Abstract
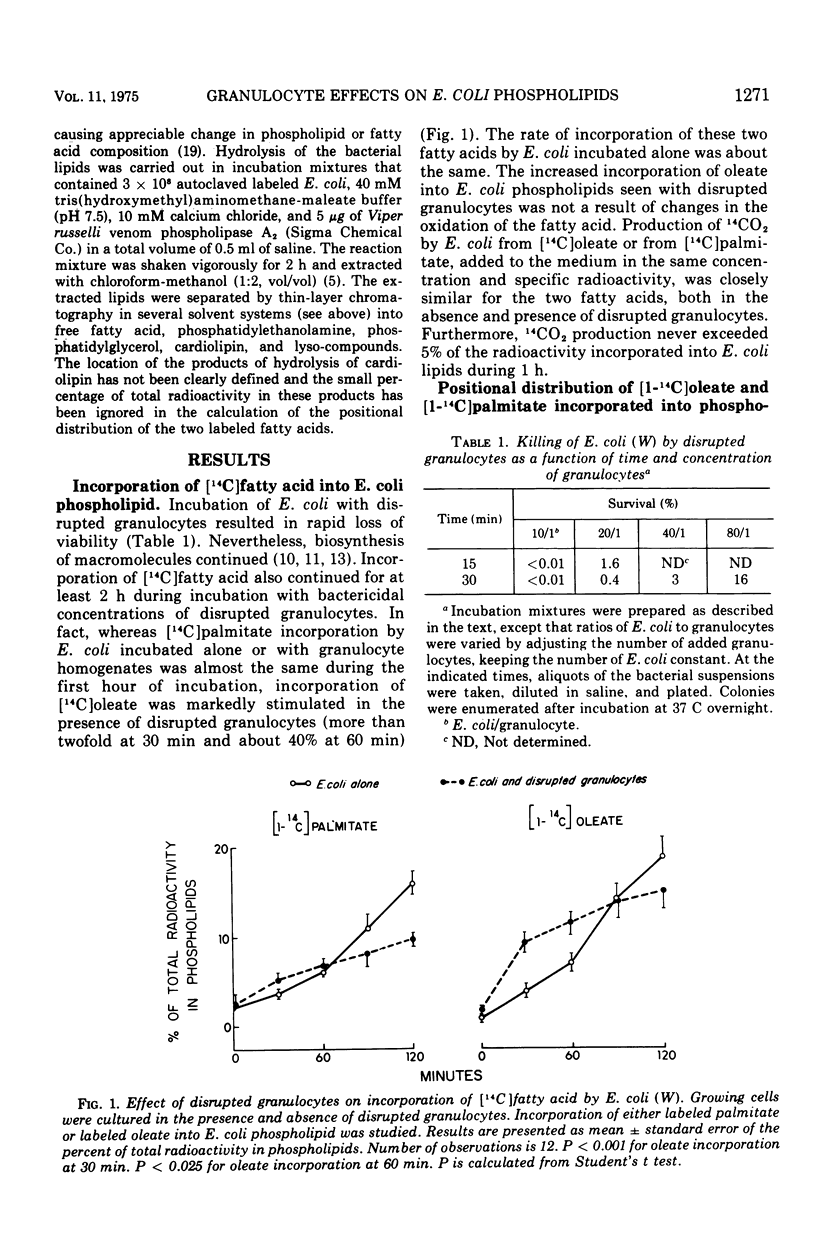
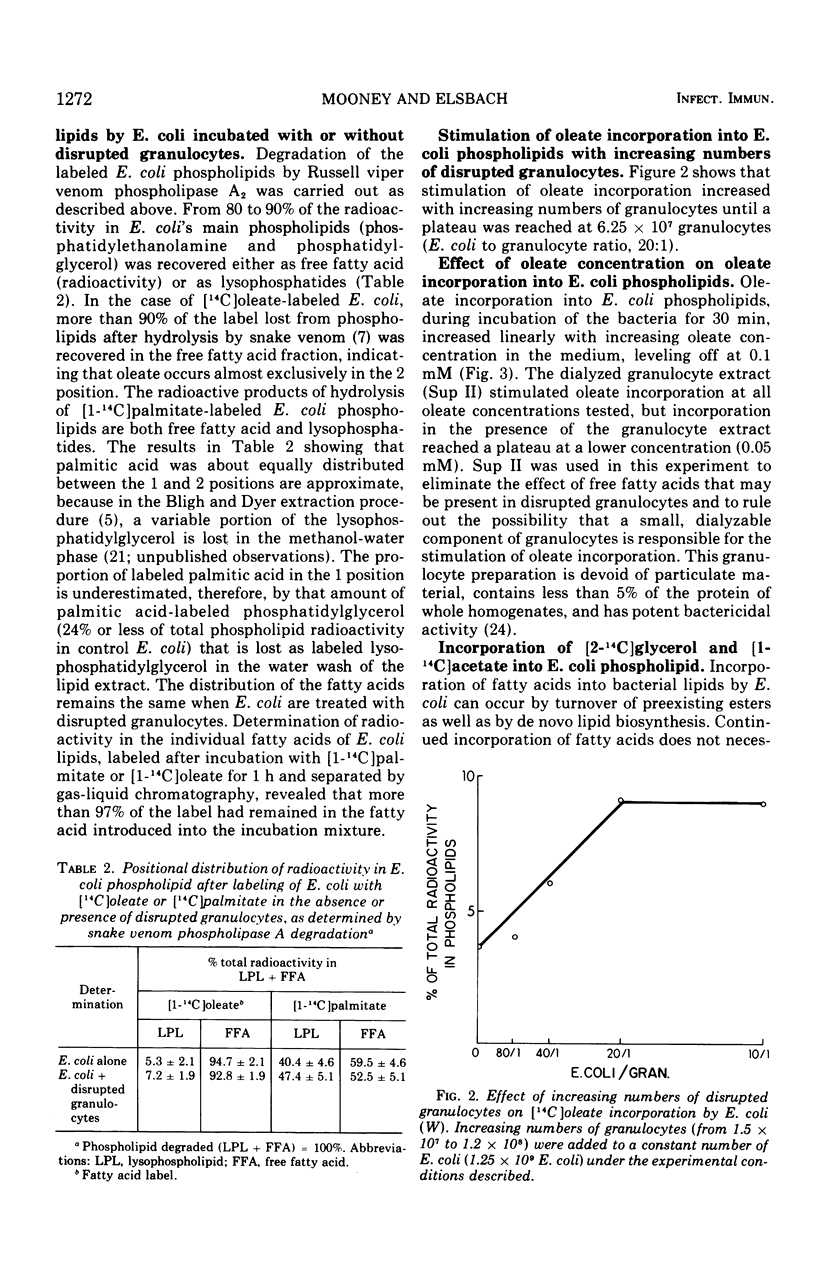
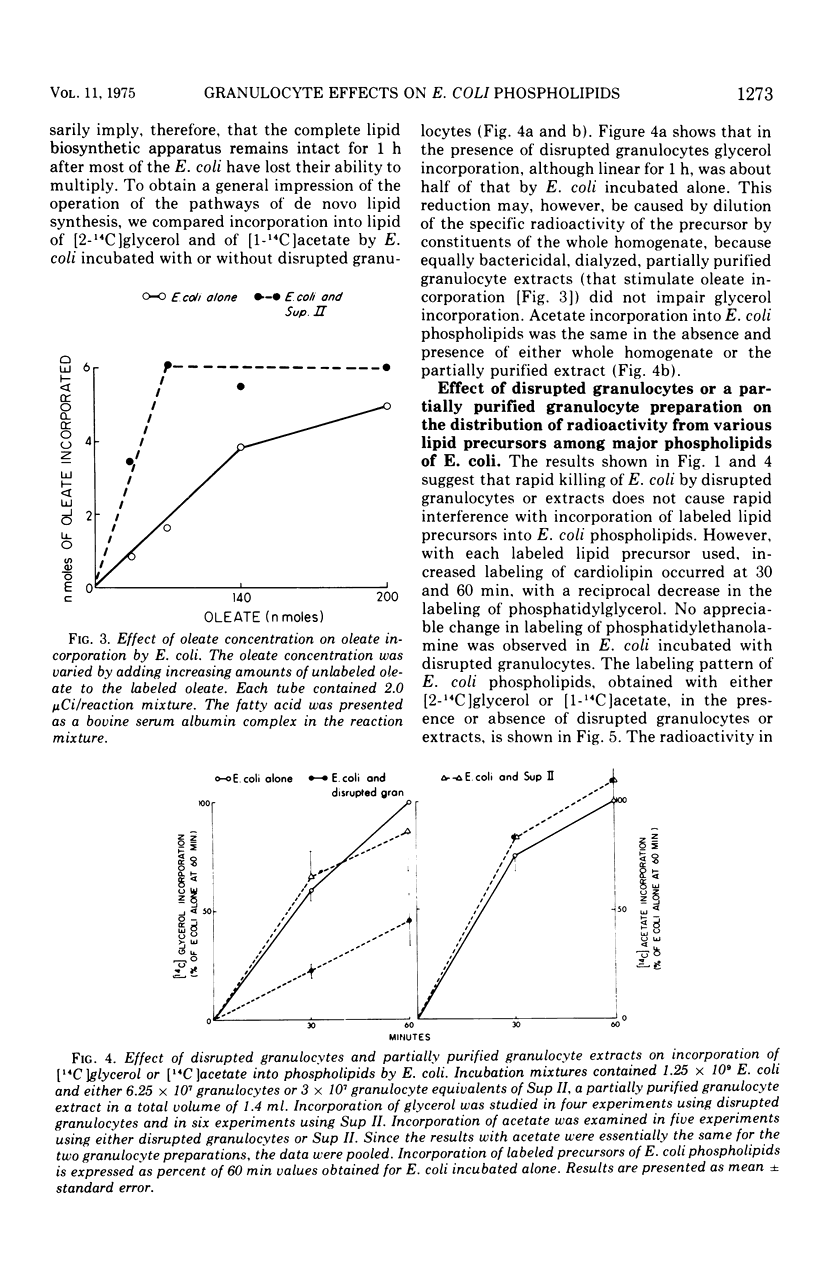
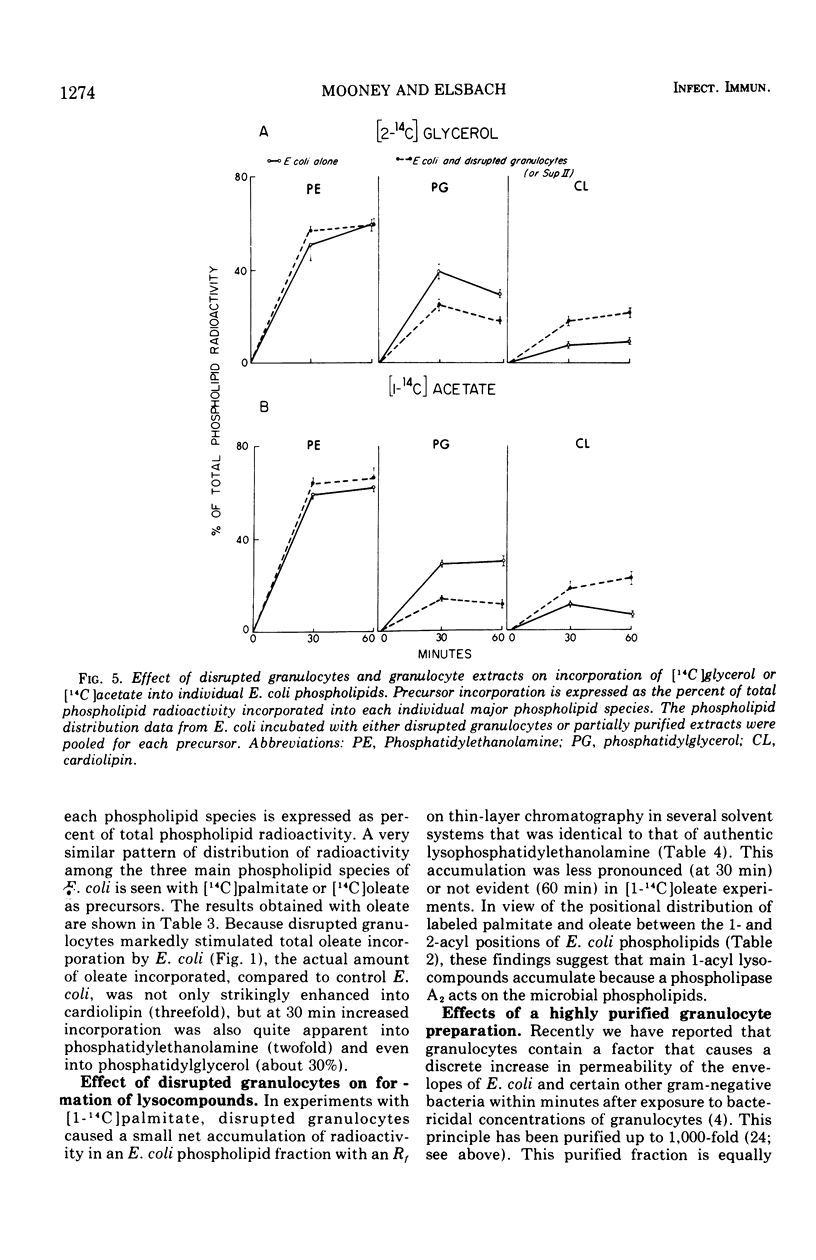
The effect of bactericidal concentrations of disrupted rabbit granulocytes and of partially purified granulocyte fractions on phospholipid metabolism by Escherichia coli has been investigated. Previous studies in this laboratory have shown that, during and after killing of E. coli by granulocytes, bacterial macromolecular synthesis continues. Similarly, despite almost complete loss of viability within 15 min, incorporation of [1-14C]palmitate, [2-14C]glycerol, and [1-14C]acetate into E. coli phospholipids, in the presence of granulocyte preparations, remains the same as in control E. coli populations for at least 1 h. Incorporation of [1-14C]oleate into E. coli phospholipids is actually stimulated during the first 60 min of incubation in the presence of granulocyte preparations (more than twofold at 30 min and 40% at 60 min). With all labeled lipid precursors, bactericidal granulocyte preparations cause a relative increase in the labeling of E. coli cardiolipin, with a corresponding drop in labeled phosphatidyl-glycerol. Labeled lyso-compounds accumulate in the presence of granulocyte preparations when [1-14C]palmitate, but not when [1-14C]oleate is the labeled precursor. Since oleate occurs mainly in the 2-acyl position of E. coli phospholipids, whereas at least 50% of palmitate occurs in the 1 position, it appears that a phospholipase A2 acts on the E. coli phospholipids. These various effects are also seen when E. coli are exposed to highly purified granulocyte preparations that possess potent bactericidal and phospholipase A2 activities. We speculate that this phospholipase A2 in the granulocyte preparations stimulates oleate but not palmitate incorporation by initiating increased turnover of the fatty acid in the 2-acyl position of E. coli phospholipids, causing formation of 1-acyl lyso-compounds likely to be preferentially reacylated with unsaturated fatty acids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright F. R., White D. A., Lennarz W. J. Studies on enzymes involved in the catabolism of phospholipids in Escherichia coli. J Biol Chem. 1973 Jun 10;248(11):3968–3977. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ballesta J. P., Schaechter M. Effect of shift-down and growth inhibition on phospholipid metabolism of Escherichia coli. J Bacteriol. 1971 Jul;107(1):251–258. doi: 10.1128/jb.107.1.251-258.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerdite S., Mooney C., Weiss J., Franson R., Elsbach P. Early and discrete changes in permeability of Escherichia coli and certain other gram-negative bacteria during killing by granulocytes. J Exp Med. 1974 Aug 1;140(2):396–409. doi: 10.1084/jem.140.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr Phospholipid alterations during growth of Escherichia coli. J Bacteriol. 1968 Jun;95(6):2054–2061. doi: 10.1128/jb.95.6.2054-2061.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Beckerdite S., Pettis P., Franson R. Persistence of regulation of macromolecular synthesis by Escherichia coli during killing by disrupted rabbit granulocytes. Infect Immun. 1974 Apr;9(4):663–668. doi: 10.1128/iai.9.4.663-668.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Goldman J., Patriarca P. Phospholipid metabolism by phagocytic cells. VI. Observations on the fate of phospholipids of granulocytes and ingested Escherichia coli during phagocytosis. Biochim Biophys Acta. 1972 Sep 7;280(1):33–44. [PubMed] [Google Scholar]
- Elsbach P. Metabolism of lysophosphatidyl ethanolamine and lysophosphatidyl choline by homogenates of rabbit polymorphonuclear leukocytes and alveolar macrophages. J Lipid Res. 1967 Jul;8(4):359–365. [PubMed] [Google Scholar]
- Elsbach P. On the interaction between phagocytes and micro-organisms. N Engl J Med. 1973 Oct 18;289(16):846–852. doi: 10.1056/NEJM197310182891610. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Pettis P., Beckerdite S., Franson R. Effects of phagocytosis by rabbit granulocytes on macromolecular synthesis and degradation in different species of bacteria. J Bacteriol. 1973 Aug;115(2):490–497. doi: 10.1128/jb.115.2.490-497.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P. Phospholipid metabolism by phagocytic cells. I. A comparison of conversion of [32P]lysolecithin to lecithin and glycerylphosphorylcholine by homogenates of rabbit polymorphonuclear leukocytes and alveolar macrophages. Biochim Biophys Acta. 1966 Dec 7;125(3):510–524. [PubMed] [Google Scholar]
- Franson R., Patriarca P., Elsbach P. Phospholipid metabolism by phagocytic cells. Phospholipases A2 associated with rabbit polymorphonuclear leukocyte granules. J Lipid Res. 1974 Jul;15(4):380–388. [PubMed] [Google Scholar]
- Gale E. F. Effect of morphine derivatives on lipid metabolism in Staphylococcus aureus. Mol Pharmacol. 1970 Mar;6(2):134–145. [PubMed] [Google Scholar]
- Gale E. F. Effects of diacetylmorphine and related morphinans on some biochemical activities of Staphylococcus aureus. Mol Pharmacol. 1970 Mar;6(2):128–133. [PubMed] [Google Scholar]
- Kanemasa Y., Akamatsu Y., Nojima S. Composition and turnover of the phospholipids in Escherichia coli. Biochim Biophys Acta. 1967 Oct 2;144(2):382–390. [PubMed] [Google Scholar]
- Onishi Y. "Phospholipids of virus-induced membranes in cytoplasm of Escherichia coli. J Bacteriol. 1971 Sep;107(3):918–925. doi: 10.1128/jb.107.3.918-925.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P., Beckerdite S., Elsbach P. Phospholipases and phospholipid turnover in Escherichia coli spheroplasts. Biochim Biophys Acta. 1972 Apr 18;260(4):593–600. doi: 10.1016/0005-2760(72)90008-2. [DOI] [PubMed] [Google Scholar]
- Proulx P. R., van Deenen L. L. Acylation of lysophosphoglycerides by Escherichia coli. Biochim Biophys Acta. 1966 Dec 7;125(3):591–593. doi: 10.1016/0005-2760(66)90046-4. [DOI] [PubMed] [Google Scholar]
- Scandella C. J., Kornberg A. A membrane-bound phospholipase A1 purified from Escherichia coli. Biochemistry. 1971 Nov 23;10(24):4447–4456. doi: 10.1021/bi00800a015. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Franson R. C., Beckerdite S., Schmeidler K., Elsbach P. Partial characterization and purification of a rabbit granulocyte factor that increases permeability of Escherichia coli. J Clin Invest. 1975 Jan;55(1):33–42. doi: 10.1172/JCI107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster N., Elsbach P., Rand J., Simon E. J. Effects of levorphanol on phospholipid metabolism and composition in Escherichia coli. Biochim Biophys Acta. 1971 Nov 5;248(2):282–292. doi: 10.1016/0005-2760(71)90016-6. [DOI] [PubMed] [Google Scholar]
- de HAAS G., DAEMEN F. J., van DEENEN L. The site of action of phosphatide acyl-hydrolase (phospholipase A) on mixed-acid phosphatides containing a poly-unsaturated fatty acid. Biochim Biophys Acta. 1962 Dec 4;65:260–270. doi: 10.1016/0006-3002(62)91045-4. [DOI] [PubMed] [Google Scholar]
- van den Bosch H., van Golde L. M., van Deenen L. L. Dynamics of phosphoglycerides. Ergeb Physiol. 1972;66:13–145. doi: 10.1007/3-540-05882-6_2. [DOI] [PubMed] [Google Scholar]



