significance in gastric cancer. Results: In immunohistochemistry study, nineteen (31.7%) normal gastric mucosa revealed Shh protein overexpression, while eighty-one (71.7%) gastric cancer revealed overexpression. The expression of Shh protein were significantly higher in gastric cancer tissues than in normal gastric mucosa (P < 0.001), which was statistically correlated with age (P = 0.006), tumor differentiation (P < 0.001), depth of invasion (P = 0.042), pathologic staging (P = 0.017), and nodal metastasis (P = 0.019). We found no significant difference in both overall and disease free survival rates between Shh overexpression and non-expression groups P = 0.168 and 0.071). However, Shh overexpression emerged as a significant independent prognostic factor in multivariate Cox regression analysis (hazard ratio 1.187, P = 0.041). Conclusions: Shh protein expression is upregulated and is statistically correlated with age, tumor differentiation, depth of invasion, pathologic staging, and nodal metastasis. The Shh protein overexpression is a significant independent prognostic factor in multivariate Cox regression analysis in gastric cancer.
Keywords: Sonic hedgehog, pathology, prognosis, gastric cancer
Introduction
Human gastric cancer (GC) is one of the most common malignant cancers in gastrointestinal tract worldwide [1], especially in China [2]. It accounts for more than 95% of gastric tumors [3]. Total resection of the cancer and adjacent lymph nodes is considered as the only effective curative treatment firstly [4]. However, despite the rate of early detection by endoscopy has increased and advances in surgical techniques, the outcome of gastric carcinoma remains dismal, the overall survival rate is still very low. Proliferation, invasion and metastasis as a whole is a major poor prognostic factor for gastric cancer patients, understanding of the complex molecular mechanisms governing the progression and aggressiveness of the disease remains a major problem. It is well known that non-resolving inflammation [5,6], especially several key chemokine receptors and their ligands play a critical role in carcinogenic, proliferative, invasive and metastatic processes in human cancers.
The Hedgehog (Hh) signaling pathway was initially identified during a mutagenesis screen conducted by the fly geneticists Christiane Nusslein-Volhard and Eric Wieschaus in 1978 [7]. The mutated gene corresponding to abnormal denticle formation (hair-like projections) resulted in flies that had the appearance like a hedgehog. In flies, the pathway is initiated by a 471-residue protein called Hedgehog. However, its prototypical mammalian counterpart was named Sonic Hedgehog after the Sega video game character. Eventually, three mammalian Hh genes activating the same signaling pathway were identified [8]. The other two gene products in addition to Shh were named Indian Hedgehog (Ihh) and Desert Hedgehog (Dhh) [9]. It is well known that Hh signaling pathway plays a critical role during vertebrate embryonic development. Hh is secreted to bind to its receptors Patched (Ptch) which is present in cytoplasmic membrane of the receiving cells. Upon Hh binding to Ptch, Smoothened (Smo) is activated to signal downstream, a seven-transmembrane-span receptor like protein essential for the transduction of Hh signaling. Smo facilitates the interaction of different Hh downstream effectors in the primary cilia, resulting in the proteolytic processing activation of glioma-associated oncogene (Gli) transcription factor family members [10].
Hh signaling pathway has been identified as one of the key players in human cancers [11], Shh is the major gene expressed in the luminal gastrointestinal tract [12]. Many studies have demonstrated that Shh is a key signaling pathway in the tumorigenesis [13]. Shh signaling pathway abnormal activation and/or Shh protein overexpression in the human body may induce a variety of malignancies, and has been shown to be contributed to many human malignant tumors progression [14]. However, is Shh signaling pathway involved in the occurrence, development, invasion and metastasis process of GC is unclear? Therefore, the aim of this study was to demonstrate the relationship of Shh expression with clinicopathological parameters and prognosis in GC.
Materials and methods
Patients and clinical data
The study was approved by the Institutional Review Board and Human Ethics Committee of Southwest Hospital, the Third Military Medical University. Written consent for using the samples for research purposes was obtained from all patients prior to surgery.
Prospectively collected data of 113 patients (73 males and 40 females) with GC who were underwent surgical resection with radical total or subtotal gastrectomy and lymph node dissection at the Southwest Hospital, the Third Military Medical University (Chongqing, China) between 2008 and 2009. All of the patients did not receive radio- or chemotherapy prior to gastrectomy. The age distribution was from 22 to 75 years, and the mean age was 54.37 ± 12.41 years. Relevant Pathological reports were all reviewed to determine pathological features including tumor size, location, macroscopic type, differentiation, depth of invasion, nodal status, local recurrence status, distant metastasis and pathological staging. The clinicopathological parameters of the patients are summarized in Table 1. Tumor pathologic staging classified according to the 7th Union International Cancer Control (UICC) TNM staging system [15].
Table 1.
Association of Shh expression with the clinicopathological features of 113 patients with GC
| Parameters | n | Shh overexpression | P | |
|---|---|---|---|---|
|
| ||||
| non-overexpression | overexpression | |||
| Gender | 113 | 32 | 81 | |
| Male | 73 | 17 | 56 | 0.109 |
| Female | 40 | 15 | 25 | |
| Age (years) | ||||
| < 60 | 75 | 15 | 60 | 0.006 |
| ≥ 60 | 38 | 17 | 21 | |
| Tumor size (cm) | ||||
| ≤ 5 | 47 | 17 | 30 | 0.118 |
| > 5 | 66 | 15 | 51 | |
| Tumor location | ||||
| Proximal 1/3 | 42 | 15 | 27 | 0.180 |
| Distal 2/3 | 71 | 17 | 54 | |
| Macroscopic type | ||||
| Early gastric carcinoma | 19 | 6 | 13 | 0.732 |
| Borrmann type 1 | 8 | 3 | 5 | |
| Borrmann type 2 | 35 | 8 | 27 | |
| Borrmann type 3 | 32 | 11 | 21 | |
| Borrmann type 4 | 19 | 4 | 15 | |
| Differentiation | ||||
| Well to moderately | 34 | 19 | 15 | < 0.001 |
| poorly | 79 | 13 | 66 | |
| Depth of invasion | ||||
| T1-T2 | 50 | 19 | 31 | 0.042 |
| T3-T4 | 63 | 13 | 50 | |
| Pathologic staging | ||||
| I-II | 54 | 21 | 33 | 0.017 |
| III-IV | 59 | 11 | 48 | |
| Nodal status | ||||
| N0 | 41 | 17 | 24 | 0.019 |
| N1-3 | 72 | 15 | 57 | |
| Distant metastasis | ||||
| Absent | 87 | 20 | 67 | 0.021 |
| Present | 26 | 12 | 14 | |
| local recurrence | ||||
| no | 102 | 28 | 74 | 0.533 |
| yes | 11 | 4 | 7 | |
Significance level: P < 0.05.
Immunohistochemistry analysis
For each case, at least one or more blocks of tumorous, non-tumorous tissues and lymph nodes were retrieved for immunohistochemistry (IHC) study. A previously described IHC staining procedure was performed [16]. Consecutive corresponding 4 mm-thick sections were cut from each study block, and the tissue sections were deparaffinized by immersion in xylene and rehydration in a series of graded concentrations of alcohol. 10 Mm citrate buffer solution (pH 6.0) was added to the samples for 10 minutes to augment the expression of antigen in the tissues. The samples were then treated with 3% hydrogen peroxide solution at 37°C for 15 minutes and rinsed with phosphate-buffered saline (PBS) to quench the endogenous peroxidase activity. The sections were blocked with 10% normal rabbit serum at room temperature for 60 minutes to prevent non-specific immune reactions. The slides were shaked lightly and then incubated overnight with primary antibody at 4°C in humid chambers. A 1:100 dilution of rabbit monoclonal anti-human to Shh antibody (EP1190Y, Epitomics Catalog No. 1843-1, Acam®) was used as the primary antibody. Subsequently, rinsed the slides with PBS, a 1:200 dilution of biotin-marked goat anti-rabbit secondary antibody was applied and the mixture were reacted at 37°C for 60 minutes and followed by a peroxidase-marked streptavidin for an additional 10 minutes. The reaction was visualized by adding 3, 3’-diaminobenzidine tetrahydrochloride, reacted for 5 minutes. The slides were finally counterstained with hematoxylin, dehydrated after a standard procedure and sealed with coverslips. Sections that had been treated with PBS instead of primary antibody were used as a negative control. A total of 3,137 lymph nodes were resected from the 113 patients and two independent observers, blinded to each patient’s data, scored all of the samples. If discrepant, a pathologist reviewed the cases, and a consensus was finally reached.
Follow-up
Follow-up data were recorded from the patient’s medical records and by a telephone survey performed every 3 months for the first 3 years after surgery, every 6 months thereafter. Overall survival (OS) was measured from the date of surgery to the date of death from any cause or date of last follow-up. Disease-free survival (DFS) was defined as from the date of surgery to the time of first relapse confirmed by imaging modalities or last follow-up. The median post-operative follow-up period was 43.6 months (range, 6~78 months) in 113 patients. During the follow-up period, forty-seven patients (41.6%) were still alive, but sixty-six patients (58.4%) died. The overall mean ± SEM survival time of the 113 patients was 38.3 ± 2.1 months. There was no perioperative mortality.
Statistical analysis
All statistical analysis was carried out using SPSS software (IBM SPSS Statistics version 19.0). Continuous variables were expressed as the mean ± standard deviation (SD, x̅ ± s). Group comparisons of categorical variables were evaluated using the chi-square test and correlation coefficient analysis. The cumulative overall survival rates and disease free survival rates were calculated using the Kaplan-Meier method and the differences in survival rates between Shh overexpression and non-expression groups were analyzed by the log-rank test. To found the significant prognostic impact of Shh overexpression compared with other established prognostic factors, overall survival was performed using the Cox proportional hazard model. Continuous variables were coded as binary variables for uni and multivariate Cox regression analysis, independent prognostic factors were also evaluated by backward multivariate analysis. The p values of less than 0.05 were considered to indicate differences statistically significant.
Results
Shh protein expression was upregulated in gastric carcinoma
Expression levels of Shh protein were determined by IHC in 113 GC samples, 81 patients (71.7%) revealed Shh protein overexpression. 19 (31.7%) cases out of 60 normal gastric tissues revealed Shh protein overexpression. The Shh immunostaining patterns are shown in Figure 1. Shh protein was observed high expressed in tumor cells but low in normal gastric glands, with the difference being statistically significant (p < 0.001).
Figure 1.
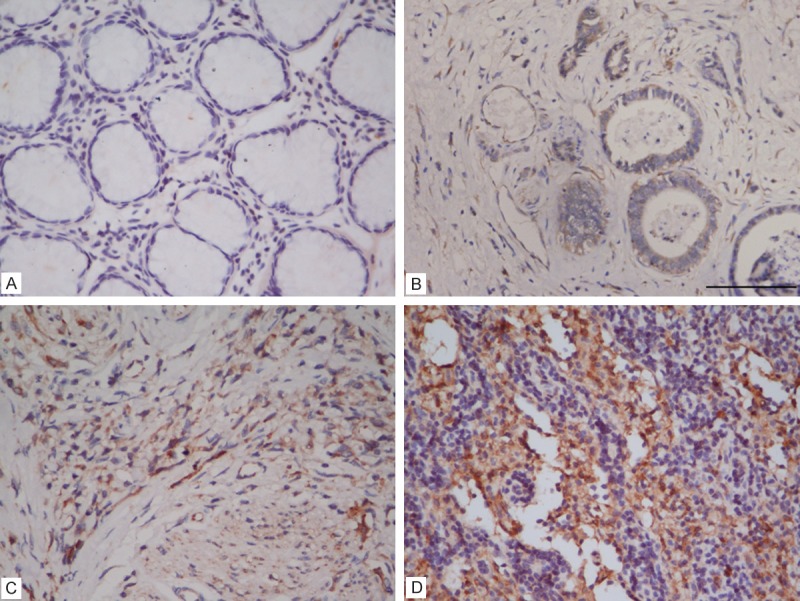
Immunoreactivity in normal gastric glands and gastric carcinoma. A: Normal gastric glands showing negative immunostaining (absent), B: Weakly positive immunostaining in a moderately-differentiated adenocarcinoma, C: Moderately positive immunostaining in a poorly-differentiated adenocarcinoma, D: Strongly positive immunostaining in a poorly-differentiated adenocarcinoma.
Overexpression of shh protein was statistically correlated with age, tumor differentiation, depth of invasion, pathologic staging, nodal status and distant metastasis
The significance of the difference between Shh overexpression and other clinicopathological parameters was determined by Chi-square test (Table 1). Gender, tumor size, tumor location, macroscopic type and local recurrence in the Shh immunostaining patterns are shown no difference. There was a statistically significant correlation between Shh protein overexpression and age (P = 0.006). Patients aged less than 60 years had a higher rate of Shh protein overexpression (80%) than those of 60 years or older (55.3%). Shh protein overexpression was statistically significant correlated with tumor differentiation (P < 0.001). Only 15 (44.1%) of well to moderately-differentiated carcinoma among the 34 cases exhibited Shh protein overexpression. However, 66 (83.5%) of poorly-differentiated carcinoma among the 79 cases displayed Shh protein overexpression. We observed that poorly-differentiated type tumors more frequently expressed Shh protein than those of the well to moderately-differentiated type. Shh protein overexpression was also noticed statistically correlated with the depth of tumor invasion (P = 0.042). 31 (62.0%) of T1 and T2 tumor (invasion not beyond muscularis propria) in 50 cases displayed Shh protein overexpression. In contrast, 50 (79.4%) of T3 and T4 tumor (invasion of subserosa or deeper) of 63 cases presented Shh protein overexpression. A statistical difference was found between Shh protein overexpression and tumor nodal status (P = 0.019). The rates of Shh protein overexpression were 79.2% (57 cases) in the 72 cases with nodal invasion and 58.5% (24 cases) in the 41 cases with no invasion respectively. Shh protein overexpression has also showed a statistical correlation with pathologic staging (P = 0.017). There were 33 (61.1%) among the 54 stage I and II cases revealed Shh protein overexpression. In contrast, 48 (81.4%) of 59 cases at stage III and IV with Shh protein overexpression. Our data thus revealed that advanced stage tumors were likely to overexpress Shh protein than tumors with early stage. Finally, Shh protein overexpression was statistically correlated with distant metastasis (P = 0.021). 14 (53.8%) out of 26 cases with distant metastasis possessed Shh protein overexpression, and 67 (77.0%) out of 87 cases with no distant metastasis showed overexpression of Shh protein. Therefore, there was a negative statistical correlation between Shh protein overexpression and distant metastasis. In addition, correlation coefficients were calculated. The correlation coefficient (r) and P value in statistically significant variables were as follows: age (r = -0.259, P = 0.006), tumor differentiation (r = 0.401, P < 0.001), depth of invasion (r = 0.191, P = 0.042), pathologic staging (r = 0.224, P = 0.017), nodal status (r = 0.220, P = 0.019) and distant metastasis (r = -0.216, P = 0.021).
Expression of shh protein was a significant independent prognostic factor in multivariate cox regression analysis
The data of 113 patients were enrolled for overall and disease free survival analysis. The overall survival rate among the 81 patients with Shh protein overexpression was 34.6%, and among the 32 without overexpression was 59.4%. The disease free survival rates in Shh protein overexpression group and non-overexpression group were 14.8% and 21.9%, respectively. There was no statistical significant difference between the Shh overexpression and non-overexpression groups in overall and disease free survival rates (log rank test P = 0.168 and 0.071), but did indicate a tendency for patients with Shh protein over-expression to have a shorter overall survival and disease free survival than those without overexpression.
The univariate Cox regression analysis of prognostic markers is summarized in Table 2. The overall survival was statistically correlated with age, tumor size, macroscopic type, tumor differentiation, depth of invasion, Pathologic staging, nodal status, distant metastasis and local recurrence. Shh protein overexpression was not statistically correlated with overall survival in univariate analysis (P = 0.093). However, backward multivariate Cox regression analysis showed that Shh protein overexpression was an independent prognostic marker for overall survival. Patients in the Shh protein overexpression group had a statistically significant shorter overall survival rate compared with patients in the non-expression group (hazard ratio 1.187, 95% confidence interval 0.614 - 4.736, P = 0.041) (Table 3). Other co-variables of prognosis included age, pathologic staging, nodal status, distant metastasis, and local recurrence.
Table 2.
Uni-variate analysis of prognostic factors in 113 patients with Gastric Carcinoma
| Variables | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Gender | |||
| Male | 1 | ||
| Female | 1.108 | 0.927-1.504 | 0.127 |
| Age (years) | |||
| < 60 | 1 | ||
| ≥ 60 | 1.768 | 0.567-3.343 | 0.013 |
| Tumor size (cm) | |||
| ≤ 5 | 1 | ||
| > 5 | 2.722 | 1.104-3.275 | 0.008 |
| Tumor location | |||
| Proximal 1/3 | 1 | ||
| Distal 2/3 | 0.804 | 0.644-1.137 | 0.171 |
| Macroscopic type | |||
| Early gastric carcinoma | 1 | ||
| Borrmann type 1-4 | 1.648 | 0.862-3.361 | 0.036 |
| Differentiation | |||
| Well to moderately | 1 | ||
| poorly | 2.014 | 1.179-4.193 | 0.006 |
| Depth of invasion | |||
| T1-T2 | 1 | ||
| T3-T4 | 1.948 | 0.709-3.467 | 0.034 |
| Pathologic staging | |||
| I-II | 1 | ||
| III-IV | 4.207 | 2.364-8.502 | <0.001 |
| Nodal status | |||
| N0 | 1 | ||
| N1-3 | 5.341 | 2.076-11.147 | <0.001 |
| Distant metastasis | |||
| Absent | 1 | ||
| Present | 5.712 | 3.576-10.218 | < 0.001 |
| local recurrence | |||
| no | 1 | ||
| yes | 2.708 | 0.973-5.066 | < 0.001 |
| SHH overexpression | |||
| negative | 1 | ||
| positive | 1.469 | 0.847-4.178 | 0.093 |
CI: confidence interval; significance level: P < 0.05.
Table 3.
Backward multi-variate analysis of SHH protein expression and other prognostic factors in 113 patients with GC
| Variables | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Age (years) | |||
| < 60 | 1 | ||
| ≥ 60 | 2.643 | 1.477-4.302 | 0.007 |
| Pathologic staging | |||
| I-II | 1 | ||
| III-IV | 2.718 | 1.629-4.833 | 0.026 |
| Nodal status | |||
| N0 | 1 | ||
| N1-3 | 3.762 | 1.824-6.416 | 0.037 |
| Distant metastasis | |||
| Absent | 1 | ||
| Present | 2.106 | 1.718-5.429 | 0.008 |
| local recurrence | |||
| no | 1 | ||
| yes | 1.638 | 0.724-3.908 | < 0.001 |
| SHH overexpression | |||
| negative | 1 | ||
| positive | 1.187 | 0.614-4.736 | 0.041 |
CI: confidence interval; significance level: P < 0.05.
Discussion
GC is an aggressive and lethal cancer. Patients with this carcinoma are usually treated at an advanced stage, and the prognosis remains poor despite the development of modern diagnostic methods and advanced open or laparoscopic surgery. Therefore, it is necessary to research the mechanisms responsible for the characteristic growth and metastasis of GC. The Hedgehog family proteins are involved in controlling almost every aspect of the vertebrate body plan, such as cell growth, survival, fate, and pattern. The function of Hh ligands have been detailed in developmental biology, recent work has focused on understanding of their role in adult tumorigenesis and tumor progression, for example, basal cell, medulloblastoma, pancreatic cancer and some types of tumors in digestive tract [17-19]. Shh gene is the most similar to the original Drosophila hedgehog and is the most important Hh isoform for the formation and progression in various carcinomas. Aberrant Shh has been reported in lung cancer, prostate cancer, superficial bladder cancer, pancreatic cancer, colorectal cancer and hepatocellular cancer [20-25].
Shh has been hypothesized to play a crucial role in the carcinogenesis and metastasis of gastrointestinal cancers. Reports showed that Shh expression level observed in the colon is higher than in the small intestine, but significantly highest in the stomach [17,26]. The specific role of Shh in gastrointestinal tumors is not quite clear. In human colon cancer the expression of Shh signaling proteins have been examined, one study reported that Shh, Ptch and Smo expression was up-regulated in hyperplastic polyps, adenomas, and adenocarcinomas, and Shh expression correlated with proliferation in all lesions examined [27], Shh may be a trigger in the colon and rectum [28]. In contrast, other studies demonstrated that Hh signaling is involved rather in constant differentiation and renewing of the colonic lining epithelium than in cancer formation, growth, or proliferation [29]. With regard to Shh signaling in gastric physiology, one study reported that disruption of Shh expression during inflammation was correlated with the development of gastric cancer [30], another also admitted that the absence of Shh correlates with neoplastic transformation [31]. However, other studies hold the opposite position, significant overexpression of Shh was detected in H. pylori-infected gastric cancer [32], and Shh was also found to promote progression and invasiveness of gastric cancer cells [33,34]. To date, the role of Shh expression in human gastrointestinal cancers is not well understood.
Although an association between Shh expression and gastric carcinoma has been documented, the clinicopathological correlations and the prognostic significance of Shh protein overexpression in gastric carcinoma had not been clearly studied. In this study, we evaluated the Shh protein expression in gastric carcinoma and non-tumor gastric tissues via IHC staining. Our data indicated that increased Shh was found in 71.7% cases of gastric carcinoma. Shh protein expression was significantly higher in GC tissues than in normal gastric mucosa. In gastric carcinoma, there is no evidence Shh overexpression was associated with the clinicopathological characteristics of gender (P = 0.109), tumor size (P = 0.118), tumor location (P = 0.180), macroscopic type (P = 0.732) and local recurrence (P = 0.533). We found Shh protein overexpression to be statistically correlated with age. Patients less than 60 years possibly more frequently expressed Shh protein than elders. Shh protein overexpression was also found to be statistically significant correlated with tumor differentiation. Poorly-differentiated tumors more frequently expressed Shh protein than well to moderately-differentiated ones in gastric carcinoma. Shh protein may be acts as a tumor promoter, and upregulates gastric carcinoma carcinogenesis, consistent with the results of most reports that increased Shh expression was related to the gastric carcinogenesis [35,36]. The finding between Shh expression and tumor differentiation has been noted in various malignancies. In prostate carcinoma, high levels of Shh expression can specifically and directly induce differentiation in pre-osteoblasts and subsequent contribute to bone metastasis [37]. In medulloblastoma, activated Shh signaling pathways are important for cell differentiated [38]. Highly expressed Shh mRNA is an early event in the development of intraductal papillary mucinous neoplasms [39]. Shh expression strongly correlated with the myogenic histopathological differentiation in human intestinal stromal tumors [40]. The activity of the Shh altered the gastric differentiation and decreased gastric acid secretion that is the predominant function of the stomach [41], this may be one of the main reasons in the development of GC.
Shh was also found to be statistically correlated with tumor progression. It has been implicated in several cancer-related processes, such as invasion and metastasis [42]. The role of Shh in regulating tumor growth and development is clearly complex and highly tissue-dependent. In some cases Shh acts as a tumor promoter [28], and in others it functions as a tumor suppressor [30]. In current immunohistochemical study, glands T3, T4 and stage III, IV were frequently observed with Shh protein overexpression than T1, T2 and stage I, II. In addition, Shh protein overexpression was noticed statistically correlated with tumor nodal status. Further more, Shh protein overexpression has also showed a statistical correlation with distant metastasis. The Shh protein is thus thought to be involved in the invasion and metastasis of gastric carcinoma.
Shh has been reported to be a prognostic marker in human cancers. In Yoshizaki’s study, Patients with higher Shh expression in human intestinal stromal tumors have a significantly higher tumor risk category and bigger tumor size [40]. Che et al demonstrated that Shh signaling activation shortened disease-free survival in patients with hepatocellular carcinoma [43]. For patients with gastric carcinoma, the prognostic significance of Shh is controversial. Saze et al reported that patients with high levels of Shh and Ptch1 mRNA had a poorer prognosis [34], but Kim et al demonstrated that Shh level may be a good prognostic indicator of gastric cancer [44]. Such a wide variety of functions is possibly attributed to the type of responding cells, the time cells are exposed to Shh, and the dose of Shh cells received. Although no statistical significance via Kaplan-Meier method, our study showed a tendency for patients without Shh protein overexpression to have a longer overall survival and disease free survival than those with Shh overexpression. In addition, Shh protein overexpression was found to be a significant independent prognostic factor for gastric carcinoma in multivariate analysis. Patients with Shh protein overexpression possibly had a statistically significant shorter survival period.
Considering the results of the current study, we concluded that in patients with advanced gastric carcinomas, Shh protein has an effect to promote carcinogenesis, progression, and increase cancer mortality. Several schools of thought with respect to the role of the Shh might account for the different findings. First, Shh protein is subjected to mediates autocrine or paracrine signaling either initiates or supports neoplastic epithelial-to-mesenchymal transformation related to immunohistochemical staining in human gastric cancer [45,46]. Second, chronic inflammation in gastric mucous is possibly to be an important mechanism to determine whether Shh expression is activated or becomes sustained [13]. Third, Shh generated by the acid-producing parietal cells is known to form a concentration gradient and in turn exert a subsequent differential effect on gene expression. Last, transcription factors Gli1 is the main nuclear mediator of mammalian Shh signaling [47]. Gli1 transcript was found two shorter isoforms, an N-terminal deletion variant (Gli1ΔN) [48] and a truncated Gli1 (tGli1) [49]. Notably, The tGli1 isoform is exclusively expressed in tumor tissue and has direct targets including CD24, VEGF-A, MMP-2, and MMP-9, leading to increased tumor size, increased cell motility, increased tumor invasiveness, and increased tumor angiogenesis [50]. Gli1 can be regulated by non-hedgehog signaling pathways in addition to canonical Shh signaling, indicates the possibility that some previous reports attributed to Shh may have been due to tGli1, so it is important to determine whether Shh pathway regulates tGli1 expression or not.
In summary, we demonstrated that Shh protein is upregulated in gastric carcinoma. Overexpression of Shh protein was statistically correlated with age, tumor differentiation, depth of invasion, pathologic staging and distant metastasis. The Shh protein overexpression was a significant independent prognostic factor for patients with gastric carcinoma in multivariate Cox regression analysis. Shh might work as a promising target for prognostic prediction in patients with gastric cancer.
Disclosure of conflict of interest
None.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Dikken JL, van Sandick JW, Maurits Swellengrebel HA, Lind PA, Putter H, Jansen EP, Boot H, van Grieken NC, van de Velde CJ, Verheij M, Cats A. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS) BMC Cancer. 2011;11:329. doi: 10.1186/1471-2407-11-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 7.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 8.Katoh Y, Katoh M. Comparative genomics on Sonic hedgehog orthologs. Oncol Rep. 2005;14:1087–1090. [PubMed] [Google Scholar]
- 9.Shimeld SM. The evolution of the hedgehog gene family in chordates: insights from amphioxus hedgehog. Dev Genes Evol. 1999;209:40–47. doi: 10.1007/s004270050225. [DOI] [PubMed] [Google Scholar]
- 10.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 11.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 12.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 13.Merchant JL, Saqui-Salces M, El-Zaatari M. Hedgehog signaling in gastric physiology and cancer. Prog Mol Biol Transl Sci. 2010;96:133–156. doi: 10.1016/B978-0-12-381280-3.00006-3. [DOI] [PubMed] [Google Scholar]
- 14.Kiesslich T, Neureiter D. Advances in targeting the Hedgehog signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:151–156. doi: 10.1517/14728222.2012.652948. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Zhao H, Lu J, Yin J, Zang L, Song N, Dong R, Wu T, Du X. Clinicopathological significance of SOX4 expression in primary gallbladder carcinoma. Diagn Pathol. 2012;7:41. doi: 10.1186/1746-1596-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saqui-Salces M, Merchant JL. Hedgehog signaling and gastrointestinal cancer. Biochim Biophys Acta. 2010;1803:786–795. doi: 10.1016/j.bbamcr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12:5924–5928. doi: 10.1158/1078-0432.CCR-06-1736. [DOI] [PubMed] [Google Scholar]
- 20.Jeng KS, Sheen IS, Jeng WJ, Yu MC, Hsiau HI, Chang FY, Tsai HH. Activation of the sonic hedgehog signaling pathway occurs in the CD133 positive cells of mouse liver cancer Hepa 1-6 cells. Onco Targets Ther. 2013;6:1047–1055. doi: 10.2147/OTT.S44828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bermudez O, Hennen E, Koch I, Lindner M, Eickelberg O. Gli1 mediates lung cancer cell proliferation and Sonic Hedgehog-dependent mesenchymal cell activation. PLoS One. 2013;8:e63226. doi: 10.1371/journal.pone.0063226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. Inhibition of prostate cancer proliferation by interference with sonic hedgehog-GLI1 signaling. Proc Natl Acad Sci U S A. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaRue H, Simoneau M, Aboulkassim TO, Lemieux P, Girard J, Hamed S, Hovington H, Jeannotte L, Fradet Y. [The PATCHED/Sonic Hedgehog signalling pathway in superficial bladder cancer] . Med Sci (Paris) 2003;19:920–925. doi: 10.1051/medsci/20031910920. [DOI] [PubMed] [Google Scholar]
- 24.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douard R, Moutereau S, Pernet P, Chimingqi M, Allory Y, Manivet P, Conti M, Vaubourdolle M, Cugnenc PH, Loric S. Sonic Hedgehog-dependent proliferation in a series of patients with colorectal cancer. Surgery. 2006;139:665–670. doi: 10.1016/j.surg.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Varnat F, Zacchetti G, Ruiz i Altaba A. Hedgehog pathway activity is required for the lethality and intestinal phenotypes of mice with hyperactive Wnt signaling. Mech Dev. 2010;127:73–81. doi: 10.1016/j.mod.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Oniscu A, James RM, Morris RG, Bader S, Malcomson RD, Harrison DJ. Expression of Sonic hedgehog pathway genes is altered in colonic neoplasia. J Pathol. 2004;203:909–917. doi: 10.1002/path.1591. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa K, Shimada M, Miyamoto H, Higashijima J, Miyatani T, Nishioka M, Kurita N, Iwata T, Uehara H. Sonic hedgehog relates to colorectal carcinogenesis. J Gastroenterol. 2009;44:1113–1117. doi: 10.1007/s00535-009-0110-2. [DOI] [PubMed] [Google Scholar]
- 29.Alinger B, Kiesslich T, Datz C, Aberger F, Strasser F, Berr F, Dietze O, Kaserer K, Hauser-Kronberger C. Hedgehog signaling is involved in differentiation of normal colonic tissue rather than in tumor proliferation. Virchows Arch. 2009;454:369–379. doi: 10.1007/s00428-009-0753-7. [DOI] [PubMed] [Google Scholar]
- 30.Sherman AE, Zavros Y. Role of Sonic Hedgehog signaling during progression from inflammation to cancer in the stomach. World J Gastrointest Pathophysiol. 2011;2:103–108. doi: 10.4291/wjgp.v2.i6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiotani A, Iishi H, Uedo N, Ishiguro S, Tatsuta M, Nakae Y, Kumamoto M, Merchant JL. Evidence that loss of sonic hedgehog is an indicator of Helicobater pylori-induced atrophic gastritis progressing to gastric cancer. Am J Gastroenterol. 2005;100:581–587. doi: 10.1111/j.1572-0241.2005.41001.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim JH, Choi YJ, Lee SH, Shin HS, Lee IO, Kim YJ, Kim H, Yang WI, Kim H, Lee YC. Effect of Helicobacter pylori infection on the sonic hedgehog signaling pathway in gastric cancer cells. Oncol Rep. 2010;23:1523–1528. doi: 10.3892/or_00000791. [DOI] [PubMed] [Google Scholar]
- 33.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- 34.Saze Z, Terashima M, Kogure M, Ohsuka F, Suzuki H, Gotoh M. Activation of the sonic hedgehog pathway and its prognostic impact in patients with gastric cancer. Dig Surg. 2012;29:115–123. doi: 10.1159/000336949. [DOI] [PubMed] [Google Scholar]
- 35.Wang LH, Choi YL, Hua XY, Shin YK, Song YJ, Youn SJ, Yun HY, Park SM, Kim WJ, Kim HJ, Choi JS, Kim SH. Increased expression of sonic hedgehog and altered methylation of its promoter region in gastric cancer and its related lesions. Mod Pathol. 2006;19:675–683. doi: 10.1038/modpathol.3800573. [DOI] [PubMed] [Google Scholar]
- 36.Lee SY, Han HS, Lee KY, Hwang TS, Kim JH, Sung IK, Park HS, Jin CJ, Choi KW. Sonic hedgehog expression in gastric cancer and gastric adenoma. Oncol Rep. 2007;17:1051–1055. [PubMed] [Google Scholar]
- 37.Zunich SM, Douglas T, Valdovinos M, Chang T, Bushman W, Walterhouse D, Iannaccone P, Lamm ML. Paracrine sonic hedgehog signalling by prostate cancer cells induces osteoblast differentiation. Mol Cancer. 2009;8:12. doi: 10.1186/1476-4598-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mascaro Cordeiro B, Dias Oliveira I, de Seixas Alves MT, Saba-Silva N, Capellano AM, Cavalheiro S, Dastoli P, Toledo SR. SHH, WNT, and NOTCH pathways in medulloblastoma: when cancer stem cells maintain self-renewal and differentiation properties. Childs Nerv Syst. 2014;30:1165–72. doi: 10.1007/s00381-014-2403-x. [DOI] [PubMed] [Google Scholar]
- 39.Ohuchida K, Mizumoto K, Fujita H, Yamaguchi H, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. Sonic hedgehog is an early developmental marker of intraductal papillary mucinous neoplasms: clinical implications of mRNA levels in pancreatic juice. J Pathol. 2006;210:42–48. doi: 10.1002/path.2019. [DOI] [PubMed] [Google Scholar]
- 40.Yoshizaki A, Nakayama T, Naito S, Wen CY, Sekine I. Expressions of sonic hedgehog, patched, smoothened and Gli-1 in human intestinal stromal tumors and their correlation with prognosis. World J Gastroenterol. 2006;12:5687–5691. doi: 10.3748/wjg.v12.i35.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng R, Xiao C, Zavros Y. The role of Sonic Hedgehog as a regulator of gastric function and differentiation. Vitam Horm. 2012;88:473–489. doi: 10.1016/B978-0-12-394622-5.00021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey JM, Singh PK, Hollingsworth MA. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem. 2007;102:829–839. doi: 10.1002/jcb.21509. [DOI] [PubMed] [Google Scholar]
- 43.Che L, Yuan YH, Jia J, Ren J. Activation of sonic hedgehog signaling pathway is an independent potential prognosis predictor in human hepatocellular carcinoma patients. Chin J Cancer Res. 2012;24:323–331. doi: 10.3978/j.issn.1000-9604.2012.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JY, Ko GH, Lee YJ, Ha WS, Choi SK, Jung EJ, Jeong CY, Ju YT, Jeong SH, Hong SC. Prognostic value of sonic hedgehog protein expression in gastric cancer. Jpn J Clin Oncol. 2012;42:1054–1059. doi: 10.1093/jjco/hys137. [DOI] [PubMed] [Google Scholar]
- 45.Fukaya M, Isohata N, Ohta H, Aoyagi K, Ochiya T, Saeki N, Yanagihara K, Nakanishi Y, Taniguchi H, Sakamoto H, Shimoda T, Nimura Y, Yoshida T, Sasaki H. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology. 2006;131:14–29. doi: 10.1053/j.gastro.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Zhu H, Lo HW. The Human Glioma-Associated Oncogene Homolog 1 (GLI1) Family of Transcription Factors in Gene Regulation and Diseases. Curr Genomics. 2010;11:238–245. doi: 10.2174/138920210791233108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimokawa T, Tostar U, Lauth M, Palaniswamy R, Kasper M, Toftgard R, Zaphiropoulos PG. Novel human glioma-associated oncogene 1 (GLI1) splice variants reveal distinct mechanisms in the terminal transduction of the hedgehog signal. J Biol Chem. 2008;283:14345–14354. doi: 10.1074/jbc.M800299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo HW, Zhu H, Cao X, Aldrich A, Ali-Osman F. A novel splice variant of GLI1 that promotes glioblastoma cell migration and invasion. Cancer Res. 2009;69:6790–6798. doi: 10.1158/0008-5472.CAN-09-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao X, Geradts J, Dewhirst MW, Lo HW. Upregulation of VEGF-A and CD24 gene expression by the tGLI1 transcription factor contributes to the aggressive behavior of breast cancer cells. Oncogene. 2012;31:104–115. doi: 10.1038/onc.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]


