Abstract
Cerium (Ce), one of the lanthanides (Ln), displays a variety of biochemical and physiological effects. However, the potential effect and mechanism of Ce on bone metabolism are not well understood. In this study, we investigated the putative role of Ce in regulating the migration and osteogenic differentiation of bone marrow stromal cells (BMSCs) and the underlying mechanism. The results indicated that Ce promoted BMSCs viability and ALP activity at lower concentrations (0.001 μM), and decreased the viability and ALP activity of BMSCs at higher concentrations (10 μM). Ce could also affect the expression of osteogenic transcription factors (Runx2, Satb2 and OCN) in BMSCs. Our results also showed that Ce promoted migration of BMSCs by increasing SDF-1 mRNA expression. As the Smad-dependent BMP signaling pathway plays an important role in migration and osteogenic differentiation of BMSCs, our results are in agreement with Ce promoting the phosphorylation of Smad1/5/8 and translocating to the nucleus by increase BMP2 expression. The activity of p-Smad1/5/8 increased SDF-1 and Runx2 expression level in BMSCs. In conclusion, our results support the notion that Ce promoted migration and osteogenic differentiation of BMSCs by Smad1/5/8 signaling pathway.
Keywords: Ce, BMSCs, migration, osteogenic differentiation, Smad1/5/8 signaling pathway
Introduction
Bone regeneration is one of the most important issues in regenerative medicine. Since the effect of drugs to inhibit bone resorption is not satisfactory, the development of bone anabolic molecules is necessary for patients who have suffered substantial bone loss. Therefore, the enhancement of bone formation is an utmost important technology in scaffold-based tissue engineering.
Cerium (Ce), one of the lanthanides (Ln), displays a variety of biochemical and physiological effects primarily based on its similarity to calcium. Early in the mid-nineteeth century, Ce was used as an anti-emetic. Several Ce (III) salts including acetate, stearate, chloride, and nitrate were reported to have antibacterial activity. It was confirmed that Cerium had broad-spectrum antibacterial activity against a range of bacteria including Pseudomonas aeruginosa and Staphylococcus aureus through a serial of systematic studies [1]. It was also reported that Ce (III) iodide had activity against solid tumors, and Ce (III) complexes with coumarin demonstrated cytotoxicity against HL-60 cell line [2].
With similar ionic radii to calcium, but a higher charge, the Ce3+ ion has a high affinity for Ca2+ sites. This high affinity suggested that Ce might intervene in bone-remodeling process and affect bone cell function. The effects of Ce on primary Osteoblasts (OBs) and MBSc proliferation, differentiation, and mineralization depended on the concentration and culture time at cell level was previously reported [3-6]. However, the potential effect and the mechanism of Ce on bone metabolism are still not well understood.
In this study, we investigated the putative role of Ce in regulating the migration and osteogenic differentiation of bone marrow stromal cells (BMSCs) and the underlying mechanism.
Materials and methods
Bone marrow cell cultures and phenotype analysis
BMSCs were collected from 6- to 8-week-old C57BL/6 male mice (Shanghai experimental animal center, Shanghai, China). This animal study was conducted in conformity with the Animal Care and Use Committee of Shandong University (Jinan, Shandong Province, China). The cells were isolated and cultured as described previously [7]. Briefly, the whole bone marrow was obtained by flushing femurs and tibias cavities with DMEM medium supplemented with 10% FBS, 50 U/mL penicillin and 50 mg/mL streptomycin. 24 hours later, non-adherent cells were removed by washes with PBS. Adherent cells were further cultured in DMEM medium supplemented with 10% FBS. The culture medium was changed every 3 days during the experiments. At passage 3, the phenotype analysis of BMSCs (anti-mouse CD44 and CD34 antibodies, Santa Cruz, USA) was performed by flow cytometer.
Cell proliferation assays
At passage 3, cells were seeded at the density of 1×104 cells/well in a 96-well plate and incubated for 24 hours. After the addition of CeCl3 at different concentrations (final concentrations of 0, 0.001, 1, 10 μM), 24 hours further incubations were performed. The adherent cells were harvested for the determination of cell proliferation with the CCK-8 kit (Santa Cruz, USA) following the manufacturer’s recommendations. Absorbance at 450 nm was determined by a FLUO star microtiter plate reader. The BMSCs proliferation rate (%) was expressed as a percentage of [ODsample-ODblank]/[ODcontrol-ODblank]×100.
ALP activity assays
BMSCs were seeded in 24-well plate at the density of 5×106 cells/well with the osteogenic induction supplement (OS) containing 5.0 mM b-glycerophosphate, 50 mg/mL ascorbic acid and 0.1 mM dexamethasone. A series of dilutions of CeCl3 (final concentrations of 0, 0.001, 1, 10 μM) were added into the medium. Cells were assayed at the 7th day for ALP activity.
Alizarine red S staining
BMSCs were seeded in 24-well plate at the density of 5×106 cells/well. 24 hours later, the medium was changed for OS medium with or without CeCl3 (0.001 μM). The formation of mineralized matrix nodules was determined by alizarine red S stainin at the 21th day.
Migration and invasion assays
For the migration assay, BMSCs (3×104) were seeded in the transwell inserts in DMEM with or without CeCl3 (0.001 μM). DMEM was then placed into the bottom chamber. For invasion assays, the filter of the transwell was coated with Matrigel. BMSCs (2×105) were plated in the upper chamber in DMEM with or without CeCl3 (0.001 μM). The bottom side of the inserts were precoated with type I collagen (0.5 μg/mL, BD Biosciences, USA). Following incubation for 24 hours, migrating or invading BMSCs were fixed with 10% formaldehyde for 1 hour and stained with hematoxylin. Micrographs were taken with a Nikon E600 microscope. 5 fields at x20 magnification images were captured and the number of cells was counted on each image. Experiments were performed in triplicate.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, USA). 2 μg of total RNA was used for reverse transcription using the Two-Step RT-PCR Kit (Invitrogen, USA) according to the manufacturer’s protocol. A quantitative real-time reverse transcription-PCR assay was performed using SYBR Green Supermix (Invitrogen) on a Bio-Rad iQ5 thermal cycler. The evaluation of relative differences in the PCR product amounts was carried out by the comparative cycle threshold method using GAPDH as a control. The primers used for qRT-PCR were shown in Table 1.
Table 1.
qRT-PCR Primers
| Gene | Primer Sequence | |
|---|---|---|
| GAPDH | Forward | 5’-AGG TCG GTG TGA ACG GAT TTG-3’ |
| Reverse | 5’-TGT AGA CCA TGT AGT TGA GGT CA-3’ | |
| Runx2 | Forward | 5’-TTCTCCAACCCACGAATGCAC-3’ |
| Reverse | 5’-CAGGTACGTGTGGTAGTGAGT-3’ | |
| Satb2 | Forward | 5’-AGGCCCAAGGAATAA TCAAGC-3’ |
| Reverse | 5’-GCGTCACAACGTGATAGACATC-3’ | |
| OCN | Forward | 5’-GAACAGACTCCGGCGCTA-3’ |
| Reverse | 5’-AGGGAGGATCAAGTCCCG-3’ | |
| SDF-1 | Forward | 5’-CTGTGCCCTTCA GATTGTAGCC-3’ |
| Reverse | 5’-GTGATGTCTCAGCATTGCCGTA-3’ | |
| CXCR4 | Forward | 5’-CCACGCCACCAACAGTCAG-3’ |
| Reverse | 5’-GGCAGGATAAGGCCAACCATG-3’ | |
| BMP2 | Forward | 5’-TGGCCCATTTAGAGGAGAACC-3’ |
| Reverse | 5’-AGGCATGATAGCCCGGAGG-3’ |
Western blot analyses
Polyacrylamide gel electrophoresis and western blot analysis were performed essentially as previously described [8]. Briefly, cells were lysed for 15 minutes in RIPA lysis buffer containing protease and phosphatase inhibitors (Sigma) on ice. Protein extracts were then run in NuPAGE 4-12% Bis-Tris gradient gels and transferred onto 0.45 μm PVDF membranes (Invitrogen). The membrane was blocked for 2 hours at room temperature and then was incubated with corresponding primary antibodies in the TBST solution overnight at 4°C, followed by 1 hour’s incubation with secondary antibodies. β-actin protein expression was used as a loading control.
Immunofluorescence experiments
Firstly, BMSCs were washed with PBS and fixed in 4% paraformaldehyde for 15 minutes at 4°C. Fixed cells were permeabilized with PBST (PBS containing 0.3% Triton X-100) and then blocked with 10% donkey serum in PBST for 1 hour at room temperature. Cells were then incubated overnight at 4°C with anti-pSmad1/5/8 antibodies. Cells were washed three times with PBST and incubated with secondary antibody for 1 hour at room temperature. After extensive washes in PBST, cells were mounted with the ProLong Gold antifade reagents with DAPI (Invitrogen). Micrographs were taken with a Nikon E600 microscope.
Statistical analysis
Data are presented as the mean ± SD. To determine significance between groups, comparisons were made using Student’s t-test. Differences between groups were considered as statistically significant when P< 0.05.
Results
Ce promotes BMSCs proliferation ex vivo
After 24 hours of primary culture, we can see that the adherent cells were at an initial rare density. After 7-10 days of primary culture, the cells replicated rapidly and reached almost 80% confluence (Figure 1A). We also characterized two phenotypes known to be associated with BMSCs by flow cytometric analysis. The cultured BMSCs were positive for CD44 (the percentage of positive cells: 99.4%), and were negative for hematopoietic lineage markers CD34 (the percentage of positive cells: 0.7%) (Figure 1B).
Figure 1.
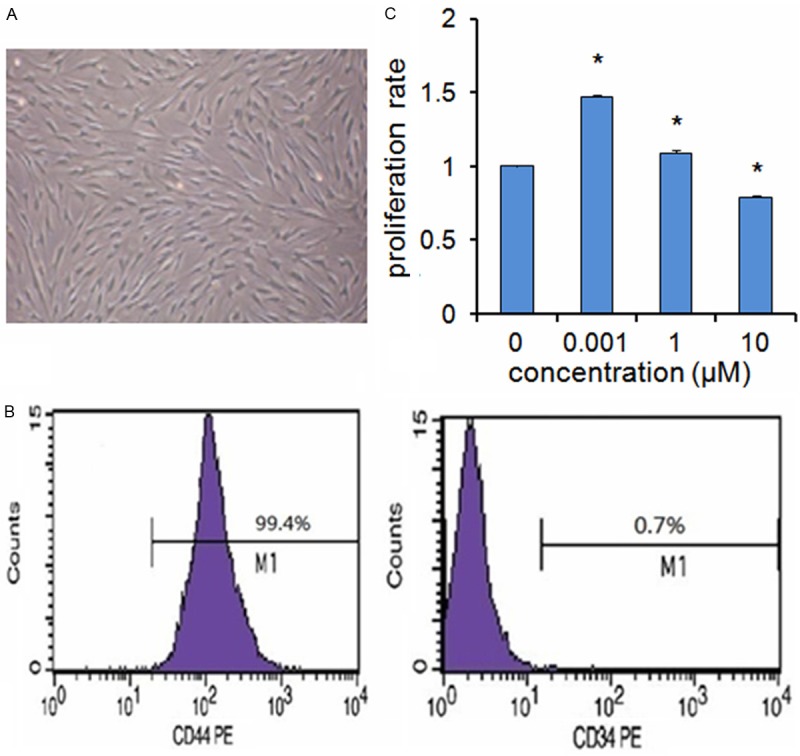
A. The primary culture of BMSCs (×10). B. Flow cytometry analysis showed that the cultured BMSCs were positive for CD44 (the percentage of positive cells: 99.4%), and were negative for CD34 (the percentage of positive cells: 0.7%). C. BMSCs were treated with various concentrations of Ce (0, 0.001, 1, 10 μM) for 24 hours and analyzed by CCK-8 kit. Data are presented as mean ± SD from a representative of three separate experiments. *P< 0.05.
As Ce was previously shown to promote MC3T3-E1 cells proliferation [9,10], we investigated the effects of Ce on the ex vivo proliferation of BMSCs. Our results revealed that Ce displayed a positive effect on the BMSCs viability at lower concentrations (0.001 μM), and decreased the viability of BMSCs at higher concentrations (10 μM) for 24 hours (Figure 1C).
Ce promotes BMSCs osteogenic differentiation ex vivo
To investigate the effects of Ce on the osteogenic differentiation of BMSCs, the ALP activity and calcium nodules stain were performed. Our results showed that after 7 days of Ce treatment, the ALP activity of BMSCs was increased at concentrations of 0.001 μM, but turn to be decreased at concentrations of 10 μM (Figure 2A).
Figure 2.
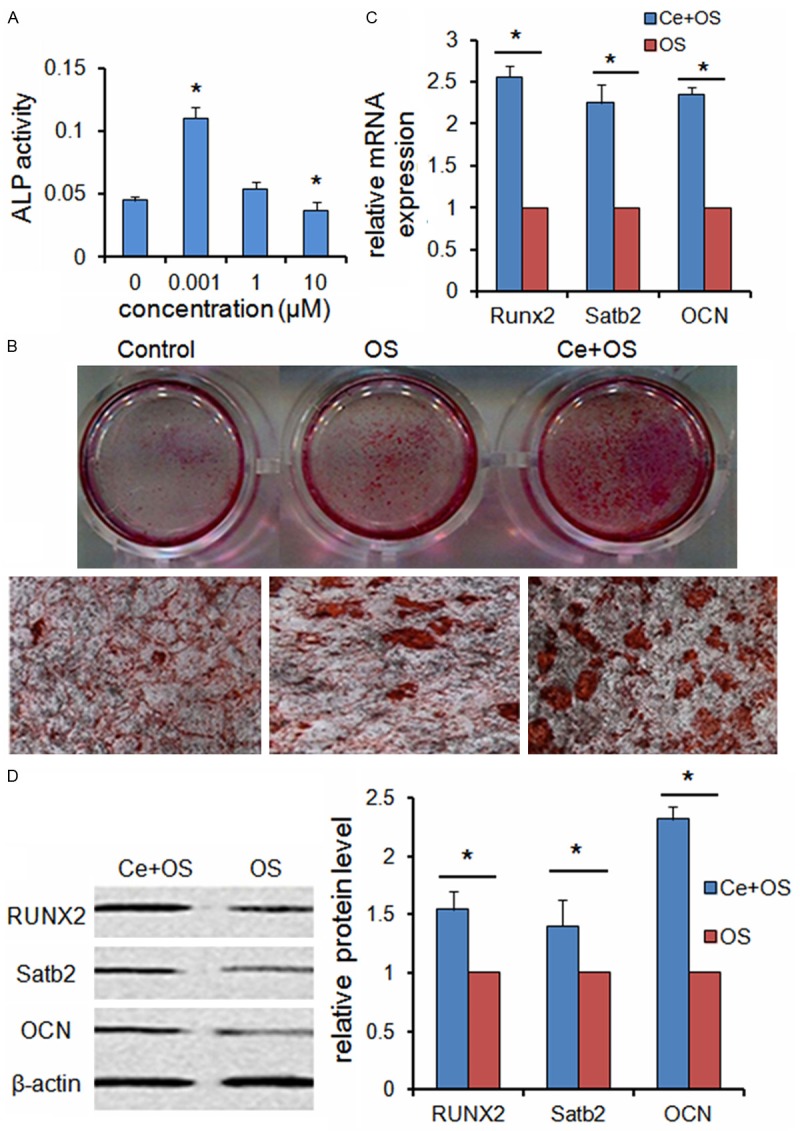
Ce promotes BMSCs osteogenic differentiation ex vivo. A. BMSCs were treated with various concentrations of Ce (0, 0.001, 1, 10 μM) for 7 days and assessed by measuring the ALP activity. B. BMSCs were treated with standard medium, OS medium and OS+Ce medium for 21 days and assessed by alizarine red S staining. C. qRT-PCR analysis indicated that the mRNA expressions of Runx2, Satb2 and OCN were significantly up-regulated in the BMSCs treated with Ce (0.001 μM) for 7 days as compared to control group. D. Western bolt analysis showed the expressions of RUNX2, Satb2 and OCN proteins were up-regulated after treatment with Ce (0.001 μM) for 7 days. Data are presented as mean ± SD from a representative of three separate experiments. *P< 0.05.
Small round alizarin red-positive nodules formed in the BMSCs cultures were also observed on the 21th day of induction with or without Ce (0.001 μM ) (Figure 2B).
We then questioned whether Ce could also affect the expression of osteogenic transcription factors in BMSCs under differentiating conditions. RT-PCR analysis indicated that the mRNA expressions of Runx2, Satb2 and OCN were significantly up-regulated in the BMSCs treated with Ce (0.001 μM) for 7 days as compared to control group (Figure 2C). As demonstrated in Figure 2D, the expressions of RUNX2, Satb2 and OCN proteins were up-regulated after treatment with 0.001 μM Ce for 7 days.
Ce promotes BMSCs migration and SDF-1 expression ex vivo
To evaluate the effect of Ce on BMSCs migration, we developed an ex vivo cell migration assay. Our results indicated that cells number migrated significantly more in the presence of Ce (0.001 μM) than in its absence (Figure 3A).
Figure 3.
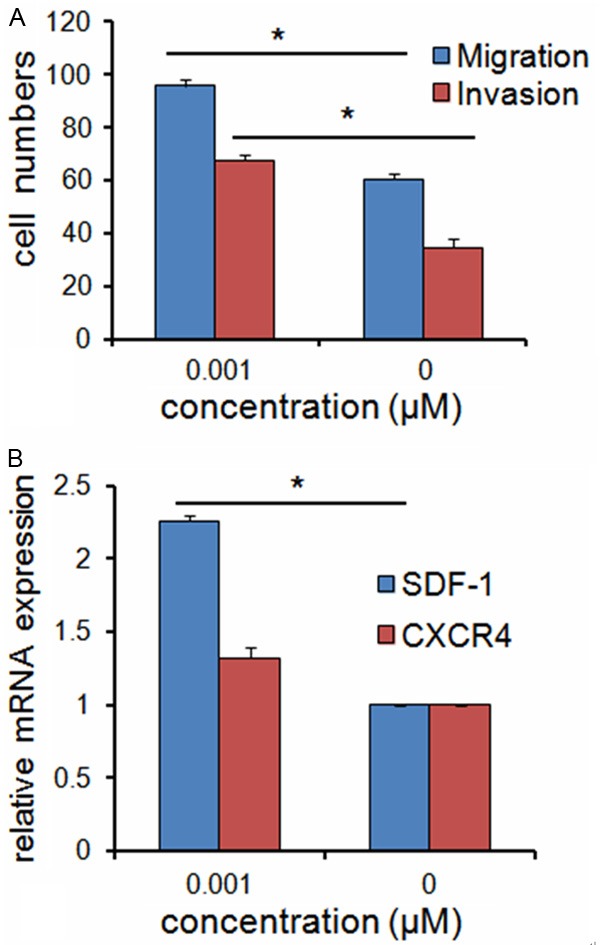
Ce promotes BMSCs migration and SDF-1 expression ex vivo. A. Ce (0.001 μM) increased the migration and invasion ability of BMSCs. B. qRT-PCR results indicated that SDF-1 mRNA expression was higher in BMSCs treated with Ce, but CXCR4 mRNA expression was not significantly affected with Ce. Data are presented as mean ± SD from a representative of three separate experiments. *P< 0.05.
As crossing the extracellular matrix (ECM) is required for BMSCs to mobilize into circulation, we examined the ability of Ce to promote BMSCs invasion in a matrigel-coated Boyden chamber assay. This invasion assay showed that Ce (0.001 μM) increased the ability of BMSCs to cross the ECM (Figure 3A).
We then compared the SDF-1 and CXCR4 mRNA expression levels in BMSCs treated with or without Ce (0.001 μM). qRT-PCR results indicated that SDF-1 mRNA expression was higher in BMSCs treated with Ce, but CXCR4 mRNA expression was not significantly affected with Ce (Figure 3B).
Ce promotes the expression of BMP2 and activates the Smad signaling pathway
It is well known that the Smad-dependent BMP signaling pathway plays an important role in osteogenic differentiation of BMSCs [11]. In fact, BMP2 controls the expression and function of SDF-1 and Runx2 through Smad signaling [12,13]. Therefore, we investigated if BMSCs migration and osteogenic differentiation promoted by Ce was related to BMP signaling pathway.
Firstly, we found that 0.001 μM Ce could increase the mRNA expression of BMP2 in BMSCs (Figure 4A). Nextly, we found that 0.001 μM Ce could cause a time-dependent increase of Smad1/5/8 phosphorylation (Figure 4B). We also examined the subcellular localization of phosphorylated Smad1/5/8 (p-Smad1/5/8). We detected p-Smad1/5/8 in the cytoplasm in the untreated BMSCs, whereas BMSCs treated with Ce (0.001 μM) for 30 minutes led to the preferential nuclear localization of p-Smad1/5/8 (Figure 4C).
Figure 4.
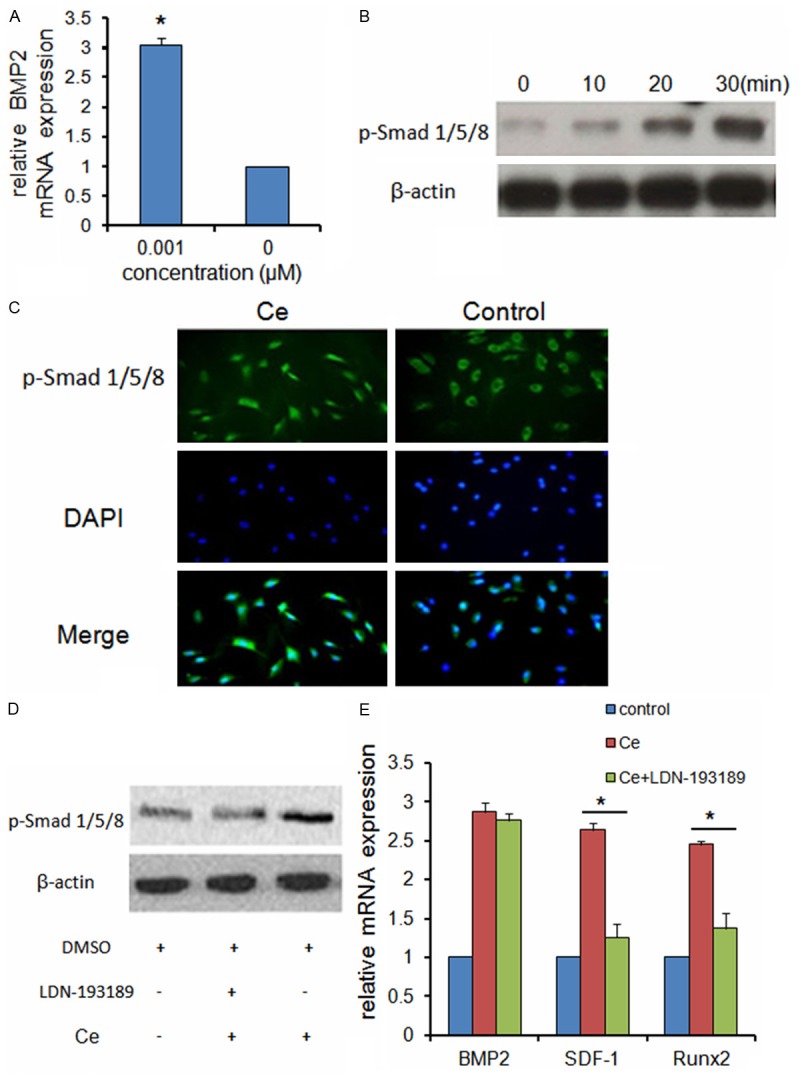
Ce promotes the expression of BMP2 and activates the Smad signaling pathway. A. qRT-PCR analysis indicated Ce increased the mRNA expression of BMP2 in BMSCs. B. Western bolt analysis showed Ce cause a time-dependent increase of Smad1/5/8 phosphorylation. C. BMSCs treated with Ce for 30 minutes led to the preferential nuclear localization of p-Smad1/5/8. D. Western blot analysis indicated that p-Smad1/5/8 was decreased in LDN-193189 pretreated BMSCs. E. qRT-PCR analysis indicated pre-treatment with LDN-193189 significantly blocked the Ce-mediated upregulation of SDF-1 and Runx2 mRNA expression, but not influence the expression of BMP2 mRNA. Data are presented as mean ± SD from a representative of three separate experiments. *P< 0.05.
Furthermore, BMSCs were pretreated with or without LDN-193189 (0.5 μM), which is an inhibitor for BMP type I receptor. Then the treated BMSCs were incubated with or without 0.001 μM Ce. Western blot analysis indicated that p-Smad1/5/8 was decreased in LDN-193189 pretreated BMSCs (Figure 4D).
Whereas Ce treatment of BMSCs increased BMP2, SDF-1 and Runx2 mRNA expression, pre-treatment with LDN-193189 (0.5 μM) significantly blocked the Ce-mediated upregulation of SDF-1 and Runx2 mRNA expression, but not influence the expression of BMP2 mRNA (Figure 4E).
Taken together, our results are in agreement with Ce promoting the phosphorylation of Smad1/5/8 and translocating to the nucleus by increase BMP2 expression. The activity of p-Smad1/5/8 increased SDF-1 and Runx2 expression level in BMSCs (Figure 5).
Figure 5.
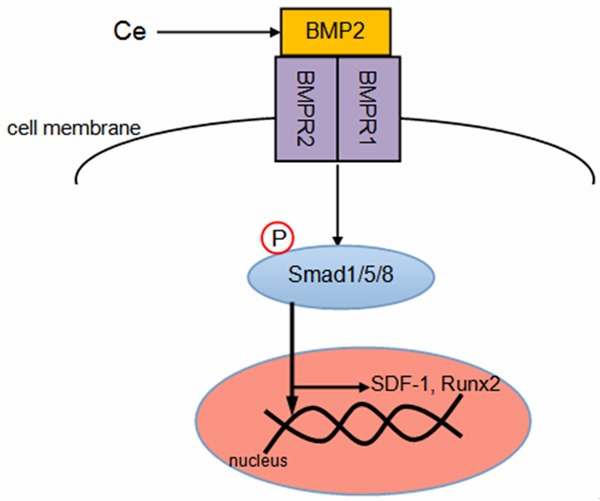
Plausible molecular mechanism of migration and osteogenic differentiation of BMSCs by Ce through Smad1/5/8 signaling pathway.
Discussion
The results from cell proliferation assay suggested that Ce increased the cell viability at lower concentration, but turned to decrease the viability at higher concentration. A substantial increase of ALP activity in BMSCs upon treatment of Ce in dose-dependent manner was also observed. As ALP activity is a marker for osteogenic differentiation [14], the experimental results showed that dose might be a key factor which affects the proliferation and differentiation of BMSCs. First, Ce promoted the proliferation and ALP activity of BMSCs at lower concentration, whereas had no effect or further turned to inhibit the proliferation and ALP activity at higher concentration.
Cells maintain their homeostasis through a comprehensive signaling network. Any perturbation of this network will affect cell function and behavior. A large number of genes which have been associated with bone cells are known to be specifically required for OB differentiation, such as Runx2, Satb2 and OCN. Runx2 is a master regulator of osteogenic gene expression and OB differentiation. Runx2 knockout mice exhibited no bone tissues or OBs, indicating that OB differentiation was completely blocked in the absence of Runx2 [15]. OCN is the most specific gene for the OB differentiation and mineralization. OCN is expressed during the post proliferative period, reaches its maximum expression during mineralization, and accumulates in the mineralized bone [16]. In our work, Ce displayed the upregulation effect on these OB specific genes (Runx2, Satb2 and OCN) at concentration of 0.001 μM. These experimental results were consistent with the observed effects of Ce on the proliferation and ALP activity of BMSCs at cell level.
The BMSCs migration to injured bone is the first step for bone repaired. Stromal cell derived factor (SDF)-1, and its receptor cysteine (C)-X-C motif chemokine receptor 4 (CXCR4) form a signaling axis that mobilizes BMSCs to an injury site. This SDF-1/CXCR4 signaling axis is critical for the recruitment of BMSCs to the fracture site to promote bone repair [17-19]. Our results showed that Ce could promote BMSCs migration by increasing SDF-1 mRNA expression.
It is well known that the Smad-dependent BMP signaling pathway plays an important role in osteogenic differentiation of BMSCs [20-22]. Ce has been shown by other investigators to increase expression of BMP2 in osteoblastic cells and BMSCs. Our results also showed that Ce could increase the mRNA expression of BMP2 with concentration of 0.001 μM. It has been demonstrated that RUNX2 cooperates with BMP-activated Smads to stimulate the osteogenic differentiation of MSCs. In addition, conditional inactivation of the SDF-1 expression was reported to decrease proliferation, impaired osteoblast differentiation in response to BMP2 and suppressed BMP2 induced activation of Smad 1/5/8 in bone tissues.
Therefore, we hypothesized that Ce-involved promotion of migration and osteogenic differentiation of BMSCs was modulated through the Smad-dependent BMP signaling pathway. BMPs are responsible for enhancing osteogenic differentiation, including the stimulation of bone structural proteins expression, such as Col-I and OCN, and the mineralization of bone matrix. BMPs belong to TGF-β superfamily. They binding to BMPR2, which recruits and phosphorylates BMPR1, regulate cellular functions including cell differentiation and growth via the phosphorylation of Smad1/5/8. P-Smad1/5/8 proteins translocate to the nucleus, bind to target genes, and regulate the transcription. Our results showed that the expression of p-Smad1/5/8 was promoted after treating with Ce, leading to subsequent up-regulation of the expression of related osteogenic differentiation genes. Our experiments with LDN-193189, a BMP inhibitor that inhibits BMP receptor type I and subsequent Smad signaling, demonstrated that inhibition of Smad 1/5/8 phosphorylation blocked Ce-induced osteogenic differentiation of BMSCs.
In conclusion, our results support the notion that Ce promoted migration and osteogenic differentiation of BMSCs by Smad1/5/8 signaling pathway.
Acknowledgements
This study was supported by Science and Technology Development Program Project of Jinan (No. 201003124) awarded to Ying Hu.
Disclosure of conflict of interest
None.
References
- 1.Havanur VC, Badiger DS, Ligade SG, Gudasi KB. Synthesis, characterization and antimicrobial study of Lanthanide (III) Complexes of 2-Anilino-N1-[pyridine-2-ylethylidene] acetohydrazide. Der Pharma Chemica. 2011;3:292–304. [Google Scholar]
- 2.Fricker SP. The therapeutic application of lanthanides. Chem Soc Rev. 2006;35:524–533. doi: 10.1039/b509608c. [DOI] [PubMed] [Google Scholar]
- 3.Zhang JC, Liu CL, Li YP, Sun J, Wang P, Di KQ, Zhao YY. Effect of cerium ion on the proliferation, differentiation and mineralization function of primary mouse osteoblasts in vitro. J Rare Earths. 2010;28:138–142. [Google Scholar]
- 4.Zhou G, Gu G, Li Y, Zhang Q, Wang W, Wang S, Zhang J. Effects of cerium oxide nanoparticles on the proliferation, differentiation, and mineralization function of primary osteoblasts in vitro. Biol Trace Elem Res. 2013;153:411–418. doi: 10.1007/s12011-013-9655-2. [DOI] [PubMed] [Google Scholar]
- 5.Schmidlin PR, Tchouboukov A, Wegehaupt FJ, Weber FE. Effect of cerium chloride application on fibroblast and osteoblast proliferation and differentiation. Arch Oral Biol. 2012;57:892–897. doi: 10.1016/j.archoralbio.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Liu DD, Zhang JC, Zhang Q, Wang SX, Yang MS. TGF-β/BMP signaling pathway is involved in cerium-promoted osteogenic differentiation of mesenchymal stem cells. J Cell Biochem. 2013;114:1105–1114. doi: 10.1002/jcb.24451. [DOI] [PubMed] [Google Scholar]
- 7.Xu QC, Hao PJ, Yu XB, Chen SL, Yu MJ, Zhang J, Yang PS. Hyperlipidemia compromises homing efficiency of systemically transplanted BMSCs and inhibits bone regeneration. Int J Clin Exp Pathol. 2014;7:1580–1587. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Tu Q, Bonewald LF, He X, Stein G, Lian J, Chen J. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res. 2011;26:1953–1963. doi: 10.1002/jbmr.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang JC, Xu SJ, Wang K, Yu SF. Effects of the rare earth ions on bone resorbing function of rabbit mature osteoclasts in vitro. Chin Sci Bull. 2003;48:2170–2175. [Google Scholar]
- 10.Wang X, Yuan L, Huang J, Zhang TL, Wang K. Lanthanum enhances in vitro osteoblast differentiation via pertussis toxin-sensitive gi protein and ERK signaling pathway. J Cell Biochem. 2008;105:1307–1315. doi: 10.1002/jcb.21932. [DOI] [PubMed] [Google Scholar]
- 11.Song B, Estrada KD, Lyons KM. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009;20:379–388. doi: 10.1016/j.cytogfr.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guang LG, Boskey AL, Zhu W. Regulatory role of stromal cell-derived factor-1 in bone morphogenetic protein-2-induced chondrogenic differentiation in vitro. Int J Biochem Cell Biol. 2012;44:1825–1833. doi: 10.1016/j.biocel.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Jang H, Kim EJ, Park JK, Kim DE, Kim HJ, Sun WS, Hwang S, Oh KB, Koh JT, Jang WG, Lee JW. SMILE inhibits BMP-2-induced expression of osteocalcin by suppressing the activity of the RUNX2 transcription factor in MC3T3E1 cells. Bone. 2014;61:10–18. doi: 10.1016/j.bone.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Rosales-Rocabado JM, Kaku M, Kitami M, Akiba Y, Uoshima K. Osteoblastic differentiation and mineralization ability of periosteum-derived cells compared with bone marrow and calvaria-derived cells. J Oral Maxillofac Surg. 2014;72:694–699. doi: 10.1016/j.joms.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 16.Chou YF, Dunn JC, Wu BM. In vitro response of MC3T3-E1 pre-osteoblasts within three-dimensional apatite-coated PLGA scaffolds. J Biomed Mater Res B Appl Biomater. 2005;75:81–90. doi: 10.1002/jbm.b.30261. [DOI] [PubMed] [Google Scholar]
- 17.Herberg S, Fulzele S, Yang N, Shi X, Hess M, Periyasamy-Thandavan S, Hamrick MW, Isales CM, Hill WD. Stromal cell-derived factor-1β potentiates bone morphogenetic protein-2-stimulated osteoinduction of genetically engineered bone marrow-derived mesenchymal stem cells in vitro. Tissue Eng Part A. 2013;19:1–13. doi: 10.1089/ten.tea.2012.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guang LG, Boskey AL, Zhu W. Age-related CXC chemokine receptor-4-deficiency impairs osteogenic differentiation potency of mouse bone marrow mesenchymal stromal stem cells. Int J Biochem Cell Biol. 2013;45:1813–1820. doi: 10.1016/j.biocel.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 19.Zhu W, Boachie-Adjei O, Rawlins BA, Frenkel B, Boskey AL, Ivashkiv LB, Blobel CP. A novel regulatory role for stromal-derived factor-1 signaling in bone morphogenic protein-2 osteogenic differentiation of mesenchymal C2C12 cells. J Biol Chem. 2007;282:18676–18685. doi: 10.1074/jbc.M610232200. [DOI] [PubMed] [Google Scholar]
- 20.Dai J, Li Y, Zhou H, Chen J, Chen M, Xiao Z. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. Int J Biol Sci. 2013;9:1089–1098. doi: 10.7150/ijbs.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Usas A, Tang Y, Lu A, Tan J, Schneppendahl J, Kozemchak AM, Wang B, Cummins JH, Tuan RS, Huard J. A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP. Biomaterials. 2014;35:6859–6870. doi: 10.1016/j.biomaterials.2014.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Z, Wang JS, Lin L, Zhang J, Liu Y, Shuai M, Li Q. Effects of BMP2 and VEGF165 on the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Exp Ther Med. 2014;7:625–629. doi: 10.3892/etm.2013.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]


