Abstract
Either colonic large cell neuroendocrine carcinoma (LCNEC) or gastric squamous-cell carcinoma (SCC) is extremely rare, with a very poor prognosis due to the high rate of distant metastases. Here, we report the first case of synchronous double malignancies in form of colonic LCNEC and gastric SCC. A 66-year male underwent a right hemicolectomy for a mass obstructing the ascending colon and an emergent gastroscopic hemostasis for another hemorrhagic stomach mass. Histopathological examination confirmed colonic LCNEC displaying the characteristic of large, vesicular nuclei with variable amounts of cytoplasm and gastroscopic biopsy revealed poorly-differentiated gastric SCC. Immunohistochemical staining of LCNEC demonstrated positive activities for chromogranin A, synaptophysin, CD56, NSE, ki-67 (>95%), but negative for CD99, CK20 and TTF-1. The patient had suffered from an accelerated growth of multiple liver metastases after surgery, suggestive of concomitant tumor resistance (CR), and survived 2 months after discharge.
Keywords: Large cell neuroendocrine carcinoma, gastric squamous-cell carcinoma, secondary primary malignancy, concomitant tumor resistance, pathogenesis, prognosis
Introduction
Neuroendocrine tumors (NETs) are frequently associated with synchronous or metachronous secondary primary malignancies (SPM) [1]. However, most of NETs are commonly termed carcinoids and their grade is either G1 (Grade 1) or G2 (Grade 2), well-differentiated NETs showing benign behavior or uncertain malignant potential. Large cell neuroendocrine carcinomas (LCNECs) are poorly differentiated, high grade and their grade is always G3 (Grade 3). LCNECs of the colon are extremely rare, representing 0.25% of colorectal cancers [2]. Furthermore, primary SCC of the stomach is much rarer with an incidence of 0.04-0.07% [3]. Additionally, an accelerated growth of metastases following tumor resection has been suspected for decades. In this report, we present our unique case and discuss the pathogenesis, prognosis and treatment of these concurrent double cancers.
Case report
A 66-year-old man presented with acute crampy abdominal pain and distention lasting several hours. He has also noted a 10-kg weight loss over the past 3 months. Physical examination revealed direct tenderness and rebound tenderness on the entire abdomen and a large mass in the middle upper quadrant of his abdomen. Abdominal CT scan revealed multiple liver lesions and a large cauliflower-like mass, the size of which suggested a malignant tumor associated with multiple new vessels, measuring 10.0 cm × 6.0 cm × 3.6 cm (Figure 1A, 1B). There were affected superior mesenteric vein, dilated ascending colon and complete bowel obstruction, suggestive of acute diffuse peritonitis. The patient underwent an emergency explorative laparotomy and a right hemicolectomy. The pathology confirmed a LCNEC locating in the hepatic flexure of transverse colon. Unfortunately, upper gastrointestinal tract bleeding abruptly happened to him in a week after surgery. To stop the bleeding an emergent gastroscopic hemoclip hemostasis was adopted (Figure 2) and a biopsy confirmed gastric squamous-cell carcinoma at fundus of stomach.
Figure 1.
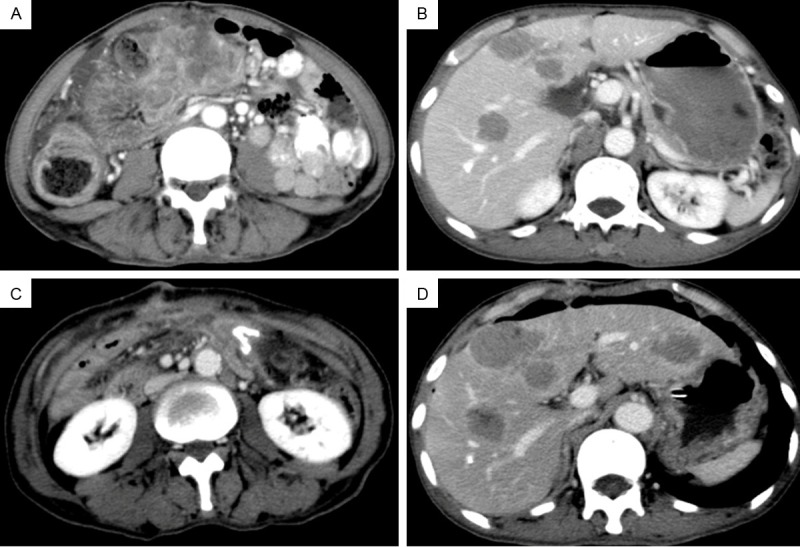
Photograph of abdominal computer tomography. A large irregular mass surrounded the transverse colon measuring 10.0 cm × 6.0 cm with dilated ascending colon (A). Multiple liver lesions considered to be metastases preoperatively (B). The colonic mass disappeared (C) and the liver metastases progressively increased (D) after surgery.
Figure 2.
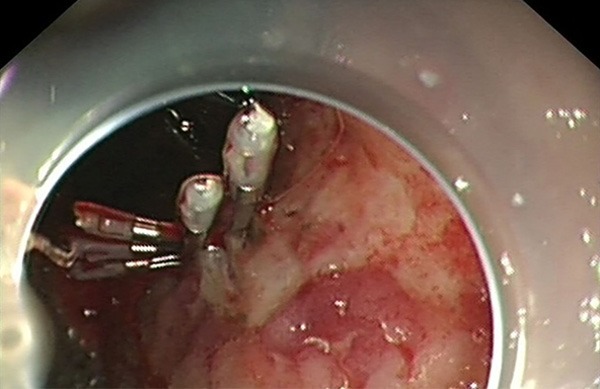
The bleeding of an ulcerative lesion at fundus of stomach measuring 1.0 cm × 1.8 cm was stopped by an emergent gastroscopic hemoclip hemostasis.
Microscopically, the colonic LCNEC invaded from the mucosa to the pericolic adipose tissue and showed poor-differentiated neuroendocrine morphology, such as nests, trabeculae, acini and rosettes (Figure 3A). The majority of the tumor cells had large, vesicular nuclei with variable amounts of cytoplasm. There were multiple necroses, apoptotic bodies and high mitotic figures (>20/10 HPF). The tumor cells had perinuclear, dot-like or cytoplasmic immunoreactivity for chromogranin A (CGA), synaptophysin (SYN), CD56, NSE (Figure 3B-E), ki-67 (>95%), but negative for CD99, CK20 and TTF-1. No cancer metastasis was found in dissected regional lymph nodes. On the other hand, the second gastric biopsy mass was a 1.0 cm × 1.8 cm ulcerated poorly-differentiated SCC (Figure 3F).
Figure 3.
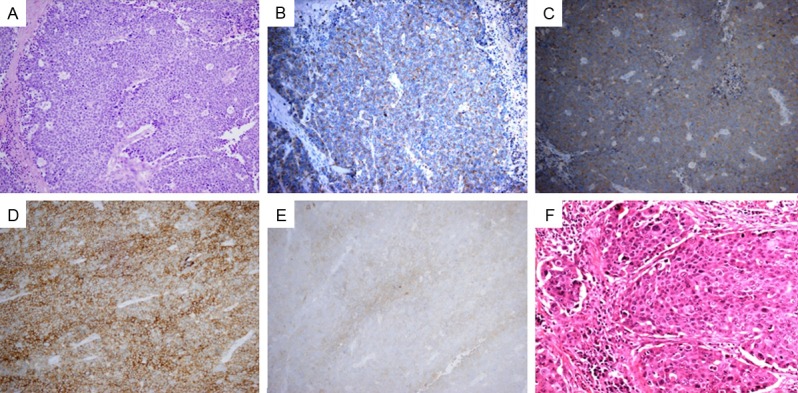
Histological and immunohistochemical examination. Colonic large cell neuroendocrine carcinoma with rosettes in the solid tumor nests by H&E staining (A); Immunohistochemical staining of LCNEC displayed diffuse positive findings for CD56 (D), and positive reaction in CGA (B), SYN (C) and NSE (E). H&E staining of the mass at fundus of stomach revealed a poorly differentiated squamous cell carcinoma (F). (Magnification: ×200).
Following completion of therapy the colon mass was no longer visible on CT scanning, while the liver metastases presented abruptly progressive increase (Figure 1C, 1D). Though we recommended postoperative chemotherapy, the patient rejected our suggestion. Moreover, due to the worsening liver failure, he died 2 months after discharge.
Discussion
There have been few reports on the synchronous double cancers consisting of LCNEC and noncarcinoid SPM locating in the gastrointestinal tract. Cases with concurrent colonic LCNEC and gastric SCC have not been reported. Due to its rarity, very little is known about its histogenesis. To our best knowledge, the origin of primary SCC of the stomach is unclear. But the theories of the separate gastric SCC can’t be used to explain our case of double cancers. In addition, there are several hypotheses to explain the pathogenesis of LCNEC-associated SPM [4-8], such as the field effect, the stem cell theory, the immunodeficiency theory, the genetic defect hypothesis and the neuropeptides theory. There is evidence that various neuropeptides secreted by neuroendocrine cells have growth factor properties, while SPMs overexpress receptors for these biologically active compounds. These neuropeptides may directly regulate malignant tissue growth in colorectal and gastric neoplasms, such as gastrin and cholecystokinin (CCK) [4]. This may be the plausible reason for colonic LCNEC coexistent with gastric SCC. Additionally, more work is necessary to clarify the precise etiology for such an infrequent case.
Sadly, the bleeding of gastric SCC and increasing multiple liver metastases took place after surgery. This is the phenomenon of concomitant tumor resistance (CR), by which the growth of distant secondary tumor implants or metastases in some tumor-bearing hosts is inhibited by the presence of a primary larger tumor. To date, different factors have been suggested as responsible for CR induction. Prehn [9] assumes two competing influences comprising circulating inhibitors and facilitating factors explain CR. Scharovsky’s results indicate angiogenic and antiangiogenic balance regulates CR [10]. Recently, Ruggiero and his colleagues [11] have identified 2 isomers of tyrosine (meta-tyrosine and ortho-tyrosine) are essential for the inhibition of secondary tumor implants together with the progressive growth of the primary tumor. Herein, due to diet resistance after surgery, the patient was administered with parenteral nutrition, which might provide a large amount of amino acids concentrating at the site of distant metastasis to counteract the inhibitory effects produced by m- and o-Tyr. Therefore, we speculate the tyrosine isomers theory might be responsible for the most universal manifestation of CR.
The poor prognostic factors [12] of gastrointestinal NETs contain metastases, invasion of the muscle propria, histological differentiation, tumor size, angioinvasion, and Ki-67 index (>30%). The median survival ranges from 5 to 10.4 months in several studies [2]. In our case, multiple liver metastases and affected superior mesenteric vein were found by abdominal CT. There were a poorly-differentiated SCC and a large sized LCNEC with the pericolic adipose tissue invasion, angioinvasion (+), and Ki-67 index (>95%). The double high-grade malignancies showed a more aggressive behavior. Furthermore, sudden acceleration of liver metastases directly resulted in a significantly reduced survival rate.
No ideal therapies are responsible for such an unusual case. In general, surgical intervention for sudden bowel obstruction is necessary. Nevertheless, tumor removal may improve the survival in patients with stage IV but only in those displaying small primary tumors and limited metastatic load. When larger primary tumors and more metastatic load are present, surgery is not recommended [13]. According to ESMO Clinical Practice Guidelines, chemotherapy is recommended in NETs, metastatic NET G2 and in NEC G3 of any site. In cases of liver metastases involving high-grade NEC G3 combination chemotherapy, using cisplatinum/etoposide is the first line therapy [14]. However, the patient refused the chemotherapy despite multidisciplinary teams (MDT) recommendation.
In conclusion, we reported the first case of synchronous double cancers of colonic LCNEC and gastric SCC with CR. Complete resection of the first lesion relieves the bowel obstruction, but more works such as the biopsy of liver metastases, the assessment of the clinical staging and postoperative chemotherapy for the unresectable lesions should be carried out and will lead to improvement in patient survival.
Disclosure of conflict of interest
None.
References
- 1.Prommegger R, Ensinger C, Steiner P, Sauper T, Profanter C, Margreiter R. Neuroendocrine tumors and second primary malignancy--a relationship with clinical impact? Anticancer Res. 2004;24:1049–1051. [PubMed] [Google Scholar]
- 2.Bernick PE, Klimstra DS, Shia J, Minsky B, Saltz L, Shi W, Thaler H, Guillem J, Paty P, Cohen AM, Wong WD. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum. 2004;47:163–169. doi: 10.1007/s10350-003-0038-1. [DOI] [PubMed] [Google Scholar]
- 3.Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. a clinicopathologic study. Cancer. 1965;18:181–192. doi: 10.1002/1097-0142(196502)18:2<181::aid-cncr2820180209>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Habal N, Sims C, Bilchik AJ. Gastrointestinal carcinoid tumors and second primary malignancies. J Surg Oncol. 2000;75:310–316. doi: 10.1002/1096-9098(200012)75:4<306::aid-jso14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Gemeinhardt M, Turck J, Piper B, Helmberger T, Nerlich A, Schepp W. [Adenocarcinoma of the stomach and neuroendocrine carcinoma of the colon in a 45-year-old male patient suffering from common variable immunodeficiency (CVID) and ulcerative colitis] . Z Gastroenterol. 2012;50:1292–1295. doi: 10.1055/s-0032-1313181. [DOI] [PubMed] [Google Scholar]
- 6.Gerstle JT, Kauffman GL Jr. Koltun WA. The incidence, management, and outcome of patients with gastrointestinal carcinoids and second primary malignancies. J Am Coll Surg. 1995;180:427–432. [PubMed] [Google Scholar]
- 7.Rivadeneira DE, Tuckson WB, Naab T. Increased incidence of second primary malignancy in patients with carcinoid tumors: case report and literature review. J Natl Med Assoc. 1996;88:310–312. [PMC free article] [PubMed] [Google Scholar]
- 8.Oberg K. Expression of growth factors and their receptors in neuroendocrine gut and pancreatic tumors, and prognostic factors for survival. Ann N Y Acad Sci. 1994;733:46–55. doi: 10.1111/j.1749-6632.1994.tb17255.x. [DOI] [PubMed] [Google Scholar]
- 9.Prehn RT. Two competing influences that may explain concomitant tumor resistance. Cancer Res. 1993;53:3266–3269. [PubMed] [Google Scholar]
- 10.Scharovsky OG, Binda MM, Rozados VR, Bhagat S, Cher ML, Bonfil RD. Angiogenic and antiangiogenic balance regulates concomitant antitumoral resistance. Clin Exp Metastasis. 2004;21:177–183. doi: 10.1023/b:clin.0000024762.32172.13. [DOI] [PubMed] [Google Scholar]
- 11.Ruggiero RA, Bruzzo J, Chiarella P, Bustuoabad OD, Meiss RP, Pasqualini CD. Concomitant tumor resistance: the role of tyrosine isomers in the mechanisms of metastases control. Cancer Res. 2012;72:1043–1050. doi: 10.1158/0008-5472.CAN-11-2964. [DOI] [PubMed] [Google Scholar]
- 12.Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen DH, Truong PT. A debate on locoregional treatment of the primary tumor in patients presenting with stage IV breast cancer. Expert Rev Anticancer Ther. 2011;11:1913–1922. doi: 10.1586/era.11.168. [DOI] [PubMed] [Google Scholar]
- 14.Oberg K, Knigge U, Kwekkeboom D, Perren A. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii124–130. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]


