Abstract
The clinical manifestation of acute corpus callosum (CC) infarction is lack of specificity and complex, so it is easily missed diagnosis and misdiagnosis in the early stage. The present study aims to describe the clinical features of the acute CC infarction. In this study, 25 patients with corpus callosum infarction confirmed by the brain MRI/DWI and the risk factors were summarized. Patients were classified into genu infarction (3 cases), body infarction (4cases), body and genu infarction (4 cases), body and splenium infarction (1 case), splenium infarction (13 cases) according to lesion location. Clinical manifestation and prognosis were analyzed among groups. The results indicated that CC infarction in patients with high-risk group accounted for 72%, moderate-risk group accounted for 20%, low-risk group (8%). The main risk factors are carotid intimal thickening or plaque formation, hypertension, hyperlipidemia, cerebral artery stenosis, and so on. The CC infarction often merged with other parts infarction, and splenium infarction had the highest incidence, the clinical symptoms in the body infarction which can appear typical signs and symptoms, but in other parts infarction which always merged many nerve defect symptoms. The body infarction prognosis is poor; the rest parts of infarction are more favorable prognosis. In conclusion, CC infarction has the highest incidence in the stroke of high-risk group; neck color Doppler and TCD examination can be found as early as possible to explore the pathogenic factors. Prognosis is usually much better by treatment according to the location and risk factors.
Keywords: Corpus callosum, cerebral infarction, clinical features
Introduction
The clinical manifestation of the acute corpus callosum infarction is lack of specificity and complex because it often merger with other location infarction [1,2]. Thus, it is easily missed diagnosis and misdiagnosis in the early stage [3]. With the widespread application of nuclear magnetic resonance (NMR), its diagnostic rate is much higher [4-6]. Now, we reviewed 25 acute corpus callosum infarction cases’ manifestation, imaging changes, risk factors, prognosis and clinical features determined by diffusion weighted imagine (DWI) who admitted from Feb. 2012 to Feb. 2013 in order to promote clinical doctors early understanding of the disease.
Materials and methods
All 25 acute corpus callosum infarction cases were identified by a conventional MR imaging protocol and a DWI sequence who met the acute cerebral infarction diagnostic criteria of the fourth Chinese national cerebrovascular disease conference in 1995. There are male 16, female 9 cases, aging from 46 to 77 years, mean age 62 years old. All patients were rapidly gathered detailed medical history and performed neurological examination, at the same time made the stroke risk factors evaluation according to the national institutes of health screening and intervention workflow in the high risk population [7]. All patients were performed NIHSS score, laboratory examination, such as blood and urine routine, liver and kidney function, blood glucose, blood coagulation function, lipid level; Electrocardiogram (ECG); Transcranial Doppler (TCD) and cervical Doppler ultrasound. All patients were assessed NIHSS in the admitting day and 2 weeks after onset or at discharge (happened before) by the same researchers.
Results
Lesion location
Corpus callosal rostrum infarction was not being found because it is difficult to be developed by routine MRI and lack of specific clinical signs. The infarction location concentrated in the corpus callosal genu, body and splenium. The isolated callosal infarction is rare, there are only 3 cases in our patients. Most of them are combining with other cerebral ischemic lesions. Especially the callosal body infarction was easy to merge with the genu or the splenium (Figure 1, Tables 1 and 2).
Figure 1.
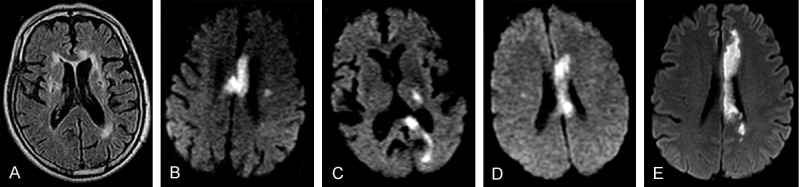
MRI images of different CC infarction location. A. Genu; B. Body; C. Splenium; D. Body & Genu; E. Body & Splenium.
Table 1.
Sites of the lesions in 25 cases of acute corpus callosum infarction
| Genu | Body | Splenium | Body & genu | Body & splenium | |
|---|---|---|---|---|---|
| Left | 2 | 2 | 7 | 3 | 1 |
| Right | 1 | 2 | 6 | 1 | 0 |
| Total | 3 | 4 | 13 | 4 | 1 |
Table 2.
MRI localization and distribution of the lesion in 25 cases of acute corpus callosum infarction
| Frontal lobe | Parietal lobe | Occipital lobe | Temporal lobe | Basal ganglia | Thalamus | Cingulate gyrus | Cerebellum brainstem | |
|---|---|---|---|---|---|---|---|---|
| Genu | 1 | - | - | - | 1 | - | - | - |
| Body | 1 | 1 | - | - | - | - | - | - |
| Splenium | - | 1 | 5 | 6 | 4 | 4 | 1 | |
| Body & genu | 1 | 2 | - | - | - | - | - | - |
| Body & splenium | 1 | - | - | - | - | - | 1 | - |
Clinical manifestation
All patients admitted to hospital with clear consciousness. There are 10 cases (40%) with cognitive or mental abnormality (slow indifference in 7 cases, depression in 3 cases); 12 cases (48%) with the language disorder (mixed aphasia in 1 case (4%), complete motor aphasia in 1 case (4%), incomplete motor aphasia 6 cases (12%), unclear words or clumsy in 4 cases (16%)); 5 cases (20%) with grope for action and forced laugher and crying; only 2 cases (4%) with lien hand syndrome; 21 cases (84%) with different degree of limb dyskinesia (including the sequelae and the new onset); 2 cases (8%) with partial body hypoalgesia in the patients who can cooperate physical examination; 1 case (4%) with visual field loss; 2 cases (8%) with incontinence (Table 3).
Table 3.
Clinical manifestation of the 25 patients with acute corpus callosum infarction
| Cognitive or mental abnormality | Language disorder | Grope for action and forced laugher and crying | Different degree of limb dyskinesia | |
|---|---|---|---|---|
| Cases | 10 | 12 | 5 | 21 |
| Percentage | 40 | 48 | 20 | 84 |
Risk factors
There are 18 cases (72%) with the hypertension history in 25 cases; 8 cases (32%) with diabetes, 1 case (4%) with atrial fibrillation; 10 cases (40%) with the long-term smoking history, 15 cases (60%) with hyperlipidemia, 14 cases (56%) with less activity, 4 cases (16%) with obesity, 2 cases (8%), with family history of stroke, 7 cases (28%) with a history of cerebral infarction (in them, 6 cases with once cerebral infarction history, 1 case with 2 times cerebral infarction), 8 cases (32%) with coronary heart disease (CHD) and 2 cases (8%) treated with the heart stents, 1 case (4%) with chronic renal failure, cases (32%) with long-term drinking. Through the laboratory test, there are 5 cases (20%) with high homocysteine levels, 9 cases (36%) with cerebral artery stenosis and 19 (76%) with cervical plaque formation by TCD and cervical Doppler ultrasound. In conclusion, according to the stroke risk factors evaluation according to the national institutes of health screening and intervention workflow in the high risk population, the high-risk group with three or more risk factors of stroke has 18 cases (72%), the group with < 3 items chronic diseases has 5 cases (20%), and the low-risk group with < 3 item disassociated with chronic diseases has 2 cases (8%) (Table 4).
Table 4.
Stroke risk factors distribution of the 25 cases of acute corpus callosum infarction
| High-risk group ≥ 3 risk factors | Mid-risk group < 3 items with chronic diseases | Low-risk group < 3 items without chronic diseases | |
|---|---|---|---|
| Cases | 18 | 5 | 2 |
| Percentage | 72 | 20 | 8 |
Early prognosis of the different parts’ infarction
To compare the difference of two NIHSS which is assessed on the admission day and 2 weeks after onset or discharge, when the difference of two NIHSS equal to 4 or higher was divided into good, and lower to 4 was divided into poor prognosis (Table 5).
Table 5.
Early prognosis of the different parts’ infarction
| NIHSS difference | Genu (3 cases) | Body (4 cases) | Splenium (13 cases) | Body & genu (4 cases) | Body & splenium (1 case) |
|---|---|---|---|---|---|
| Admission NIHSS | 5 | 7.3 | 4.7 | 7.7 | 12 |
| ≥4 | 2 | 3 | 12 | 2 | 0 |
| <4 | 1 | 1 | 1 | 2 | 1 |
Discussion
Infarcts of the corpus callosum are not common and are attributed to a rich blood supply from three main arterial systems: the anterior communicating artery, the pericallosal artery, and the posterior pericallosal artery [8]. A detailed description of the vascular supply to the corpus callosum was published by Ture et al. [9], including variations in the main arterial supply. The pericallosal branch of the anterior cerebral artery is most often the main vascular supply to the body. The subcallosal and medial callosal arteries, branches of the anterior communicating artery, provide the main supply for the anterior portion of the corpus callosum. The posterior pericallosal artery, a branch of the posterior cerebral artery, supplies the splenium, disposing them to generalized atherosclerosis. Only 3 cases in our series are isolated corpus callosum infarction, the others all merge with other parts infarction, conforming to the 8characteristics of vascular supply of corpus callosum. And the corpus callosum rostrum infarction is not to be scanned by MRI.
According to the stroke risk factors evaluation according to the national institutes of health screening and intervention workflow in the high risk population, 18 cases (72%) has three or more risk factors and it indicates the corpus callosum infarction accord with the susceptibility characteristics of stroke population. The high risk factors are carotid intimal thickening or plaque formation (76%), hypertension (72%), hyperlipidemia (60%), cerebral artery stenosis (36%), long-term smoking history (40%), diabetes (32%) and coronary heart disease (32%). These factors all indicate that there is wide range arteriosclerosis and vascular lesions, especially in the carotid lesions. Chrysikopoulos et al. [10] note that the majority of strokes are thromboembolic in origin, and emboli tend to favor the middle cerebral artery distribution because of hemodynamic factors. Moreover, the penetrating vessels of the corpus callosum are small in size and generally run perpendicular to the parent artery, thus protecting the corpus callosum from emboli. And in our study, 13 cases corpus callosum infarction (62%) are multiple lacunar infarctions, so the main pathogenesis of corpus callosum infarction is due to the hemodynamic disorder after the cerebral large vascular lesions.
The corpus callosum (CC) is the largest fiber bundle that connects cortical and subcortical regions of the brain [11]. It also interconnects both cerebral hemispheres, promoting functional integration of sensory and motor functions [12]. It is anatomically divided into rostrum, genu, body and splenium. The corpus callosum plays an integral role in replaying sensory, motor and cognitive information from homologous regions in the two cerebral hemispheres. Lesions of the corpus callosum can present a diagnostic dilemma, both for the radiologist and the clinician. Clinically, they are associated with neuropsychiatric symptoms, mainly interhemispheric disconnection syndromes. Patients may experience gait disorders, apraxia, agraphia, tactile anomia, alien hand syndrome and so on [13,14].
In our series, we found that the splenium of the corpus callosum was affected more often than the body and genu as previously described by Chrysikopoulos et al. [15]. They attributed this to the greater incidence of posterior cerebral artery infarcts compared with anterior cerebral artery infarcts. Due to the isolated callosal infraction is rare, the symptom of the callosal infarction is non-characteristic mostly. In our series, prominent clinical symptom manifests for movement disorders (84%), the language barrier (48%), cognitive and mental abnormality (40%), and grope for action and the strong cry laugh in 5 cases (20%), typical alien hand syndrome (AHS) only 2 cases (8%). One type of Alien hand syndrome is characterized by reflexive grasping, groping, and compulsive manipulation of tools, and is usually associated with frontal release signs. The second type is characterized primarily by the presence of intermanual conflict and the absence of frontal release signs [16,17]. Although 7 cases located in the body infarction, these typical features were found only in 2 patients. One reason is that the clinicians are not familiar with the signs, and the more important reason is that the symptom is concealed by the combined lesion. When the combined frontal, parietal or brainstem lesion caused severe neurologic deficits in cognition, mental disorder and paralysis, the interhemispheric disconnection syndrome can’t be considered. That’s why the callosal infarction is common, but the typical symptoms are rare and it’s difficult to localization diagnosis for the clinician.
Most of the patients with cognitive and mental state have improved after treatment, but the clinical symptoms of most body infarction are serious, and progressing. One patient was dead after three months because of secondary infection. The callosal genu and splenium infarction clinical symptom is lighter; most prognoses is improving, having no effect on the daily life. The following reasons are [18,19]: ① the rich callosal vascular supply, collateral circulation is abundant; ② the corpus callosum is white matter fibers arcuate which presented primary demyelinating change after ischemia whereas neuron degeneration and necrosis.
In conclusion, the corpus callosum infarction is lack of typical symptoms and signs because it often occurs with other cerebral ischemic lesion. It is reminded of callosal infarction when the patient has consciousness or cognitive change, apraxia, such as alien hand syndrome with mild paralysis. The patients are at higher risk of stroke and the main risk factors are carotid intima thickening or plaque formation, hypertension, hyperlipidemia and the cerebral artery stenosis. The hemodynamic disorder after the cerebral large vascular lesions may be the main pathogenesis. TCD and cervical Doppler ultrasound examination can found early hemodynamic disorder to guide the prevention and treatment.
Disclosure of conflict of interest
None.
References
- 1.Murthy SB, Chmayssani M, Shah S, Goldsmith CE, Kass JS. Clinical and radiologic spectrum of corpus callosum infarctions: Clues to the etiology. J Clin Neurosci. 2013;20:175–7. doi: 10.1016/j.jocn.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Ishizaki M, Ueyama H, Nishida Y, Imamura S, Hirano T, Uchino M. Crossed aphasia following an infarction in the right corpus callosum. Clin Neurol Neurosurg. 2012;114:161–5. doi: 10.1016/j.clineuro.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Goenka AH, Mukund A, Ahuja J, Kumar A. Reversible lesion in the splenium of the corpus callosum in a child with influenza-associated encephalitis, encephalopathy (IAEE) J Clin Neurosci. 2010;17:607, 678. [PubMed] [Google Scholar]
- 4.Yuan JL, Wang SK, Guo XJ, Hu WL. Acute infarct of the corpus callosum presenting as alien hand syndrome: evidence of diffusion weighted imaging and magnetic resonance angiography. BMC Neurol. 2011;9:142. doi: 10.1186/1471-2377-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang K, Choi NC. Ipsilateral hemiparesis and spontaneous horizontal nystagmus caused by middle cerebral artery territory infarct in a patient with agenesis of the corpus callosum. Neurol Sci. 2012;33:1165–8. doi: 10.1007/s10072-011-0871-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhang LL, Tang Q, Wang Z, Zhang XS. Alveolar soft part sarcoma of the uterine corpus with pelvic lymph node metastasis: case report and literature review. Int J Clin Exp Pathol. 2012;5:715–719. [PMC free article] [PubMed] [Google Scholar]
- 7.The stroke risk score scale of the stroke screening and prevention engineering of China health ministry. Beijing: Acadamy of China; 2012. The stroke screening prevention engineering committee office of China health ministry. [Google Scholar]
- 8.Saito Y, Matsumura K, Shimizu T. Anter-ograde amnesia associated with infarction of the anterior fornix and genu of the Corpus Callosum. J Stroke Cerebrovasc Dis. 2006;15:176–7. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Ture U, Yasargil MG, Krisht AF. The arteries of the corpus callosum: a microsurgical anatomic study. Neurosurgery. 1996;39:1075–85. doi: 10.1097/00006123-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kasow DL, Destian S, Braun C, Quintas JC, Kagetsu NJ, Johnson CE. Corpus callosum infarcts with atypical clinical and radiologic presentations. AJNR Am J Neuroradiol. 2000;21:1876–80. [PMC free article] [PubMed] [Google Scholar]
- 11.Aloumanis KP, Papapetrou PD. Corpus callosum aplasia in a young patient with a parathyroid adenoma. J Clin Neurosci. 2007;11:1124–6. doi: 10.1016/j.jocn.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Hofer S, Frahm J. Topography of the human corpus callosum revisited: comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–94. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Shin HW, Song SK, Sohn YH. Is progressive upper-body apraxia a corticobasal syndrome? J Clin Neurosci. 2013;20:319–22. doi: 10.1016/j.jocn.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Fujishima I. Usefulness of ice massage in triggering the swallow reflex. J Stroke Cerebrovasc. 2013;22:378–82. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Wu FY, Leong CP, Su TL. Alien hand syndrome: report of two cases. Changgeng Yi Xue Za Zhi. 1999;22:660–5. [PubMed] [Google Scholar]
- 16.Kim YD, Lee ES, Lee KS, Kim JS. Callosal alien hand sign following a right parietal lobe infarction. J Clin Neurosci. 2010;17:796–7. doi: 10.1016/j.jocn.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Spinicci G, Conti M, Cherchi MV, Mancosu C, Murru R, Carboni N. Unusual clinical presentations in subjects carrying novel NOTH3 gene mutations. J Stroke Cerebrovasc Dis. 2013;22:539–44. doi: 10.1016/j.jstrokecerebrovasdis.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Zheng JL, Hu YS. Clinical analysis of callosal infarction (38 retrospective cases) J Apoplexy and Nervous Disease. 2011;28:366. [Google Scholar]
- 19.Hashmi S, Al-Salam S. Loss of dystrophin staining in cardiomyocytes: a novel for detection early myocardial infarction. Int J Clin Exp Pathol. 2013;6:249–257. [PMC free article] [PubMed] [Google Scholar]


