Abstract
Intravascular large B-cell lymphoma (IVLBCL) is a rare type of extranodal large B-cell lymphoma characterized by the selective growth of lymphoma cells within the lumina of vessels, particularly within capillaries, with exception of larger arteries and veins. The authors reported a case of a 45-year-old woman who was admitted in hospital with refractory fever, cough and progressive dyspnea despite of receiving broad-spectrum antibiotics. Computed tomography (CT) of the lung showed bilateral patchy ground-glass opacities with some pleural effusion in the left lung. A CT-guided percutaneous lung biopsy was performed and primary pulmonary intravascular large B-cell lymphoma was diagnosed by histopathology, immunophenotype and fluorescence in situ hybridization. The patient’s general status was improved after chemotherapy with R-CHOP. CT-guided percutaneous biopsy of lung is a safe and accurate diagnostic procedure in IVLBCL.
Keywords: Lung, intravascular large B-cell lymphoma, CT-guided percutaneous lung biopsy
Introduction
Intravascular large B-cell lymphoma (IVLBCL) is a rare subtype of non-Hodgkin lymphoma that is characterized by proliferation of lymphoma within the lumina of small blood vessels and capillaries and was recently listed as a rare subtype of diffuse large B-cell lymphoma in the World Health Organization (WHO) classification [1-3]. The central nervous system, liver and other organs are often involved. The most common clinical manifestations of IVLBCL often shows the central nervous system presentations, cutaneous lesions, fever of unknown origin or hemophagocytic syndrome, but abnormal findings related to lymph nodes, bone marrow and other solid organs are relatively rare in Western countries [4]. There are only several case reports and studies of primary pulmonary IVLBCL in the literature. IVLBCL in the lung is often suspected with dyspnea and abnormal chest findings on chest x-ray and/or CT and is usually diagnosed pathologically by transbronchial lung biopsy (TBLB), skin biopsy from the site of a rash or a peripheral nerve biopsy when neuritis is present [5,6].
We described the case of an IVLBCL patient, who was once treated as viral pneumonia and then developed refractory fever and severe dyspnea, despite of receiving broad-spectrum antibiotics. She also had elevated levels of serum lactic dehydrogenase (LDH) and C-reactive protein (CRP) and bilateral patchy ground-glass opacities with some pleural effusion in the left lung which is rarely described as a complication of IVLBCL.
Case presentation
A 45-year-old woman who presented complaining of persistent cough and intermittent high fever with temperatures greater than 39°C for about a month was admitted to local hospital. She had progressive dyspnea and hypoxia, and chest CT revealed worsened bilateral ground glass opacities with some pleural effusion in the left lung. Lymph nodes were not palpable, and liver function tests and spleen size were normal. However, other laboratory data showed anemia (hemoglobin 9.6 g/L) and elevated serum LDH (1443.9 U/L) and CRP (14 mg/L) levels. A concomitant infection of viral and bacterial and viral myocarditis were initially suspected, and administration of broad-spectrum antibiotics (imipenem-cilastatin and teicoplanin), antiviral (Ganciclovir) and steroids (Methylprednisolone), was started. However, her symptoms of cough, fever, dyspnea and general condition were deteriorated, and she was admitted to Xiangya hospital as a case of pyrexia of unknown origin (PUO) after intermittent antibiotic administration for one months. On admission, high fever persisted and dyspnea and hypoxia were present.
On physical exam, her body temperature was 38.5°C, her pulse rate was 120 counts per minute, respiratory rate was 25 breaths per minute without rales, and her BP was 100/50 mm Hg. She had no murmur or gallop on cardiac exam. Arterial blood gas analysis showed: pH 7.508, PaO2 57.0 mm Hg, and PaCO2 28.5mm Hg. Also, there were no skin lesions, lymphadenopathy, or neurological signs. CT scanning of the chest (Figure 1A, 1B) showed ground glass opacities with some pleural effusion in the left lung and mild hepatosplenomegaly. Enlarged lymph nodes and masses were not detected. Laboratory data on admission were as follows: white blood cell count 7.3×109/L (N 74%, Eo 1.4%, Baso 0.4%, Mono 9.2%, and Ly 15%), hemoglobin 96 g/L, platelet count 167×109/L, total protein 53.6 g/L, creatinine 52.6 umol/L, total bilirubin 8 umol/L, AST52.0 U/L, ALT19.0 U/L, LDH 1991.8 U/L and CRP14 mg/L. These laboratory findings showed inflammation and anemia. Infection, collagen diseases, and lymphoproliferative diseases were suspected as the primary disease resulting in the high fever and elevated serum LDH and CRP levels. However, microbiological examinations, such as sputum, urine, and blood cultures, were negative. The evaluation for collagen disease was unremarkable. Rheumatoid factor and antinuclear antibodies were negative. Examinations for a broad range of serum tumor markers were all negative. Bone marrow aspiration was performed, showing an active proliferation of granulocyte with obvious toxic reaction marrow but no lymphoma cells were observed.
Figure 1.
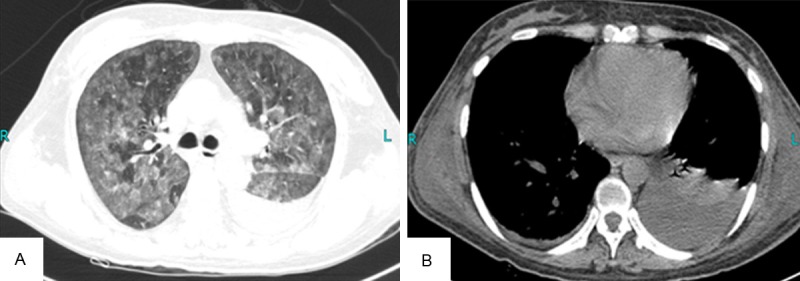
CT scanning of the chest. A: CT scan of chest revealed ground glass opacities in bilateral lung fields; B: CT scan of chest showed pleural effusion in the left lung field.
Tuberculosis (TB) was suspected and fungal infection cannot be excluded after consultation. Therefore diagnostic anti-TB therapy (isoniazid+rifapentine+ethambutol+pyrazinamide) was started. Pulmonary CT showed no obvious improvement after antituberculosis treatment for 20 days. Pulmonary CT showed that the patient with the lung image was no obvious improvement after empirical anti-tuberculosis treatment. We suspected IVLBCL for the reason that the patient was general symptoms along with an increased evidence of hypoxia. At the same time, CT-guided percutaneous lung biopsy was performed. Eight samples were obtained from the left lung with 1.0 cm×0.5 cm×0.5 cm sizes. Large neoplastic lymphoid cells were showed within small pulmonary arteries, veins and capillaries, but not outside the vessels (Figure 2).
Figure 2.
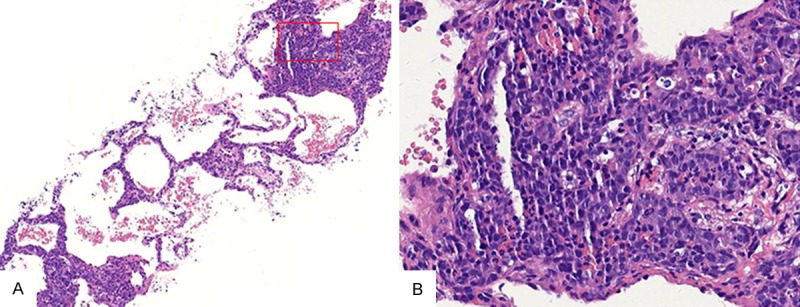
Hematoxylin-eosin staining (H&E staining) of pulmonary interstitium intravascular lymphoma from the CT-guided percutaneous lung biopsy specimen. A: the low microscopic view showed the alveolar septae was widen and filled with tumour cells (H&E staining, ×100); B: the high microscopic view showed invasion of many atypical lymphoid cells into the capillary vessels of the alveolar septae (H&E staining, ×400)
. Immunohistochemistry results showed that intravascular neoplastic cells were positive expression of leukocyte common antigen (LCA) (Figure 3A) and B-cell marker CD20 (Figure 3B). Then vascular endothelium cells were positive immunostaining for CD34, but the tumor cells were no staining of CD34 (Figure 3C). Proliferative marker, KI-67 showed distinctive nuclear reaction involving 75% neoplastic cells (Figure 3D). The tumor cells also positive immunostaining for B-cell marker such as CD79a, PAX-5, MUM1, BCL6. The intravascular neoplastic cells were seen the positive expression of Vim. All intravascular neoplastic cells were negative immunostaining for CD3, CD45RO, CD3, EMA, CK-pan, EMA, CgA, TTF-1, CK-H, CK-L, CK5/6, P63, 34βE12, CK7, Syn, CD56, NSE, CD30, HMB45, MC, MPO.
Figure 3.
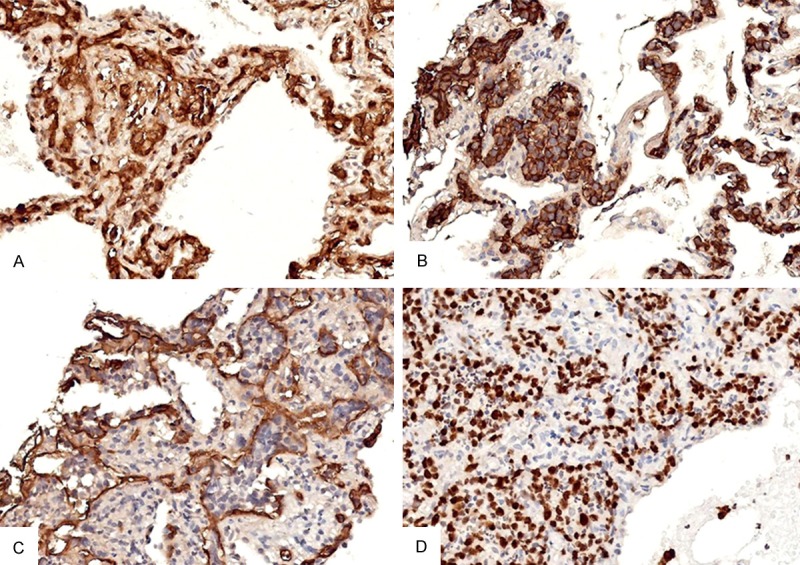
Immunohistochemical staining of the neoplastic intravascular tumor cells. A: Positive expression of LCA in the intravascular neoplastic cells (400X); B: Positive expression of CD20 in the intravascular neoplastic cells (400X); C: CD34 immunostaining shows the neoplastic lymphocytes located in the alveolar capillaries (400X); D: KI-67 immunostaining highlights the proliferation of intravascular lymphoma cells (400X).
Rearrangement of BCL6 gene on the IVLBCL cells was detected by FISH. BCL6 gene translocation was assessed using the LSI BCL6 Dual Color Break Apart Rearrangement probes (Vysis Inc, IL, USA). Nonrearranged BCL6 gene is represented by orange/green fusion signal. Rearrangement results in separation of the two colors and their appearance on two separate chromosomes. Fluorescence in situ hybridization analysis with a dual-color, break-apart probe set confirmed rearrangement of BCL6 gene (Figure 4).
Figure 4.
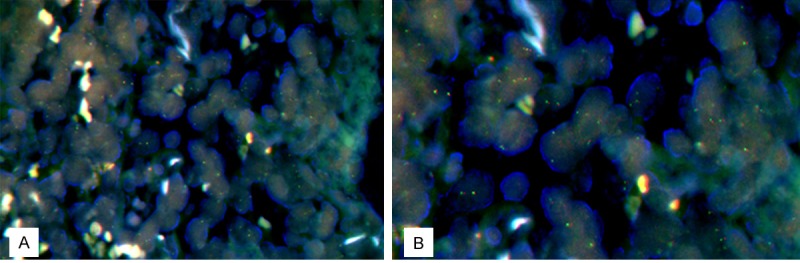
Rearrangement of BCL6 gene on the IVLBCL cells was detected by FISH using the BCL6 dual color break apart rearrangement probes. Non-rearranged BCL6 gene is represented by orange/green fusion signal. Rearrangement results in separation of the two colors and their appearance on two separate chromosomes (A, 400X, B, 1000X).
IVLBCL was the final diagnosis. Systemic chemotherapy (CHOP) consisting of vinorelbine on day 1, cyclophosphamide and epirubicin on days 1-3, and prednisolone on days 1-5 was started immediately after the final definitive diagnosis of IVLBCL. In this case, no severe adverse effects were occurred and the patient condition was improved. A second course of chemotherapy was started 4 weeks after the first course and rituximab was added to the original regimen. The patient has been well without recurrence and will be regularly admitted to hospital to undergo chemotherapy.
Discussion
IVLBCL once was described as malignant epithelioid hemangioendothelioma, and intravascular lymphoma and so on. Symptoms such as fever, night sweats and weight loss are seen in the majority of cases and these are thought to be partially caused by blood flow disturbances due to proliferation of lymphoma cells within the lumina of the small vessels [7]. In Western countries, IVLBCL patients usually show symptoms of central nervous system (CNS) and skin involvement [7]. In contrast, IVLBCL patients in Japan usually present with fever, hepatosplenomegaly, and thrombocytopenia, very rarely with CNS and skin involvement [7-10]. In our reported case, the patient presented with high fever and cough, developed dyspnea, though no CNS and skin symptoms.
The lung is not a common site used to make the diagnosis of IVLBCL, autopsies have revealed changes in the lung in approximately 60% of the cases [3]. Although lymphoma cell invasion of the lung was observed at autopsy in almost all cases, there have been only 19 cases diagnosed by TBLB [11,12]. In many cases, chest CT revealed interstitial infiltrates, patchy consolidations or ground glass opacities but there have been only three cases in which chest CT showed no abnormal findings. Despite the presence of dyspnea, hypoxia and abnormal chest radiography and CT findings, these symptoms might be nonspecific [13]. In our present case, IVLBCL was suspected on the basis of clinical symptoms and laboratory data such as fever, dyspnea, cough and elevated serum LDH and CRP levels. CT-guided percutaneous lung biopsy of the left lung was performed. Histologically, the most of the affected vessels were located in bronchial submucosal interstitial and alveolar septa. The thoracoscopic lung biopsy and open lung biopsy have also been reported as promising for diagnosing IVLBCL with lung involvement [13]. CT-guided percutaneous lung biopsy is one of the most useful methods to diagnose IVLBCL. These data also strongly suggested that CT-guided percutaneous lung biopsy is necessary for diagnosis of IVLBCL, despite the absence of abnormal findings on chest x-ray or CT.
There are high-frequency translocation of the 3, 14 chromosome in DLBCL involving excessive expression of BCL6 procarcinogenic gene. In the previous paper shows at least 40% of the DLBCL involving 3 q27 chromosome translocation or BCL6 rearrangement of genes [14]. Chromosome translocation in DLBCL such as t (3; 14), t (2; 3), t (3; 22), t (3; 11) and so on is related to 3 Chromosomes and involving 3 q27 belt. Detection of BCL6 gene rearrangement is important for auxiliary diagnosis of DLBCL. BCL6 gene translocation site such as t (3; 14), t (3; 22) and t (2, 3) are basically more complex and is formed by the translocation 3q27, immunoglobulin Ig heavy chain, lambda light chain and κ light chain respectively. In recent years, the breaking point separation probe FISH technology is the ideal methods of testing BCl-6 genes rearrangement [15]. BCL6 rearrangement is relationship with prognosis of DLBCL patients and BCL6 gene is independent index for detection and evaluation of the survival rate and the recovery rate [16].
In summary, the patient presented symptoms of cough, fever, progressive dyspnea and hypoxia, and had the abnormal findings with ground glass opacities on chest CT, IVLBCL should be suspected and CT-guided percutaneous lung biopsy should be performed earlier to confirm the diagnosis.
Acknowledgements
This work was supported by Department of Radiology and Department of Oncology, Xiangya Hospital, Central South University.
Disclosure of conflict of interest
None.
References
- 1.Nakamura S, Ponzoni M, Campo E. Intravascular large B-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp. 252–253. [Google Scholar]
- 2.Kotake T, Kosugi S, Takimoto T, Nakata S, Shiga J, Nagate Y, Nakagawa T, Take H, Katagiri S. Intravascular large B-cell lymphoma presenting pulmonary arterial hypertension as an initial manifestation. Intern Med. 2010;49:51–54. doi: 10.2169/internalmedicine.49.2774. [DOI] [PubMed] [Google Scholar]
- 3.Murase T, Yamaguchi M, Suzuki R, Okamoto M, Sato Y, Tamaru J, Kojima M, Miura I, Mori N, Yoshino T, Nakamura S. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109:478–485. doi: 10.1182/blood-2006-01-021253. [DOI] [PubMed] [Google Scholar]
- 4.Ferreri AJ, Dognini GP, Campo E, Willemze R, Seymour JF, Bairey O, Martelli M, De Renz AO, Doglioni C, Montalban C, Tedeschi A, Pavlovsky A, Morgan S, Uziel L, Ferracci M, Ascani S, Gianelli U, Patriarca C, Facchetti F, Dalla Libera A, Pertoldi B, Horvath B, Szomor A, Zucca E, Cavalli F, Ponzoni M International Extranodal Lymphoma Study Group (IELSG) Variations in clinical presentation, frequency of hemophagocytosis and clinical behavior of intravascular lymphom a diagnosed in different geographical regions. Haematologica. 2007;92:486–492. doi: 10.3324/haematol.10829. [DOI] [PubMed] [Google Scholar]
- 5.Goh SG, Chuah KL, Tan PH. Intravascular lymphomatosis of the lung and liver following eyelid lymphoma in a Chinese man and review of primary pulmonary intravascular lymphomatosis. Pathology. 2002;34:82–85. doi: 10.1080/00313020120105688-2. [DOI] [PubMed] [Google Scholar]
- 6.Asada N, Odawara J, Kimura S, Aoki T, Yamakura M, Takeuchi M, Seki R, Tanaka A, Matsue K. Use of random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2007;82:1525–1527. doi: 10.1016/s0025-6196(11)61097-5. [DOI] [PubMed] [Google Scholar]
- 7.Ferreri AJ, Campo E, Seymour JF, Willemze R, Ilariucci F, Ambrosetti A, Zucca E, Rossi G, Lopez-Guillermo A, Pavlovsky MA, Geerts ML, Candoni A, Lestani M, Asioli S, Milani M, Piris MA, Pileri S, Facchetti F, Cavalli F, Ponzoni M International Extranodal Lymphoma Study Group (IELSG) Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127:173–183. doi: 10.1111/j.1365-2141.2004.05177.x. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura T, Sando Y, Tajima S, Hosono T, Sato M, Maeno Y, Maeno T, Suga T, Kurabayashi M, Nagai R. Pulmonary intravascular lymphoma complicated with Pneumocystis carinii pneumonia: a case report. Jpn J Clin Oncol. 2001;31:333–336. doi: 10.1093/jjco/hye069. [DOI] [PubMed] [Google Scholar]
- 9.Shimada K, Murase T, Matsue K, Okamoto M, Ichikawa N, Tsukamoto N, Niitsu N, Miwa H, Asaoku H, Kosugi H, Kikuchi A, Matsumoto M, Saburi Y, Masaki Y, Yamamoto K, Yamaguchi M, Nakamura S, Naoe T, Kinoshita T IVL Study Group in Japan. Central nervous system involvement in intravascular large B-cell lymphoma: A retrospective analysis of 109 patients. Cancer Sci. 2010;101:1480–1486. doi: 10.1111/j.1349-7006.2010.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada K, Matsue K, Yamamoto K, Murase T, Ichikawa N, Okamoto M, Niitsu N, Kosugi H, Tsukamoto N, Miwa H, Asaoku H, Kikuchi A, Matsumoto M, Saburi Y, Masaki Y, Yamaguchi M, Nakamura S, Naoe T, Kinoshita T. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26:3189–3195. doi: 10.1200/JCO.2007.15.4278. [DOI] [PubMed] [Google Scholar]
- 11.Taura Y, Yamazaki H, Katou T. Two cases of intravascular lymphomatosis diagnosed by transbronchial lung biopsy. Nihon Kokyuki Gakkai Zasshi. 2009;47:875–880. (in Japanese) [PubMed] [Google Scholar]
- 12.Shinoda H, Maejima A, Shimizu K, Onaka A, Boku T, Oyamada Y. A case of intravascular lymphoma with diffuse centrilobular opacities. Nihon Kokyuki Gakkai Zasshi. 2010;48:76–78. [PubMed] [Google Scholar]
- 13.Kitanaka A, Kubota Y, Imataki O, Ohnishi H, Fukumoto T, Kurokohchi K, Tanaka T. Intrava- scular large B-cell lymphoma with FDG accumulation in the lung lacking CT/(67) gallium scintigraphy abnormality. Hematol Oncol. 2009;27:46–49. doi: 10.1002/hon.876. [DOI] [PubMed] [Google Scholar]
- 14.Akasaka T, Ueda C, Kurata M, Akasaka H, Yamabe H, Uchiyama T, Ohno H. Nonimmunoglobulin (non-Ig)/BCL6 gene fusion in diffuse large B-cell lymphoma results in worse prognosis than Ig/BCL6. Blood. 2000;96:2907–2909. [PubMed] [Google Scholar]
- 15.Paternoster SF, Brockman SR, McClure RF, Remstein ED, Kurtin PJ, Dewald GW. A new method to extract nuclei from paraffin-embedded tissue to study lymphom as using interphase fluorescence in situhybridization. Am J Pathol. 2002;160:1967–1972. doi: 10.1016/S0002-9440(10)61146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Offit K, Lo Coco F, Louie DC, Parsa NZ, Leung D, Portlock C, Ye BH, Lista F, Filippa DA, Rosenbaum A, et al. Rearrangement of the bcl-6 gene as a prognostic marker in diffuse large-cell lymphoma. N Engl J Med. 1994;331:74–80. doi: 10.1056/NEJM199407143310202. [DOI] [PubMed] [Google Scholar]


