Abstract
We report an 8-month-old female infant with the fatal enterovirus 71 infection here. Clinically, she developed respiratory failure and severe pulmonary edema rapidly. Histologically, the lung specimen showed diffuse, severe pulmonary congestion and edema with focal intra-alveolar hemorrhage and typical features of acute encephalitis were easily identified under light microscope. Immunohistochemically, enterovirus 71 antigen was positive in the cerebella and brainstem. We measured the viral loads of different tissues and found that the brainstem and mesenteric lymph nodes showed the highest viral loads among all tissues. We hope that our case report may help to have a better understanding of the enterovirus 71 infection and provide clues to the prevention and treatment of this disease.
Keywords: Enterovirus 71, autopsy, clinical features, histology, immunohistochemistry, viral loads of different tissues
Introduction
Enterovirus 71 (EV71) is a small, single-stranded, positive-sense RNA virus, which belongs to the family of Picornaviridae. An EV71 infection typically causes hand, foot, and mouth disease (HFMD) with mild symptoms, most frequently affecting children. However, several reports from Asia-Pacific region have indicated that patients present neurological manifestations, suggesting EV71 is a neurotropic pathogen [1-5]. Patients with severe EV71 infection often have brainstem encephalitis, aseptic meningitis and cardiorespiratory dysfunction. The pathogenesis of this disease is incompletely understood. In this case, we report an 8-month-old infant with the fatal EV71 infection and analyze the histopathological and virological features.
Case report
An 8-month-old female infant with fever and drooling was brought to hospital on May 1, 2008 (day 1). On physical examination, blisters were found on hands and feet. An ulcer was seen in pars laryngea pharyngis. Therefore, anti-inflammatory and anti-viral therapeutic regimes were administered in outpatient service. At 4:35 pm of May 5 (day 5), the baby was transported by ambulance to the emergency department of our hospital because her parents noted that the baby had developed hyperspasmia and become unresponsive. On admission, the body temperature was 38.1°C, heart rate was 200/min, and respiratory rate was 60/min. She had severe tachypnea and lung rales on auscultation. Physical examination revealed a few erythematous macular rashes and papules on her palms and soles. The throat was congested and multiple ulcers scattered in the oral cavity. Muscular spasm was found in her limbs with positive reflex of Babinski’s sign. Laboratory examinations revealed: white blood cell count, 14.6×109 cells/L; neutrophilic granulocyte count, 9.5×109 cells/L. During hospitalization, her condition deteriorated rapidly and a large amount of pink frothy fluid was aspirated from the lung when she was endotracheally intubated. Despite the resuscitation efforts, she was declared dead with respiratory failure and severe pulmonary edema at 6:50 pm shortly after arriving at the hospital. Past medical history revealed that she was delivered vaginally at 40 weeks’ gestation after an uncomplicated pregnancy and had received scheduled immunizations.
Informed consent for autopsy was obtained from the child’s parent and a complete autopsy was performed 6 hours after death. The brain revealed mild cerebral edema with shallow sulci and flattened gyrus. But there was no evidence of herniation. No obvious hemorrhage and necrosis were seen in the brain parenchyma. Both lungs showed significant pulmonary congestion, edema, and local hemorrhage. Some enlarged mesenteric lymph nodes were seen. The heart appeared normal. No visible abnormal findings were noted in other organs.
On histology, the lung specimen showed diffuse, severe pulmonary congestion and edema with focal intra-alveolar hemorrhage. Neutrophil and histomonocyte infiltration were prominent focally. Notably, viral inclusions were absent. Regeneration and hyperplasia of the bronchi were observed. Some sections showed features of early exudative-stage diffuse alveolar damage (DAD) (Figure 1A). Although encephalitis and cortical laminar necrosis were not macroscopically diagnosed, typical features of acute encephalitis were easily identified under light microscope. These features include mild lymphocytic infiltration in the meninges, dense perivascular lymphocytic infiltration in the parenchyma, extensive microglial proliferation, formation of some glial nodules, and cuff like inflammatory cells infiltrating around the blood vessel (Figure 1B-D). Thymus tissue showed mild thymic dysplasia with follicular hyperplasia. Spleen was infiltrated with phagocytes and monocytes, which indicated slight hypersplenism. To further characterize the histological changes, we also observed the secondary lymphoid organs among which the mesenteric lymph nodes appeared mild lymphoid hyperplasia and congestion (Figure 1E-G). In addition, there were some small scattered areas of lymphoid necrosis with hemorrhage, edema, and fibrin accumulation. The histological features of other tissues were nonspecific. There were slight changes in the mucosa of the alimentary tract with focal monocytes, lymphocytes infiltrating and some intestinal epithelial cell necrosis, but without formation of pseudo membrane (Figure 1H). The heart showed mild exudation with a small number of monocytes infiltrating focally in the myocardium. Ultrastructural observation showed that organelles within the myocardial cell were intact without degeneration and rupture. Nevertheless, no myocyte damage or viral inclusion was observed (Figure 1I).
Figure 1.
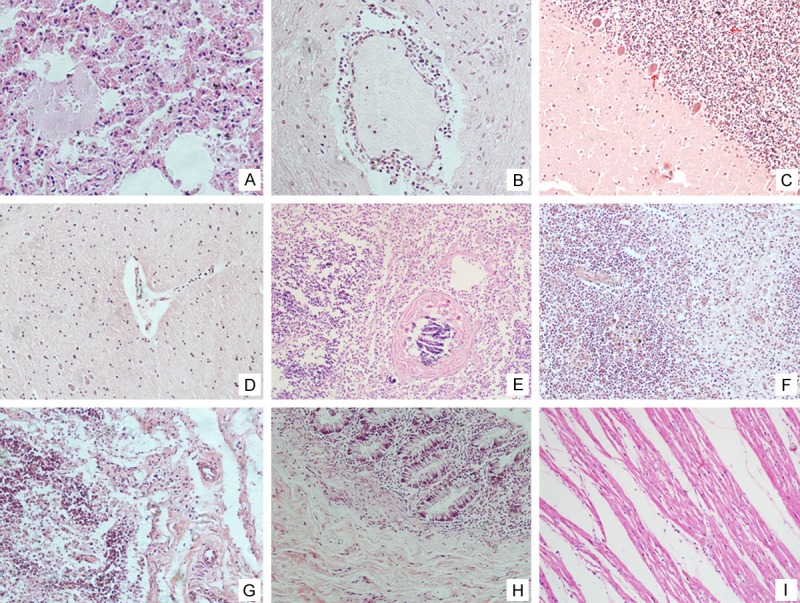
Histological findings of the main tissues of the autopsy. Lungs manifested as typical severe pulmonary congestion and edema with focal intra-alveolar hemorrhage (A); Sleevelet-like inflammatory cells infiltrated around the blood vessel in the brain. The cerebellum and brain stem showed the inflammatory cell infiltration (B-D); The thymus showed follicular hyperplasia (E); The spleen was congested with phagocytes and mononuclear cells (F); The mesenteric lymph nodes showed mild lymphoid hyperplasia and congestion (G); There was slight histological change in the mucosa of the alimentary tract with only a mild degree of focal monocytes, lymphocytes infiltration (H); The heart showed mild local exudation with a small focus of mononuclear cell infiltration in the myocardium. The myocardial cells were intact without degeneration and rupture (I).
The immunohistochemical analysis for EV71 clearly showed positive staining in the cytoplasm and nucleus of neurocytes at the areas of the cerebrum, cerebella and brainstem. Approximately 2/3 cerebellar granule cells were strongly positive. Moderate numbers of Purkinje cells displayed an intracytoplasmic and intranuclear staining. Neuron was negative for EV71 antigen in the brain, but it was partly positive in the brainstem (Figure 2A-C).
Figure 2.
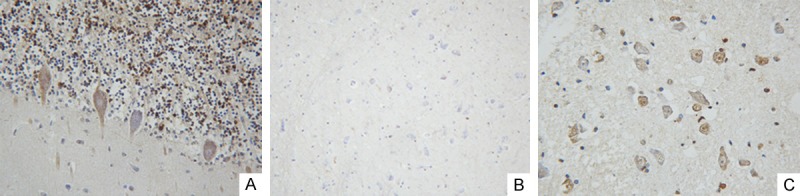
Detection of VP1 protein of EV71 by immunohistochemistry staining method. In cerebella, 2/3 cerebellar granule cells were strongly positive and moderate numbers of Purkinje cells displayed an intracytoplasmic and intranuclear staining (A); Neuron was negative for EV71 antigen in the brain (B); Neuron were partly positive in the brainstem (C).
To further study the distribution of EV71, we also measured the viral loads of different tissues by the method of Real-time PCR. The brainstem and mesenteric lymph nodes showed the highest viral loads among all tissues, while the ileum and stomach were lowest (Figure 3).
Figure 3.
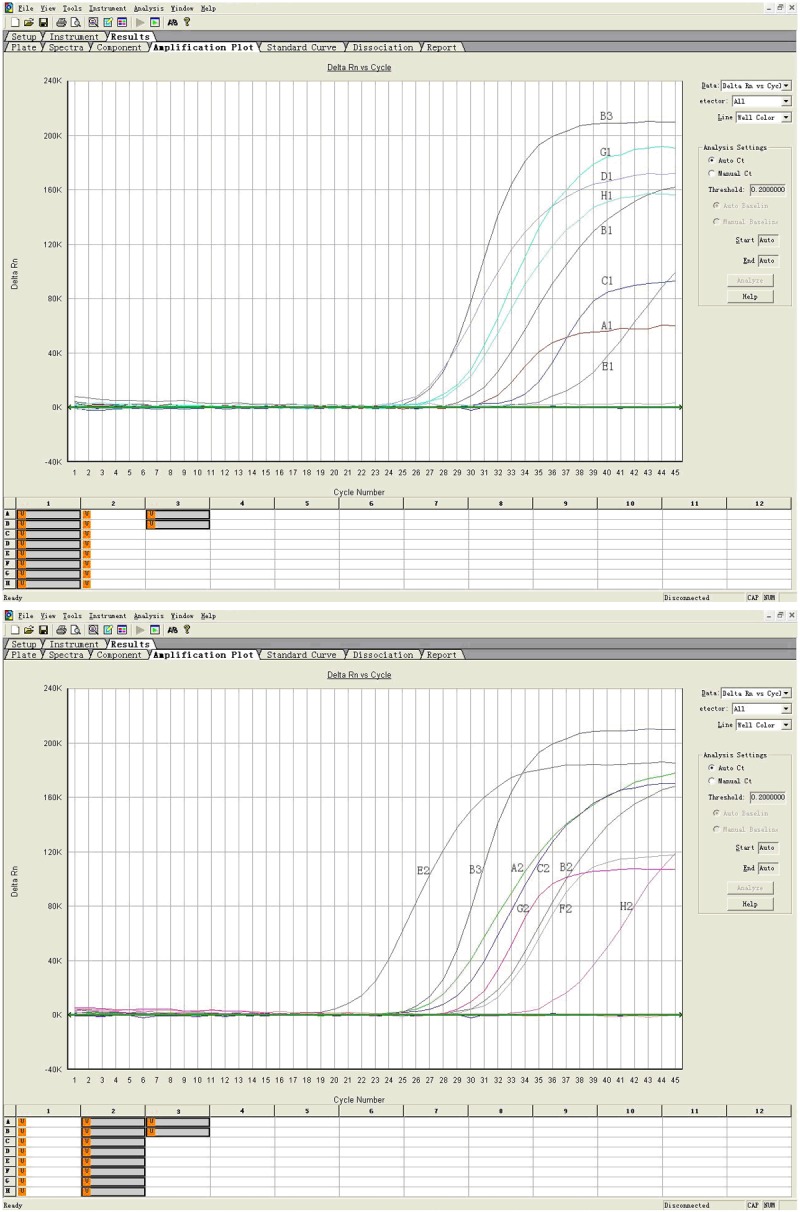
Detection of EV71 in each tissue by real-time RT-PCR. A1: Heart blood; B1: Thymus; C1: Heart; D1: Mesenteric lymphoid node; E1: Ileum; G1: left lower lung; H1: Right lower lung; A2: Spleen; B2: Liver; C2: Kidney; E2: Brain stem; F2: Cerebellum; G2: Cerebrum; H2: Gastric wall; B3: Positive control.
Discussion
EV71 was firstly isolated in California, USA in 1969. Its infection has been a major public health issue across the Asia-Pacific region [1]. Neurological complication and cardiopulmonary dysfunction have become notable features in EV71 epidemics in Asia, and were the primary cause of death [2-4]. However, the biological determinants of these differences are poorly understood. Only a few autopsy cases are reported and the pathologic features of EV71 infection are not well documented.
Human beings are the only known natural hosts of human enterovirus. The speculated replication cycle of EV71 is similar to those known polioviruses [5]. Generally speaking, initial polioviruses replication is presumed to occur in the lymphoid tissues of the tonsils and small bowel. Then the viruses invade mesenteric lymph nodes, giving rise to mild virusemia. Further dissemination of enterovirus to the reticuloendothelial system, liver, spleen, bone marrow, heart, lung, pancreas, skin, mucous membranes and CNS coincides with the onset of clinical features. In this case, the powerful evidence for viremia was clear, for various tissues were positive for viral nucleic acid. Elevated serum enzymes also indicated that viremia may occur. The viral loads implied disseminated infection and the differences in viral tropism. The brainstem and mesenteric lymph nodes showed the highest viral loads of all tissues, while the ileum and stomach were lowest. Based on the above results, we therefore assume that digestive system is not the primary target organ during the period of viremia, which is consistent with clinical manifestation of patients who often have no or only slight gastrointestinal symptoms in EV71 infections. Therefore, our result supported the opinion that mesenteric lymph nodes are viral replication sites and the brain may be the primary target organ.
The features of this case were well documented with a series of pathological analysis. We believe that the main cause of death was pulmonary failure because both lungs showed marked congestion, edema, and focal hemorrhage while the heart showed only foci of monocyte infiltration without obvious myocyte necrosis and fibr inolysis. The pathological evidence was insufficient to prove that cardiac dysfunction was the major contributor to pulmonary edema. Different from previous study that EV 71 RNA was not detected in the lung [6-8], we found high viral loads in lungs. It is unknown whether the virus directly damage lung tissue or fulminant pulmonary edema is neurogenic. Its mechanism needs further investigation. In previous reviews, the pathogenesis of brain stem encephalitis has been the main subject, but is not entirely clear. In our case, the presence of brain injury was panencephalitis, including cerebrum, cerebella and brain stem. Although both previous reports [9] and our animal models (the results were not published) proved that spinal cord was damaged, pathological examination of this autopsy case demonstrated very mild inflammation and non-specific changes. It was not sufficient to explain that EV 71 involvement of CNS resulted in neurogenic cardiopulmonary failure and even death.
The virus in this case has been identified as the human enterovirus type 71, belonging to Cluster C4a, basically similar to the epidemic genotype in mainland China and neighboring countries. Correspondingly, the deduced amino acid sequences revealed that there were amino acid changes in H22R and A289T [10]. For polioviruses, the 5’untranslated region and VP1 genes contain virulence determinants. Phylogenetic analysis of EV71 based on the structural VP1 gene identified three independent lineages of EV71, designated A, B, and C [11]. Subgroup C4 has become the predominant circulating subtype in mainland China, Japan, Vietnam, and Taiwan after 2000 [4,12-14]. The relationship between the gene types of EV71 virus and the factors that contributed to its survival, transmission and evasion of immunity is still unclear [15]. Several researchers have, therefore, examined the relevant nucleotide sequences to compare isolates from fatal and non-fatal cases, but most isolates have been identical or nearly identical without unanimous conclusion [16,17]. In our study, there were amino acid changes in H22R and A289T [10], but more strains from fatal cases and more data are required to prove that the virulence determinants of the strain have key roles in the pathogenesis of severe neurological complications. As a matter of fact, the biological determinants of enterovirus 71 are poorly understood. Further research is needed on virus epidemiology, pathogenesis, and control. Our studies should provide clues to the prevention and treatment of this devastating illness.
Acknowledgements
This work was supported by Guangdong Natural Science Foundation (No.S2011040003732 and No.S2012010009540).
Disclosure of conflict of interest
None.
References
- 1.Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, Wang JR, Shih SR. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341:929–935. doi: 10.1056/NEJM199909233411301. [DOI] [PubMed] [Google Scholar]
- 2.Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med. 1999;341:936–942. doi: 10.1056/NEJM199909233411302. [DOI] [PubMed] [Google Scholar]
- 3.Prager P, Nolan M, Andrews IP, Williams GD. Neurogenic pulmonary edema in enterovirus 71 encephalitis is not uniformly fatal but causes severe morbidity in survivors. Pediatr Crit Care Med. 2003;4:377–381. doi: 10.1097/01.PCC.0000074274.58997.FE. [DOI] [PubMed] [Google Scholar]
- 4.Tu PV, Thao NT, Perera D, Huu TK, Tien NT, Thuong TC, How OM, Cardosa MJ, McMinn PC. Epidemiologic and virologic investigation of hand, foot, and mouth disease, southern Vietnam, 2005. Emerg Infect Dis. 2007;13:1733–1741. doi: 10.3201/eid1311.070632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jesus NH. Epidemics to eradication: the modern history of poliomyelitis. Virol J. 2007;4:70. doi: 10.1186/1743-422X-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin TY, Chang LY, Hsia SH, Huang YC, Chiu CH, Hsueh C, Shih SR, Liu CC, Wu MH. The 1998 enterovirus 71 outbreak in Taiwan: pathogenesis and management. Clin Infect Dis. 2002;34(Suppl 2):S52–57. doi: 10.1086/338819. [DOI] [PubMed] [Google Scholar]
- 7.Lum LC, Wong KT, Lam SK, Chua KB, Goh AY, Lim WL, Ong BB, Paul G, AbuBakar S, Lambert M. Fatal enterovirus 71 encephalomyelitis. J Pediatr. 1998;133:795–798. doi: 10.1016/s0022-3476(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 8.Chang LY, Lin TY, Hsu KH, Huang YC, Lin KL, Hsueh C, Shih SR, Ning HC, Hwang MS, Wang HS, Lee CY. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet. 1999;354:1682–1686. doi: 10.1016/S0140-6736(99)04434-7. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Wang H, Gong E, Du J, Zhao X, McNutt MA, Wang S, Zhong Y, Gao Z, Zheng J. Neuropathology in 2 cases of fatal enterovirus type 71 infection from a recent epidemic in the People’s Republic of China: a histopathologic, immunohistochemical, and reverse transcription polymerase chain reaction study. Hum Pathol. 2009;40:1288–1295. doi: 10.1016/j.humpath.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Gao LL, Chen Q, Zhong XZ, Tan XL, Zhou J, Guo J, Yu SY. An analysis of the characteristics and etiology of hand, foot and mouth disease in a Guangzhou sentinel hospital from May to December, 2008. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:1333–5. [PubMed] [Google Scholar]
- 11.Brown BA, Oberste MS, Alexander JP Jr, Kennett ML, Pallansch MA. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J Virol. 1999;73:9969–9975. doi: 10.1128/jvi.73.12.9969-9975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin KH, Hwang KP, Ke GM, Wang CF, Ke LY, Hsu YT, Tung YC, Chu PY, Chen BH, Chen HL, Kao CL, Wang JR, Eng HL, Wang SY, Hsu LC, Chen HY. Evolution of EV71 genogroup in Taiwan from 1998 to 2005: an emerging of subgenogroup C4 of EV71. J Med Virol. 2006;78:254–262. doi: 10.1002/jmv.20534. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu H, Utama A, Onnimala N, Li C, Li-Bi Z, Yu-Jie M, Pongsuwanna Y, Miyamura T. Molecular epidemiology of enterovirus 71 infection in the Western Pacific Region. Pediatr Int. 2004;46:231–235. doi: 10.1046/j.1442-200x.2004.01868.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, Mao N, Xu S, Zhu S, Cui A, Zhang Y, Yan D, Li Q, Dong X, Zhang J, Zhao Y, Wan J, Feng Z, Sun J, Wang S, Li D, Xu W. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J. 2010;7:94. doi: 10.1186/1743-422X-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee TC, Guo HR, Su HJ, Yang YC, Chang HL, Chen KT. Diseases caused by enterovirus 71 infection. Pediatr Infect Dis J. 2009;28:904–910. doi: 10.1097/INF.0b013e3181a41d63. [DOI] [PubMed] [Google Scholar]
- 16.Shih SR, Ho MS, Lin KH, Wu SL, Chen YT, Wu CN, Lin TY, Chang LY, Tsao KC, Ning HC, Chang PY, Jung SM, Hsueh C, Chang KS. Genetic analysis of enterovirus 71 isolated from fatal and non-fatal cases of hand, foot and mouth disease during an epidemic in Taiwan, 1998. Virus Res. 2000;68:127–136. doi: 10.1016/s0168-1702(00)00162-3. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Poh CL, Chow VT. Complete sequence analyses of enterovirus 71 strains from fatal and non-fatal cases of the hand, foot and mouth disease outbreak in Singapore (2000) Microbiol Immunol. 2002;46:801–808. doi: 10.1111/j.1348-0421.2002.tb02767.x. [DOI] [PubMed] [Google Scholar]


