Abstract
Granular cell tumor (GCT) is an uncommon tumor of soft tissue, and rarely occurs in thyroid. In this article, we report the FNAC results and pathological analysis of a 14-year-old female who presented with a painless mass in the right lobe of thyroid gland. A resection of the right lobe and isthmus of thyroid were applied after cells with abundant strong eosinophilic cytoplasma, indistinct border and inconspicuous nucleolus were found in the FNAC of the mass. Postoperative pathology and immunohistology helped diagnosis the lesion as thyroid GCT. Differential diagnosis from five diseases and cell types were performed and a review of all eleven papers reporting thyroid GCT was provided.
Keywords: Fine needle aspiration cytology, granular cell tumor, thyroid gland
Granular cell tumor (GCT) is an uncommon tumor of soft tissue which could occur in many locations. The most common site is known to be the tongue [1], while GCTs of the thyroid gland have been rarely reported. Here we reported the fine needle aspiration cytology (FNAC) result of a patient with GCT of the thyroid gland and reviewed relevant literatures.
Case report
A 14-year-old Chinese female presented to our hospital complaining of a painless mass in the neck for more than three months. She denied dysphagia, nausea, vomiting or dyspnea. Physical examination revealed I° thyroid enlargement. A non-tender, smooth, firm, and irregular mass with a diameter of 2 cm was palpated in the right lobe, which was movable when swallowing.
A thyroid ultrasound revealed a hypoechoic nodule (2.4 cm x 1.9 cm x 1.7 cm) in the right lobe with undefined margin, irregular shape and non-uniform density. Several anechoic nodules were detected in both lobes. It suggested a potential carcinoma in the right lobe and nodular goiters in other areas.
Accompanied by ultrasound guided thyroid fine needle aspiration, short-spindle and round-shaped cells with abundant strong eosinophilic cytoplasma and indistinct border were observed in the smears and cell blocks. The nuclei were round or oval, with inconspicuous nucleolus. No colloid or scattered lymphocytes were found (Figures 1, 2). As FNAC indicated a tumor couldn’t be excluded, and surgery was suggested to remove this mass.
Figure 1.

FNAC smears observed abundant cells distributing as sheets. Short-spindle and round- shaped cells were observed, with abundant strong eosinophilic cytoplasma. HEX400.
Figure 2.
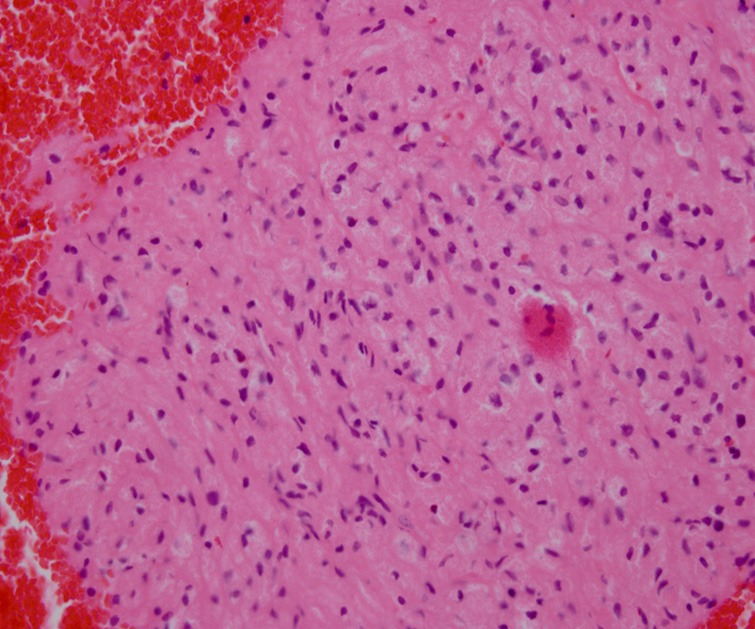
Cell blocks observed tumor cells with abundant strong eosinophilic cytoplasma. The nuclei were round or oval. HEX400.
During surgery, a solid mass of a size 3.3 cm x 3.2 cm x 2.6 cm was removed from the right lobe of thyroid, invading the right cricothyroid muscle. The section of the mass was grey. As no suspected mass was suggested in the left lobe, the right lobe and isthmus of thyroid were resected.
Post-surgical pathology observed tumor cells distributing as patchy or nests, with undefined margin from the normal tissue. The tumor cells were large, uniformly-shaped, often presented in round, polyhedral or spindle shapes. The tumor cells had abundant cytoplasm filled with eosinophilic granules, and appeared as syncytial clusters with indistinct border. The nucleus of the tumor cells was small, round, oval, occasionally spindle in shape, with inconspicuous nucleolus. No nuclear division was detected (Figures 3, 4).
Figure 3.

Tumor cells distributed as patchy or nests, pushing surrounding normal thyroid tissue, with undefined margins. HEX100.
Figure 4.

The tumor cells were large and often presented in round, polyhedral or spindle shapes. They had abundant cytoplasma filled with eosinophilic granules. The nucleus of the tumor cells was small, with inconspicuous nucleolus. HEX400.
Immunohistochemistry showed the tumor cells were positive for S-100, CD68, and NSE in both cytoplasm and nucleus, while uniformly negative with TTF1, TG, and calcitonin (Figures 5, 6 and 7). On the basis of morphology and immunophenotype, the lesion was diagnosed as GCT in the thyroid. No recurrence was observed one year after surgery.
Figure 5.

Immunohistochemistry showed the tumor cells were positive for S-100 in both cytoplasma and nucleus. DABX200.
Figure 6.

Immunohistochemistry showed the tumor cells were positive for NSE in both cytoplasma and nucleus. DABX200.
Figure 7.

Immunohistochemistry showed the tumor cells were positive for CD68 in both cytoplasma and nucleus. DABX200.
Discussion
Clinical features and pathogenesis
GCT could occur in patients of all ages. Previous studies reported GCT occurring in patients between 11 to 85 years old but most commonly in middle-aged women of 40 to 50 years old [2]. GCT could occur all throughout the human body, such as tongue, skin, subcutaneous tissue, breast, esophagus, stomach, and larynx [3-7]. But tongue is considered as the most common site. Thyroid GCT is very rare, with a PubMed search result of merely 11 cases (shown in Table 1) [8-18]. Most of the cases were young female (10 out of 11) between 11.5 and 47 years old, with the diameter of tumor between 8 mm to 42 mm. Most of them presented as painless mass. Five of the 11 patients underwent FNAC before surgery, one of which was found with atypical cell morphology but the final diagnosis was all based upon histopathology [14-18].
Table 1.
Granular cell tumors of thyroid reported to date
| Reference | Age/Gender | Macroscopic size | Immunohistochemistry |
|---|---|---|---|
| Vladimir, et al [8] | 21/M | 1.8 cm | “+” S-100, Calretinin; “-” AE1-3, Thyroglobulin, TTF1, Inhibin-A |
| Baloch, et al [9] | 47/F | 2.5 cm | “+” S-100; “-” TTF1, Thyroglobulin, Calcitonin, Chromogranin |
| Milias, et al [10] | 43/F | 2.5 cm | “+” S-100, CD68, Vimentin, Laminin, NSE; “-” AE1/3, CAM5.2, EMA, CEA, Calcitonin, SMA |
| Paproski, et al [11] | 23/F | 1.5 cm | “+” S-100; “-” Thyroglobulin, Electron microscopy |
| Mahoney, et al [12] | 11.5/F | 1.5 cm | “+” S-100; “-” Thyroglobulin, calcitonin, chromograninA, EMA, vimentin, ER, PR |
| Chang, et al [13] | 12/F | 1.4 cm | “+” S-100, vimentin; “-” Thyroglobulin, TTF-1, ChromograninA, Synaptophysin, Calcitonin, Cytokeratin |
| Bowry, et al [14] | 36/F | 0.8 cm | “+” S-100, CD68, Calretinin, Inhibin A, NSE; “-” AE1/3, CK7,TTF1, Thyroglobulin, Synaptophysin, Chromogranin A, Calcitonin |
| Min, et al [15] | 24/F | 1.8 cm | “+” S-100, CD68, NSE; “-” GFAP, TTF-1 |
| Cimino-Mathews, et al [16] | 28/F | 3.0 cm | “+” S-100, CD68; “-” Thyroglobulin, TTF-1, Calcitonin |
| Harp, et al [17] | 27/F | 4.2 cm | “+” S-100, CD68; “-” Synaptophysin, Chromogranin |
| Singh, et al [18] | 11/F | 3.5 cm | “+” S-100 |
| Current report | 14/F | 3.3 cm | “+” S-100, CD68, NSE; “-” Thyroglobulin, TTF-1, Calcitonin |
The genesis of GCT has been argued and later confirmed to be Schwann cells in the neurolemma via immunohistochemistry and electronic microscope. Tumor cells in this case were S-100, NSE and CD68 positive, consistent with which from the source of Schwann cells.
Pathological diagnosis
The preoperational FNAC results of this patient were consistent with those of the five reported cases [14-18]. The tumor cells were large in shape, syncytial, with abundant eosinophilic granular cytoplasm and indistinct order. Nuclei of the cells were polymorphic, presenting in round, oval, or spindle shapes. Nucleolus was inconspicuous. It has been difficult to make the diagnosis of GCT via cytology before surgery. And differential diagnosis should be made from the following diseases and cell types.
Firstly, Hürthle cell tumor and dysplastic nodules should be differentiated. Hürthle cells are generally larger, with round nucleus, and granular pink cytoplasm. The cell boarders are usually well-defined. In lesions derived from Hürthle cells metaplasia,lymphocytes can be observed in the background, and the nucleolus is usually predominant. Hürthle cell tumor can be classified into large and small pleomorphism. Large pleomorphism presents as loosely attached and obviously enlarged Hürthle cells with rich granular cytoplasm, the nuclear size of which is various with difference between cells of more than two times. Large nucleolus can be observable in this type. Small pleomorphism presents as loosely attached Hürthle cell cluster with the size of cells and cytoplasm varying from giant cells with abundant cytoplasm and large nucleolus to uniform small cells with high nuclei-cytoplasm ratio. A mixture of large and small cells can be seen in some cases [19].
Secondly, macrophages with high nuclei-cytoplasm ratio are often observed in benign follicular nodular cystic changes of thyroid. However, macrophages are often less viscous than GCT cells, with vesicular nucleus and foamy cytoplasm, usually containing tawny hemosiderin.
Thirdly, cells of the eosinophilic subtype of medullary thyroid carcinoma can be diagnosed from a presentation of rough karyosome, protruding nucleolus and intranuclear inclusions, and pink-stained amyloid substance around tumor cells. Moreover, positive immunohistochemical staining for synaptophysin, chromogranin A, CEA and calcitonin is of diagnostic significance [17].
Fourthly, non-neoplastic follicular cells are uniformly small, with distinct cell borders and oval uniform nuclei with a size similar to lymphocytes [14,15].
Fifthly, rhabdomyoma cells are presented with defined cell borders, striated cytoplasm and clear cellular background. Special staining and immunohistochemical staining results showing differentiation towards skeleton muscle can help differentiate the two diseases [20].
Cytological diagnosis of GCT should take the five cellular pathological changes into differentiation. Although it is difficult to make the diagnosis of GCT via cytology independently, FNAC could potentially suggest the diagnosis of GCT before surgery. Cell blocks and immunohistochemistry can give evidence to the differentiation from GCT to other pathological changes.
Histopathologically benign granular cell tumors, as our current case, are presented with tumor cells distributing as patchy or nests, pushing normal thyroid tissue with an indistinct border. The tumor cells are large but polygonal, presented in round, polyhedral, or spindle shapes with indistinct borders. Some cells fuse together with abundant cytoplasm and eosinophilic granules. The tumor cell nuclei are small, majority of which present in round or oval shape, minority of which present in spindle shapes with inconspicuous nucleolus and no nuclear division.
Malignant GCT is rare. Non-malignant GCT in Thyroid gland was reported according to our knowledge. It presents as rapid in growth with a diameter larger than 5 cm. Early regional recurrence or metastasis is common. According to the histological diagnostic criteria established by Fanburg-Smith et al [21] and Wang et al [22], six items should be evaluated: necrosis, presence of spindle cells, presence of vesicular nucleus and large nucleolus, more than 2 nuclear division per 10 HPF (more than 5 nuclear division per 50 HPF according to Wang, et al.), high nuclei-cytoplasm ratio, and pleomorphism. Histological malignant tumor can be diagnosed if at least three items are matched. An atypical diagnosis is suggested if two items match. Benign pathological change is considered if at most one item is matched. An atypical GCT case has been reported with spindle tumor cell shape, increased nuclei-cytoplasm ratio and conspicuous nucleolus [16]. On the basis of histological features and immunophenotype, our case was diagnosed as benign GCT.
Treatment
According to our knowledge, all reported thyroid GCTs are benign and grow slowly. Surgery was used in all reported cases. Excellent prognosis was observed after local excision.
Surgery is also the main treatment of benign GCT in other sites according to previous reports. A good prognosis is supposed by complete remove of the tumor. Regarding malignant GCTs, wide local excision combining with regional lymph node dissection if necessary are adapted currently. Clinical outcomes could hardly be improved by radiotherapy or chemotherapy [23]. Follow-up is necessary, especially for those with the risk of reoccurrence and metastasis.
In conclusion, thyroid GCT is rare but usually benign. All diagnosis of reported cases is made according to histopathology. However, cytology is potentially diagnostically significant due to its specific characteristics in the future.
Acknowledgements
We thank Dr. Jingqiang Zhu from Department of Thyroid and Breast Surgery, West China Hospital, Sichuan University for giving surgical treatment to the patient. This work is supported by the Research Supporting Project from Science and Technology Department of Sichuan Province (No. 2013SZ0093) and the Scientific Research Project of Health Department of Sichuan Province (No. 120203).
Disclosure of conflict of interest
None.
References
- 1.Vered M, Carpenter WM, Buchner A. Granular cell tumor of the oral cavity: updated immunohistochemical profile. J Oral Pathol Med. 2009;38:150–159. doi: 10.1111/j.1600-0714.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 2.Eguia A, Uribarri A, Gay Escoda C, Crovetto MA, Martínez-Conde R, Aguirre JM. Granular cell tumor: report of 8 intraoral cases. Med Oral Patol Oral Cir Bucal. 2006;11:E425–E428. [PubMed] [Google Scholar]
- 3.Qureshi NA, Tahir M, Carmichael AR. Granular cell tumour of the soft tissues: a case report and literature review. Int Semin Surg Oncol. 2006;3:21. doi: 10.1186/1477-7800-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celebi AS, Toksoy G, Ozel A, Caliskan KC, Kabukcuoglu F. Granular cell tumor of the breast. JBR-BTR. 2012;95:235–236. [PubMed] [Google Scholar]
- 5.Mohammad S, Naiditch JA, Jaffar R, Rothstein D, Bass LM. Granular cell tumor of the esophagus in an adolescent girl. J Pediatr Gastroenterol Nutr. 2012;54:715. doi: 10.1097/MPG.0b013e3182446b6a. [DOI] [PubMed] [Google Scholar]
- 6.Gilg MM, Mrak K, Vieth M, Langner C. Granular cell tumor of the stomach. Pathologe. 2012;33:61–64. doi: 10.1007/s00292-011-1547-7. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Do NY, Cho SI, Choi JY. Granular cell tumor on larynx. Clin Exp Otorhinolaryngol. 2010;3:52–55. doi: 10.3342/ceo.2010.3.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa-de-Los-Monteros-Franco VA, Martínez-Madrigal F, Ortiz-Hidalgo C. Granular cell tumor (Abrikossoff tumor) of the thyroid gland. Ann Diagn Pathol. 2009;13:269–271. doi: 10.1016/j.anndiagpath.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Baloch ZW, Martin S, Livolsi VA. Granular cell tumor of the thyroid: a case report. Int J Surg Pathol. 2005;13:291–294. doi: 10.1177/106689690501300312. [DOI] [PubMed] [Google Scholar]
- 10.Milias S, Hytiroglou P, Kourtis D, Papadimitriou CS. Granular cell tumour of the thyroid gland. Histopathology. 2004;44:190–191. doi: 10.1111/j.1365-2559.2004.01776.x. [DOI] [PubMed] [Google Scholar]
- 11.Paproski SM, Owen DA. Granular cell tumor of the thyroid. Arch Pathol Lab Med. 2001;125:544–546. doi: 10.5858/2001-125-0544-GCTOTT. [DOI] [PubMed] [Google Scholar]
- 12.Mahoney CP, Patterson SD, Ryan J. Granular cell tumor of the thyroid gland in a girl receiving high-dose estrogen therapy. Pediatr Pathol Lab Med. 1995;15:791–795. doi: 10.3109/15513819509027014. [DOI] [PubMed] [Google Scholar]
- 13.Chang SM, Wei CK, Tseng CE. The cytology of a thyroid granular cell tumor. Endocr Pathol. 2009;20:137–140. doi: 10.1007/s12022-009-9071-5. [DOI] [PubMed] [Google Scholar]
- 14.Bowry M, Almeida B, Jeannon JP. Granular cell tumour of the thyroid gland: a case report and review of the literature. Endocr Pathol. 2011;22:1–5. doi: 10.1007/s12022-011-9146-y. [DOI] [PubMed] [Google Scholar]
- 15.Min KW, Paik SS, Jun YJ, Han H, Jang KS. Fine needle aspiration cytology of a granular cell tumour arising in the thyroid gland. Cytopathology. 2012;23:411–412. doi: 10.1111/j.1365-2303.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 16.Cimino-Mathews A, Illei PB, Ali SZ. Atypical granular cell tumor of the thyroid: cytomorphologic features on fine needle aspiration. Diagn Cytopathol. 2011;39:608–611. doi: 10.1002/dc.21509. [DOI] [PubMed] [Google Scholar]
- 17.Harp E, Caraway NP. FNA of thyroid granular cell tumor. Diagn Cytopathol. 2013;41:825–828. doi: 10.1002/dc.22851. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Gupta N, Sharma S, Azad RK. Aspiration cytology in the preoperative diagnosis of granular cell tumor of thyroid region in an 11-years-old female child. J Cytol. 2013;30:218–219. doi: 10.4103/0970-9371.117639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria and Explanatory Notes. New York: Springer Science + Business Media; 2010. pp. 59–74. [Google Scholar]
- 20.Bellis D, Torre V, Nunziata R, Demarchi A, Fornaseri V, Coverlizza S, Beatrice F. Submandibular rhabdomyoma: A case report. Acta Cytol. 2006;50:557–559. doi: 10.1159/000326015. [DOI] [PubMed] [Google Scholar]
- 21.Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998;22:779–794. doi: 10.1097/00000478-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Zhu XZ, Zhang RY. Malignant granular cell tumor: a clinicopathologic analysis of 10 cases with review of literature. Zhonghua Bing Li Xue Za Zhi. 2004;33:497–502. [PubMed] [Google Scholar]
- 23.Lack EE, Worsham GF, Callihan MD, Crawford BE, Klappenbach S, Rowden G, Chun B. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301–316. doi: 10.1002/jso.2930130405. [DOI] [PubMed] [Google Scholar]


