Abstract
Primary malignant lymphoma of the urinary bladder is a rare disease constituting less than 1% of neoplasms of the urinary bladder. The most prevalent histological subtype is extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue type (MALT lymphoma). It is frequently associated with chronic cystitis and predominantly occurs in females. On the other hand, malakoplakia is thought to be a reactive granulomatous lesion occurring most prevalently in the genitourinary tracts. It is frequently found in females and often associated with bacterial infection in immunosuppressive status. Here we report a rare case of concurrent primary MALT lymphoma and malakoplakia in the urinary bladder in a 78-year-old Japanese female. Presumably, both lymphoma and malakoplakia are considered to be involved in the antecedent cystitis and might contribute to the development of the urinary bladder tumor of the patient, leading to the occlusion of the right ureter with subsequent hydronephrosis.
Keywords: MALT lymphoma, malakoplakia, urinary bladder, cystitis
Introduction
Primary malignant lymphoma of the urinary bladder is a rare disease constituting less than 1% of neoplasms of the urinary bladder [1]. Its frequency is reported to be as low as 0.2% of the extranodal lymphomas [2]. The most prevalent histological subtype of the primary malignant lymphoma of the urinary bladder is extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue type (MALT lymphoma). It is frequently associated with chronic cystitis and predominantly occurs in females [1]. Its prognosis is reported to be better than that of secondary involvement of the urinary bladder by diffuse large B-cell lymphomas [1].
Malakoplakia is thought to be a reactive granulomatous lesion composed of histiocytes containing Michaelis-Gutmann body, which is detected by Kossa staining [3]. Malakoplakia is reported to occur most prevalently in the genitourinary tracts including the urinary bladder [3]. Malakoplakia in the urinary tract shows female predominance [3]. It is considered to be associated with inflammatory changes of the urinary bladder, probably due to impairment of bacterial killing. Therefore, it is often observed in immunosuppressive status [3]. It was reported that malakoplakia is associated with malignant as well as benign lesions [3]. Sometimes a reactive character of malakoplakia may obscure a malignant nature of the primary lesion, providing a diagnostic challenge for pathologists [3].
Here we report a case of concurrent primary MALT lymphoma with malakoplakia of the urinary bladder in a 78-year-old Japanese female. Although both MALT lymphoma and malakoplakia are associated with cystitis, their concurrence in the urinary bladder has rarely been reported.
Case report
A 78-year-old Japanese female had been followed up by clinic for hyperlipidemia and cholelithiasis for around five years. Since 9 months before she was admitted to our hospital, she had been medicated for cystitis. One month before her admission, she had been presented to the clinic with complaint of fever. Examination of the peripheral blood on admission suggested anemia and renal dysfunction. She was referred to the urology department of our hospital for further examination and treatment of refractory chronic cystitis. Examination of the peripheral blood revealed that white blood cell count was 8910 /μl (normal range: 4000-9000) with 69% of neutrophils (normal range: 38.0-58.0), hemoglobin 10.1 g/dl (normal range: 11.5-15.0), total protein 8.2 g/dL (normal range: 6.6-8.7), albumin 3.5 g/dL (normal range: 3.7-4.7), IgG 2299 mg/dL (normal range: 870-1700), CRP 10.3 mg/dL (normal range: less than 0.3), blood urea nitrogen 24 mg/dL (normal range: 8-20), and creatinine 1.89 mg/dL (normal range: 0.36-1.06). Serological test and urine examination showed no evidence of monoclonal protein (M protein). Urine analysis showed microhematuria and E. coli, but urine cytology was negative for malignant cells.
Magnetic resonance imaging (Figure 1A) and computerized tomography (Figure 1B) of the abdomen and the pelvis revealed a huge mass covering the anterior to right side wall of the urinary bladder with around 15 mm of thickness (Figure 1). The mass obliterated the right ureter, resulting in hydronephrosis (data not shown). 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT examination showed positive signals in four 10 mm-sized lymph nodes in the mediastinum, suggesting metastasis (data not shown). A bladder cancer and its mediastinal metastasis were suspected.
Figure 1.

Magnetic resonance imaging (MRI) and computerized tomography (CT) of the urinary bladder tumor. A. Sagittal view of the tumor by MRI. The left side is the anterior portion of the abdomen. The tumor is indicated by arrowheads. Bar: 5 cm; B. Horizontal view of the tumor by CT. The upper side is the anterior portion of the pelvis. The tumor is indicated by arrowheads. Bar: 4 cm.
Transurethral biopsy for the bladder tumor was performed. The hematoxylin and eosin (HE) image of the biopsy specimen showed vaguely nodular proliferation of small to medium-sized lymphoid cells with urothelial erosion (Figure 2A and 2B). The germinal centers were not evident (Figure 2B). The tumor nodule showed prominent plasmacytic differentiation particularly at the periphery of the nodule (data not shown). Immunohistochemical studies demonstrated that the proliferating small lymphoid cells were positive for CD20 (Figure 2C), CD79a (data not shown), BCL2 (data not shown), and negative for CD3 (Figure 2D), CD10 (data not shown), CD23 (data not shown), and cyclin D1 (data not shown). Part of the tumor cells were positive for CD138, consistent with the plasmacytic differentiation (data not shown). In situ hybridization of immunoglobulin light chains showed restricted expression of immunoglobulin κ chain compared with λ chain (Figure 2E and 2F). On the other hand, no apparent evidence for urothelial carcinoma was observed (Figure 2A). From these histopathological findings, a diagnosis of MALT lymphoma of the urinary bladder was made. The lymphoma lesion was associated with sheets or aggregates of epithelioid histiocytes (Figure 3A), which was confirmed by CD68 immunohistochemistry (Figure 3B). Kossa staining of the aggregated histiocytes showed Michaelis-Gutmann bodies (Figure 3C), confirming the association of malakoplakia with MALT lymphoma. Together with the results of systemic examination and imaging studies, a final diagnosis of primary MALT lymphoma with malakoplakia of the urinary bladder of clinical stage III was established.
Figure 2.
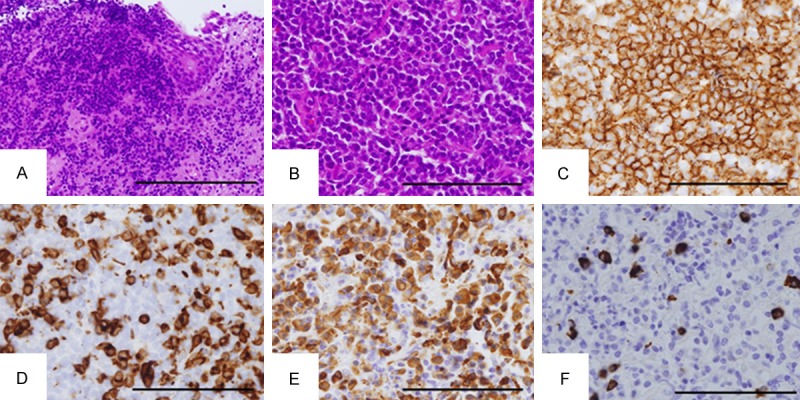
Histology of the urinary bladder tumor. A. Hematoxylin and eosin (HE) image of the urothelial erosion by infiltration of the tumor cells. The tumor cells proliferate in a vaguely nodular fashion. Residual urothelial epithelium is observed in the right-upper corner of the figure. Original magnification: ×200. Bar: 200 μm; B. HE image of the tumor nodule. The tumor cells are small and medium-sized. Germinal centers are not evident. Original magnification: ×400. Bar: 100 μm; C. CD20 immunohistochemistry. The tumor cells are stained brown and CD20-positive. Original magnification: ×400. Bar: 100 μm; D. CD3 immunohistochemistry. Most of the tumor cells are stained negative. Original magnification: ×400. Bar: 100 μm; E, F. In situ hybridization of immunoglobulins (Ig) κ light chain (E) and λ light chain (F). Most of the tumor cells are positive for Igκ (E) but negative for Igλ (F), suggesting monoclonal proliferation of the B lymphoid cells. Original magnification: ×400. Bar: 100 μm.
Figure 3.
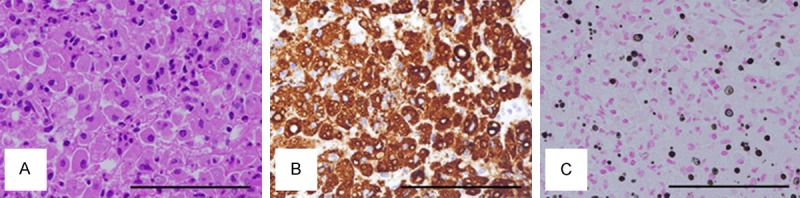
Histology of malakoplakia. A. HE image. Epithelioid histiocytes are aggregated in a sheet-like manner; B. CD68 immunohistochemistry. The aggregated cells are positively stained; C. Kossa staining. The histiocytes contain black calcified Michaelis-Gutmann bodies. Original magnification: ×400. Bar: 100 μm.
After the subsequent four courses of treatment with rituximab, the thickness of the lymphoma lesion decreased from 15 mm to 5 mm by abdominal ultrasonography (data not shown).
Discussion
Primary MALT lymphoma of the urinary bladder is primarily a localized disease [1]. Its transformation into diffuse large B-cell lymphoma was reported [4]. Although large cell components were not apparent in the urinary bladder in our case, PET/CT examination revealed a mediastinal lesion, which was presumed to be a distant involvement of the bladder lymphoma.
Primary MALT lymphoma of the urinary bladder shows female predominance [1]. It is frequently associated with chronic cystitis [1]. One plausible pathological basis may be an induction of MALT tissue in the urinary bladder by chronic cystitis, a close parallel of MALT lymphomagenesis by gastric infection with Helicobacter pylori. It is generally known that MALT lymphoma may be associated with autoimmune diseases. However, in our case, there were no apparent evidences suggesting the presence of autoimmune disorders except the 40 fold titer of anti-nuclear antibody (normal range: less than 40 fold). In addition, IgG4-positive plasma cells were only scatteredly observed immunohistochemically, excluding the possibility of IgG4-related disease (data not shown).
In our case, malakoplakia was associated with the MALT lymphoma in the urinary bladder. The majority of the bladder tumor was occupied by the lymphoma cells, and malakoplakia was observed in part of the tumor. Supposedly, the lymphoma and the malakoplakia may share cystitis as a common etiology, or malakoplakia may be a reactive lesion against the lymphoma. Various histiocytic lesions were reported to be associated with MALT lymphoma, such as crystal-storing histiocytosis [5-14], hemophagocytosis [15], and Langerhans cell histocytosis [16]. However, no definite case of concurrent MALT lymphoma and malakoplakia was reported. There was only one case report on lymphoma and malakoplakia in the urinary bladder [17]. However, in that case, the malakoplakia preceded the lymphoma and the histological subtype of the lymphoma was not shown [17]. In general, malakoplakia occurs in immunocompromised hosts. It is possible that the rarity of malakoplakia may be related to the level of immunosuppression required.
Here we report a rare case of concurrent primary MALT lymphoma and malakoplakia in the urinary bladder in a 78-year-old Japanese female. In our case, both lymphoma and malakoplakia may contribute to the development of the urinary bladder tumor of the patient, leading to the occlusion of the right ureter with subsequent hydronephrosis. It is important to keep in mind that malakoplakia sometimes coexists with malignant lesions as in our case.
Acknowledgements
We thank all the colleagues in the Department of Surgical Pathology, Hyogo College of Medicine for preparation of pathological specimen.
Disclosure of conflict of interest
None.
References
- 1.Marx A. Lymphomas. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: International Agency for Research on Cancer (IARC); 2004. p. 147. [Google Scholar]
- 2.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Yousef GM, Naghibi B, Hamodat MM. Malakoplakia outside the urinary tract. Arch Pathol Lab Med. 2007;131:297–300. doi: 10.5858/2007-131-297-MOTUT. [DOI] [PubMed] [Google Scholar]
- 4.Bates AW, Norton AJ, Baithun SI. Malignant lymphoma of the urinary bladder: a clinicopathological study of 11 cases. J Clin Pathol. 2000;53:458–461. doi: 10.1136/jcp.53.6.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llobet M, Castro P, Barcelo C, Trull JM, Campo E, Bernado L. Massive crystal-storing histiocytosis associated with low-grade malignant B-cell lymphoma of MALT-type of the parotid gland. Diagn Cytopathol. 1997;17:148–152. doi: 10.1002/(sici)1097-0339(199708)17:2<148::aid-dc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Coupland SE, Foss HD, Hummel M, Stein H. Extranodal marginal zone B-cell lymphoma of the lacrimal gland associated with crystal-storing histiocytosis. Ophthalmology. 2002;109:105–110. doi: 10.1016/s0161-6420(01)00837-5. [DOI] [PubMed] [Google Scholar]
- 7.Fairweather PM, Williamson R, Tsikleas G. Pulmonary extranodal marginal zone lymphoma with massive crystal storing histiocytosis. Am J Surg Pathol. 2006;30:262–267. doi: 10.1097/01.pas.0000178093.99889.f7. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Tawfiqul B, Valderrama E, Kline G, Kahn LB. Pulmonary crystal-storing histiocytosis and extranodal marginal zone B-cell lymphoma associated with a fibroleiomyomatous hamartoma. Ann Diagn Pathol. 2003;7:47–53. doi: 10.1053/adpa.2003.50008. [DOI] [PubMed] [Google Scholar]
- 9.Kusakabe T, Watanabe K, Mori T, Iida T, Suzuki T. Crystal-storing histiocytosis associated with MALT lymphoma of the ocular adnexa: a case report with review of literature. Virchows Arch. 2007;450:103–108. doi: 10.1007/s00428-006-0323-1. [DOI] [PubMed] [Google Scholar]
- 10.Kurabayashi A, Iguchi M, Matsumoto M, Hiroi M, Kume M, Furihata M. Thymic mucosa-associated lymphoid tissue lymphoma with immunoglobulin-storing histiocytosis in Sjogren’s syndrome. Pathol Int. 2010;60:125–130. doi: 10.1111/j.1440-1827.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- 11.Rossi G, De Rosa N, Cavazza A, Mengoli MC, Della Casa G, Nannini N, Colby TV. Localized pleuropulmonary crystal-storing histiocytosis: 5 cases of a rare histiocytic disorder with variable clinicoradiologic features. Am J Surg Pathol. 2013;37:906–912. doi: 10.1097/PAS.0b013e31827b1618. [DOI] [PubMed] [Google Scholar]
- 12.Yu SC, Yao M, Liao SL. Crystal-storing histiocytosis in a patient with ocular extranodal marginal zone lymphoma. Br J Haematol. 2013;160:419. doi: 10.1111/bjh.12149. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Myers JL. Crystal-storing histiocytosis complicating primary pulmonary marginal zone lymphoma of mucosa-associated lymphoid tissue. Arch Pathol Lab Med. 2013;137:1199–1204. doi: 10.5858/arpa.2013-0252-CR. [DOI] [PubMed] [Google Scholar]
- 14.Radhakrishnan S, Maneksha V, Adulkar N. Crystal-storing histiocytosis masquerading ocular adnexal lymphoma: a case report and review of literature. Ophthal Plast Reconstr Surg. 2014;30:e67–69. doi: 10.1097/IOP.0b013e31829c41f7. [DOI] [PubMed] [Google Scholar]
- 15.Ohno H, Takimoto K. Gastric mucosa-associated lymphoid tissue lymphoma complicated with hemophagocytic syndrome in an elderly woman. Ann Hematol. 2010;89:1175–1176. doi: 10.1007/s00277-010-0929-x. [DOI] [PubMed] [Google Scholar]
- 16.Licci S, Boscaino A, De Palma M, Del Nonno F, D’Antonio A. Concurrence of marginal zone B-cell lymphoma MALT-type and Langerhans cell histiocytosis in a thyroid gland with Hashimoto disease. Ann Hematol. 2008;87:855–857. doi: 10.1007/s00277-008-0489-5. [DOI] [PubMed] [Google Scholar]
- 17.Batchelor JS, Philp NH, Ramsden KL, Scott KW. Primary lymphoma of the bladder arising from an area of malakoplakia. Br J Urol. 1991;68:550–551. doi: 10.1111/j.1464-410x.1991.tb15405.x. [DOI] [PubMed] [Google Scholar]


