Abstract
Repair of skeletal muscle after injury is a key aspect of maintaining proper musculoskeletal function. Studies have suggested that regenerative processes, including myogenesis and angiogenesis, are impaired during advanced age, but evidence from humans is limited. This study aimed to compare active muscle regeneration between healthy young and older adults. We evaluated changes in clinical, biochemical, and immunohistochemical indices of muscle regeneration at precisely 2 (T2) and 7 (T3) days following acute muscle injury. Men and women, aged 18-30 and ≥70 years, matched for gender and body mass index, performed 150 unilateral, eccentric contractions of the plantar flexors at 110% of one repetition maximum. Data were analyzed using analysis of covariance, adjusted for gender, habitual physical activity, and baseline level of the outcome. A total of 30 young (n = 15; 22.5 ± 3.7 yr) and older (n = 15; 75.8 ± 5.0 yr) adults completed the study. Following muscle injury, force production declined 16% and 14% in young and older adults, respectively, by T2 and in each group, returned to 93% of baseline strength by T3. Despite modest differences in the pattern of response, postinjury changes in intramuscular concentrations of myogenic growth factors and number of myonuclear (4′,6-diamidino-2-phenylindole+ and paired box 7+) cells were largely similar between groups. Likewise, postinjury changes in serum and intramuscular indices of inflammation (e.g., TNF-α and monocyte chemoattractant protein-1) and angiogenesis (e.g., VEGF and kinase insert domain receptor) did not differ significantly between groups. These findings suggest that declines in physical activity and increased co-morbidity may contribute to age-related impairments in active muscle regeneration rather than aging per se.
Keywords: aging, skeletal muscle, myogenesis, inflammation, angiogenesis
as the worldwide population continues to age, the prevention and treatment of age-related medical conditions have become an important public health priority. The maintenance of independent functioning is one key factor in preserving the health of seniors. The capacity to perform basic physical functions is not only a central tenet of health-related quality of life (35) but is also a key predictor of hospitalization, surgical outcome, and mortality (1, 39, 46). Although the etiology of functional decline is complex, sarcopenia—the age-related loss of muscle mass and quality—is generally considered to be a primary contributor to its development and progression. Hence, additional knowledge of mechanisms underlying sarcopenia development is necessary to advance prevention and treatment efforts that will improve the quality of life for millions of older adults.
Among the numerous processes thought to contribute to the development and progression of sarcopenia is an age-related impairment in the processes that govern repair of skeletal muscle following injury. Skeletal muscle may be injured through acute injuries, such as crush or laceration, musculoskeletal diseases, or by the performance of high-intensity or eccentric (i.e., lengthening) contractions. Under ideal conditions, repair of injured skeletal muscle occurs within four inter-related and time-dependent phases: degeneration, inflammation, regeneration, and remodeling/repair (11). Proper timing and coordination of these phases are essential components of muscle recovery following injury, as impairments are likely to result in the formation of scar tissue (fibrosis) rather than remodeling and functional recovery (24, 52).
When faced with trauma, skeletal muscle fibers lose their cytoskeletal integrity, and gaps are created between disrupted myofibers (21). These gaps may contain destroyed sarcomeric tissue, muscle progenitor (satellite) cells released from the disrupted basal lamina, fibroblasts, fragments of intramuscular nerve branches, and blood from broken capillaries (34). Shortly after injury, neutrophils and macrophages migrate quickly to the area to initiate the inflammatory process and facilitate muscle healing (47). These leukocytes are responsible for the release of proinflammatory cytokines and chemokines, which attract additional macrophages to the area. Typically, the inflammatory process peaks ∼48 h following injury, before a subsequent shift in the role of resident macrophages. Indeed, these macrophages shift from a proinflammatory to anti-inflammatory state to begin the obligatory resolution of inflammation (2). Additionally, this shift in the inflammatory state is critical to the initiation of the myogenic process through the activation and proliferation of satellite cells (2, 28, 33). This process of active muscle regeneration is also supported by the reperfusion of damaged tissue (36, 45) and typically occurs within 7–10 days of injury, although continued healing may be observed for several weeks (20, 22).
Although an age-related impairment in muscle repair has been reported in animals (41, 42), definitive evidence is lacking to document this impairment in humans. Conceptually, impaired muscle repair among older adults is plausible, given that prior studies have reported age-related changes in resting satellite cell number (25, 43, 49), although contradictory evidence does exist (44). Data have also suggested potential age-related differences in acute satellite cell responsiveness to hypertrophic stimuli (16, 32, 51). However, follow-up in prior studies has largely been limited to the initial 24- to 48-h postexercise, making it difficult to fully evaluate the regenerative process. Additionally, confounding factors, such as physical activity level of participants and baseline level of the dependent outcome, have traditionally been overlooked in these analyses. Therefore, the objective of the present study was to evaluate and compare regenerative responses with acute muscle injury between apparently healthy young and older adults. We aimed to evaluate changes in key regenerative processes, including aforementioned biologic processes and the recovery of force production, at 2 and 7 days postinjury. We hypothesized that regenerative processes of older adults would demonstrate significant impairments compared with younger counterparts.
METHODS
Participants.
The primary objective of this study was to compare the time course of skeletal muscle repair between young and older adults following eccentric exercise-induced muscle injury. Interested individuals, aged 18–30 and ≥70 years, matched for gender, were recruited to participate in this study. To eliminate potentially confounding effects on study outcomes, care was taken to recruit older adults free of age-related diseases. Accordingly, the study's exclusion criteria included active treatment for cancer, stroke (<6 mo), peripheral vascular disease, coronary artery disease (myocardial infarction <6 mo), state III or IV congestive heart failure, valvular heart disease, severe anemia, liver or renal disease, diabetes, anticoagulant therapy (aspirin use permitted), anticholinesterase inhibitor, anabolic medications, upper- or lower-extremity fracture in the past 6 mo, severe osteoarthritis, upper- or lower-extremity amputation, vein or artery replaced in leg, blindness, deafness, history of significant head injury, major psychiatric disease, dementing illness, Parkinson's disease, significant cognitive impairment [Mini–Mental State Examination (MMSE), <24], smoking, excessive alcohol use (more than two drinks/day), pregnancy, contraindications to MRI (e.g., cardiac pacemaker, implanted cardiac defibrillator, aneurysm clip, claustrophobia, etc.), and current use of antidepressant medications. Participant recruitment was performed by the Recruitment Core of the University of Florida Claude D. Pepper Older Americans Independence Center. Participants were recruited through a variety of methods, including media articles, direct mailings, newspaper announcements, and presentations to community groups. Following telephone screening, potentially eligible persons were invited to attend a screening visit, during which, the purposes and procedures of the study were explained. Prior to participation in this study, participants were required to sign a written, informed consent form, approved by the University of Florida's Institutional Review Board.
Research design.
Following the evaluation of screening criteria, a general assessment was performed that included questionnaires to collect information regarding medical history, cognitive function (i.e., MMSE), as well as a brief physical exam to determine participant height, weight, and body mass index (BMI). Next, isometric and isotonic strength of the plantar flexor muscles was assessed, prior to collection of fasting venous blood samples and skeletal muscle biopsy samples. Following completion of the baseline assessment, participants were then provided a validated physical activity monitor (SenseWear; BodyMedia, Pittsburgh, PA) (30) to wear for 7 days to evaluate their physical activity habits. Participants were also scheduled for their next study visit, during which, they performed a bout of eccentric-resistance exercise, an established method of inducing skeletal muscle injury in humans (15). This bout consisted of 150 repetitions of unilateral, eccentric-resistance exercise at 110% of one repetition maximum using standard, cable-loaded, resistance-training equipment (Life Fitness, Schiller Park, IL). They then returned to the laboratory, 2 (T2) and 7 (T3) days following the exercise bout for follow-up assessments (Fig. 1).
Fig. 1.
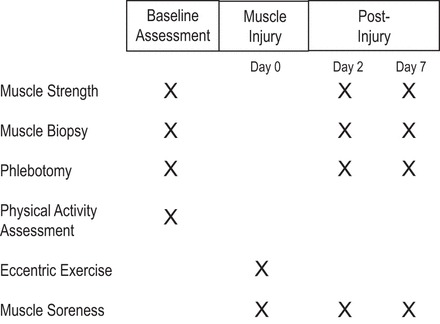
Graphical overview of study design.
Magnetic resonance imaging.
T1-weighted MRI was used to quantify tissue volumes of the tibiofibular region, as published previously (10). Briefly, images were obtained from each leg using a Philips 3.0 tesla magnet (Philips Medical Systems, Bothell, WA). Three-dimensional data were collected using a fast gradient echo sequence, with repetition time = 100 ms, echo time = 10 ms, and flip angle = 30°, and a chemically selective fat suppression was used. Images were acquired with an encoding matrix of 256 × 256, field of view of 16–24 cm, and 7 mm slice thickness. Images were collected using an eight-channel, sensitivity-encoding, receive-only extremity coil. All images were exported and analyzed on a separate workstation. For image analyses, 11 contiguous axial slices (10 mm thickness) were selected, beginning at the maximal cross-sectional area of the tibio fibular region. Muscle and intermuscular adipose tissue (IMAT) were measured volumetrically.
Images were analyzed using the freely available software program, Medical Image Processing, Analysis, and Visualization (MIPAV; version 1.3; Center for Information Technology, National Institutes of Health, Bethesda, MD; http://mipav.cit.nih.gov). To quantify muscle-fat infiltration, IMAT was defined as the visible, low-signal-intensity pixels between muscle groups and within muscle fascia. The image segmentation process was performed using a nonparametric, nonuniform intensity normalization algorithm that corrects smoothly varying shading, caused by poor radiofrequency coil uniformity or gradient-driven eddy currents. Next, a volume of interest was created for bone and the fascia lata. Once bone and subcutaneous adipose tissue were removed, muscle and IMAT were segmented using automated pixel clustering using the Fuzzy c-means approach. Muscle quality was defined as the ratio between muscle mass and IMAT.
Isometric plantarflexor strength.
Maximum voluntary isometric strength of plantarflexors of the dominant limb was measured by dynamometer (Biodex Medical Systems, Shirley, NY). Participants sat upright with the chair's backrest inclined to 85°, and their knee was 20° of flexion. The axis of the ankle was aligned with the rotational axis of the dynamometer. For the test, participants were asked to perform a maximal voluntary contraction of the plantarflexor muscles for 5 s and then rest for 15 s. Participants completed this cycle three times, and the mean peak torque value was taken. Specific torque—the ratio of muscle strength relative to muscle mass—was calculated as [isometric muscle strength (N × m)/skeletal muscle volume (cm3)] × 10.
Tissue collection.
Venous blood samples were collected by venipuncture and centrifuged to obtain serum, according to standard laboratory practices. A percutaneous muscle biopsy was then collected, as described previously (7, 27). Briefly, muscle samples were collected from the medial gastrocnemius under a 2% lidocaine local anesthetic using a six-gauge needle with suction applied. Muscle samples were gently blotted on saline-moistened gauze to remove excess blood, weighed, and separated from adipose and/or connective tissue under microscope. Muscle for immunoblotting was then placed into cryovials, snap frozen in liquid nitrogen, and stored at −80°C until analysis. Tissue used for immunohistochemistry was mounted cross sectionally on cork in Tissue-Tek Optimum Cutting Temperature (OCT) Compound (Sakura, Torrance, CA) and frozen in liquid nitrogen-cooled isopentane.
Perceived muscle soreness.
As a subjective indicator of the severity of muscle injury, perceived soreness was assessed using a visual analog scale, as published previously (8, 9). Briefly, participant soreness level was evaluated using a 10-cm scale (0 = no soreness; 10 = extreme soreness). Participants rated their level of soreness by drawing an intersecting line across the continuum line, extending from 0 to 10.
Serum analyses.
Serum concentrations of creatine kinase (CK) were determined by the Shands Hospital Clinical Laboratory (University of Florida, Gainesville, FL). Concentrations of TNF-α and monocyte chemoattractant protein-1 (MCP1) were determined using a multiplex bead array kit from Millipore (Billerica, MA). Additionally, serum concentrations of VEGF were determined by ELISAs from R&D Systems (Minneapolis, MN). All assays were performed in duplicate. Intraplate coefficients of variation for each analyte were as follows: VEGF, 2.8%; TNF-α, 8.1%; and MCP1, 9.0%.
Immunoblotting.
Immunoblotting was performed according to previously published procedures (23, 27, 31). Briefly, 50 μg whole muscle protein extract was separated by gel electrophoresis on 8–15% polyacrylamide gels and transferred to nitrocellulose membranes. Transfer efficiency was verified by staining the gels with GelCode Blue Stain Reagent (Pierce Biotechnology, Rockford, IL) and the membranes with Ponceau S (Sigma-Aldrich, St. Louis, MO). Ponceau S staining was also used as a loading control. Blots were blocked for 1 h in StartingBlock T20 Blocking Buffer (Thermo Fisher Scientific, Waltham, MA) and probed with the appropriate primary antibody. Primary antibodies for myogenic regulatory factors [MRF4; myogenic factor 5, and myogenic differentiation antigen (MyoD)], hepatocyte growth factor (HGF) and its receptor (cMET), and VEGF were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used at a 1:200 dilution. The primary antibody for kinase insert domain receptor (KDR), or VEGF receptor 2, was purchased from Cell Signaling Technology (Danvers, MA) and used at a 1:1,000 dilution. Following primary antibody incubation, blots were incubated with anti-rabbit secondary antibody, conjugated with horseradish peroxidase at a dilution of 1:5,000 (Cell Signaling Technology). Blots were washed in Tris-buffered saline (TBS)/Tween 20 (3 × 5 min) and proteins subsequently visualized with a DuoLuX enhanced chemiluminescence kit (Vector Laboratories, Burlingame, CA) and detected using a ChemiDoc XRS imager (Bio-Rad Laboratories, Hercules, CA). Spot density of target bands was normalized to the amount of protein loaded in each lane, as determined by densitometric analysis of the full lane on the corresponding Ponceau S-stained membranes. Bands were quantified using Image Lab software from Bio-Rad Laboratories (Fig. 2).
Fig. 2.
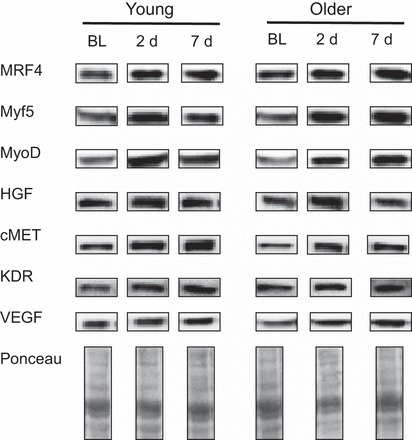
Representative blots of target proteins and Ponceau S staining used for immunblotting. BL, baseline; d, days; MRF4, myogenic regulatory factor 4; Myf5, myogenic factor 5; MyoD, myogenic differentiation antigen; HGF, hepatocyte growth factor; cMET, HGF receptor; KDR, kinase insert domain receptor.
Immunohistochemistry.
Prepared blocks were stored at −80°C until cryosectioned at a thickness of 7 μm for cross-sectional viewing using a CM1850 cryostat (Leica Microsystems, Buffalo Grove, IL). To improve adherence, sections were air dried overnight at room temperature before staining. A standard hemotoxylin and eosin stain was performed for each tissue to evaluate morphology (Fig. 3). For immunostaining, residual OCT was removed from the slides with a 5-min wash in 1× TBS. Slides were blocked in 2% horse serum for 60 min, followed by the application of primary antibody overnight at 4°C. The antibodies used consisted of mouse monoclonal anti-laminin (1:100; Leica) and rabbit polyclonal anti-paired box 7 (Pax7; 1:150; Sigma-Aldrich) to identify satellite cells and mouse MAb to CD31 (1:50; DakoCytomation, Carpinteria, CA) to identify capillaries. Positive control tissues and concentration-matched Ig controls were included with each immunoassay. Immunoreactivity was detected using 1:500 dilutions of species-appropriate Alexa Fluor secondary antibodies, raised in donkey (Molecular Probes, Eugene, OR). In the case of Pax7 staining, anti-rabbit Alexa Fluor 488 secondary was selected. For both CD31 and laminin, anti-mouse Alexa Fluor 594 secondary was used. Following 1 h of incubation at room temperature and two, 5-min washes, sections were postfixed for 4 min in 10% neutral-buffered formalin (Thermo Fisher Scientific). Sections were mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories) prior to imaging. High-resolution, bright-field and fluorescent images were captured using a DM2500 microscope (Leica), equipped with Plan-Apochromat objectives and an Optronics digital camera, supported with Magnafire software. Images were then visualized and analyzed using Adobe Photoshop Lightroom (Adobe Systems, San Jose, CA) to quantify the number of DAPI+, Pax7+, and CD31+ cells/muscle fiber.
Fig. 3.
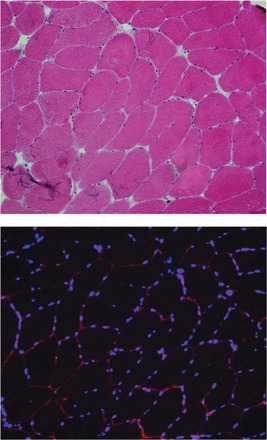
Top: representative muscle cross-section stained for hemotoxylin and eosin. Bottom: representative section-stained laminin to identify the sarcolemmal membrane (shown in red) and 4′,6-diamidino-2-phenylindole (DAPI) to identify myonuclei (shown in blue).
Statistical analysis.
Data were analyzed initially for normality and homogeneity of variance prior to computation of descriptive statistics. Categorical descriptive data were analyzed using the χ2 test. Continuous descriptive data and baseline outcome data, meeting the assumptions of parametric tests, were analyzed using Student's t-test for independent samples. When assumptions were violated, the nonparametric Mann-Whitney (U)-test was used. Data used to evaluate changes following the eccentric exercise bout were analyzed by repeated measures analyses of covariance with linear contrasts applied. Data were adjusted for gender, physical activity level, and baseline level of the outcome measure. The log transformation was used to normalize concentrations of circulating analyses. The Huynh-Feldt correction was applied when the assumption of sphericity was not met. Models of multiple linear regressions were also used to evaluate predictors of change-in-force production at T2 and T3. Factors included in the regression models are: demographic characteristics, tibiofibular muscle and fat mass, physical activity level (steps/day), baseline strength, and immunohistochemical measures. All analyses were performed using IBM SPSS Statistics for Windows version 20.0 (IBM, Armonk, NY), and data are presented as mean ± SD, unless otherwise noted.
RESULTS
The two groups were similar with respect to BMI and gender composition (Table 1). As shown in Table 1, young adults were modestly more physically active than older adults, with the majority of the difference accounted for by moderate-intensity activities. A statistical main effect for age was observed for tibiofibular muscle mass (P = 0.047), as younger adults displayed significantly greater muscle volume than older adults (Table 1). Older adults also displayed a greater volume of IMAT than younger adults (P < 0.001). Accordingly, muscle quality—the ratio between the volume of muscle and that of IMAT—was significantly greater among younger compared with older adults (P < 0.001). The younger group also had significantly higher isotonic (P = 0.021) and isometric (P = 0.008) plantar flexor strength (Table 1). Accordingly, specific torque—representing the ratio between strength and muscle mass—was also significantly higher in the young compared with older participants (P = 0.028).
Table 1.
Descriptive characteristics and physical activity habits of study participants by age group
| Age Group | Young Adults, n = 15 | Older Adults, n = 15 | P Value |
|---|---|---|---|
| Demographic/health characteristics | |||
| Age, yr | 22.5 ± 3.7 | 75.8 ± 5.0 | <0.001 |
| Body mass index, kg/m2 | 24.3 ± 4.7 | 25.9 ± 4.6 | 0.359 |
| Systolic blood pressure, mmHg | 119.0 ± 9.6 | 136.1 ± 15.6 | 0.001 |
| Diastolic blood pressure, mmHg | 71.5 ± 9.5 | 74.4 ± 10.0 | 0.417 |
| Pulse, beats/min | 70.4 ± 10.1 | 63.6 ± 9.9 | 0.072 |
| Vascular function, ankle brachial index | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.128 |
| Cognitive function, MMSE score | 28.9 ± 1.6 | 28.6 ± 0.9 | 0.571 |
| Women, n | 5 | 5 | N/A |
| Physical activity habits | |||
| Energy expenditure, Kcal/day | 2,702 ± 710 | 2,139 ± 448 | 0.017 |
| Active energy expenditure, Kcal/day | 687 ± 540 | 315 ± 224 | 0.024 |
| Physical activity, min/day | 140 ± 86 | 64 ± 39 | 0.006 |
| Steps, n/day | 8,254 ± 3,381 | 6,092 ± 2,649 | 0.067 |
| METs, average daily | 1.6 ± 0.3 | 1.3 ± 0.2 | 0.001 |
| Sedentary activity, min/day | 986 ± 296 | 1,086 ± 290 | 0.364 |
| Moderate activity, min/day | 125 ± 66 | 63 ± 38 | 0.005 |
| Vigorous activity, min/day | 6 ± 13 | 2 ± 3 | 0.223 |
| Very vigorous activity, min/day | 3 ± 12 | 0 ± 0 | 0.324 |
| Skeletal muscle size, quality, and strength | |||
| Skeletal muscle volume, cm3 | 273.2 ± 44.8 | 241.3 ± 39.3 | 0.047 |
| Intermuscular adipose tissue (IMAT), cm3 | 38.1 ± 8.3 | 57.5 ± 14.1 | <0.001 |
| Muscle quality, arbitrary units | 7.2 ± 1.2 | 4.3 ± 0.7 | <0.001 |
| Isotonic plantarflexor muscle strength, kg | 56.5 ± 19.2 | 41.9 ± 13.2 | 0.021 |
| Isometric plantarflexor muscle strength, N × m | 148.9 ± 50.1 | 104.2 ± 31.4 | 0.008 |
| Specific torque, arbitrary units | 5.5 ± 1.5 | 4.4 ± 1.0 | 0.028 |
Values are expressed as means ± SD.
MMSE, Mini-Mental State Examination; METs, metabolic equivalents: Sedentary = <3; Moderate = 3–6; Vigorous = 6–9; Very vigorous = >9. Muscle quality = muscle volume/IMAT volume. Specific torque = [isometric muscle strength (N × m)/skeletal muscle volume (cm3)] × 10.
During the eccentric exercise bout, the average rating of perceived exertion (RPE) reported by participants' exercise bout (17.7 ± 1.9) corresponded with a perceived intensity of “very hard” on the Borg scale. No significant difference was observed between the average RPE reported by younger adults (18.1 ± 1.3) and that reported by older adults (17.4 ± 2.4). Following the bout, adjusted changes in force production were similar between young and older adults (P = 0.951; Fig. 4), as peak torque declined by ∼15% at T2 and returned to 93% of baseline strength by T3. Likewise, no significant difference between young and older adults was observed for postinjury muscle soreness (P = 0.338) or serum CK (P = 0.403), despite significant increases across both groups (P < 0.05). Despite the lack of statistical significance, the pattern of response differed slightly for CK, as serum levels among older adults peaked at T2 but in young adults, continued to increase at T3. Following the exercise bout, changes in serum mediators of inflammation were largely similar between age groups (Fig. 4). No statistically significant effects were observed for TNF-α (P = 0.606) or MCP1 (P = 0.234). However, a weak trend toward an age × time interaction (P = 0.092) was observed at T3 for MCP1, as circulating concentrations continued to increase among young adults but declined among older adults.
Fig. 4.
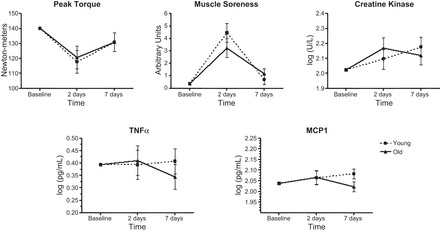
Clinical markers of muscle injury and serum indices of inflammation in young and older adults before and after exercise-induced skeletal muscle injury. Dashed lines represent values for young adults; solid lines represent values for older adults. Symbols represent means adjusted for gender, physical activity (steps/day), and baseline measure of the outcome; error bars represent SE. The age × time interaction was not statistically significant for any variable (P > 0.05). Adjusted main effect for time: peak torque, P = 0.744; muscle soreness, P < 0.001; creatine kinase, P = 0.101; serum TNF-α, P = 0.929; serum monocyte chemoattractant protein-1 (MCP1), P = 0.735.
For muscular indices of myogenesis, a significant main effect for time was observed for MRF4 (P = 0.021), HGF (P = 0.027), DAPI+ cells (P = 0.001), and Pax7+ cells (P = 0.002), with a trend toward significance observed for MyoD (P = 0.073). Across all time points, we did not observe a significant age × time interaction for any of the myogenic proteins or DAPI+ cells (Table 2), although there was a trend toward significance for cMET (P = 0.054). Likewise, the age × time interaction was not significant for Pax7+ cells (P = 0.857; Fig. 5). Linear contrasts did reveal a significant difference between groups in the change in MyoD at T3 (P = 0.026) and cMET at T2 (P = 0.017). Additionally, a significant time × gender interaction was observed at T3, as adjusted DAPI+ (P = 0.034) and Pax7+ (P = 0.024) cell number each increased to a greater extent among women than men across age groups (27% and 34% for DAPI+ and Pax7+ cells, respectively).
Table 2.
Indices of myogenesis, 2 and 7 days following exercise-induced muscle injury
| Age Group | Young Adults, n = 15 | Older Adults, n = 15 | Main Effect for Time | Age × Time Interaction |
|---|---|---|---|---|
| MRF4, A.U. | P = 0.021 | P = 0.440 | ||
| Baseline | 2.1 ± 0.0 | 2.1 ± 0.0 | ||
| 2 Days post (T2) | 2.8 ± 0.8 | 4.0 ± 0.7 | ||
| 7 Days post (T3) | 3.0 ± 0.6 | 4.1 ± 0.5 | ||
| Myf5, A.U. | P = 0.547 | P = 0.620 | ||
| Baseline | 3.8 ± 0.0 | 3.8 ± 0.0 | ||
| 2 Days post (T2) | 4.8 ± 1.0 | 5.8 ± 0.9 | ||
| 7 Days post (T3) | 4.3 ± 0.8 | 5.6 ± 0.7 | ||
| MyoD, A.U. | P = 0.073 | P = 0.311 | ||
| Baseline | 1.6 ± 0.0 | 1.6 ± 0.0 | ||
| 2 Days post (T2) | 2.5 ± 0.4 | 2.8 ± 0.4 | ||
| 7 Days post (T3) | 2.3 ± 0.2 | 3.1 ± 0.2 | ||
| HGF, A.U. | P = 0.027 | P = 0.914 | ||
| Baseline | 0.8 ± 0.0 | 0.8 ± 0.0 | ||
| 2 Days post (T2) | 1.0 ± 0.1 | 1.1 ± 0.1 | ||
| 7 Days post (T3) | 1.0 ± 0.2 | 1.1 ± 0.2 | ||
| cMET, A.U. | P = 0.177 | P = 0.054 | ||
| Baseline | 2.2 ± 0.0 | 2.2 ± 0.0 | ||
| 2 Days post (T2) | 4.3 ± 0.5 | 2.5 ± 0.4 | ||
| 7 Days post (T3) | 3.3 ± 0.6 | 3.7 ± 0.5 | ||
| DAPI+ cells/fiber | P = 0.001 | P = 0.149 | ||
| Baseline | 6.0 ± 0.0 | 6.0 ± 0.0 | ||
| 2 Days post (T2) | 6.7 ± 0.5 | 6.5 ± 0.5 | ||
| 7 Days post (T3) | 6.0 ± 0.5 | 7.1 ± 0.4 |
Values are expressed as adjusted means ± SE.
Analyses were adjusted for confounding factors, including gender, physical activity (steps/day), and baseline measure of the outcome. MRF, myogenic regulatory factor 4; A.U., arbitrary units; Myf5, myogenic factor 5; MyoD, myogenic differentiation antigen; HGF, hepatocyte growth factor; cMET, HGF receptor; DAPI, 4′,6-diamidino-2-phenylindole.
Fig. 5.
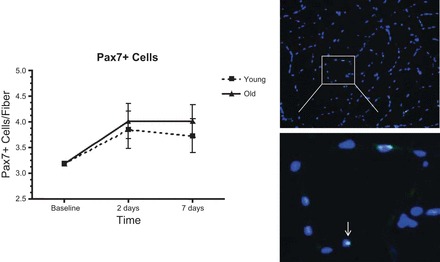
Skeletal muscle paired box 7 (Pax7)+ (satellite) cells in young and older adults before and after exercise-induced skeletal muscle injury. Dashed line represents value for young adults; solid line represents value for older adults. Symbols represent means adjusted for gender, physical activity (steps/day), and baseline measure of the outcome; error bars represent SE. A significant main effect was observed for time (P = 0.002), but the age × time interaction was not significant (P = 0.857). Right: representative muscle cross-section stained for DAPI (blue) and Pax7 (green) to identify muscle satellite cells.
Similar to indices of myogenesis, the changes in serum and skeletal muscle variables related to angiogenesis were largely similar between age groups. Following the bout, serum VEGF increased significantly across both groups (P = 0.039). However, the age × time interaction did not suggest age-related differences in the response to the injury protocol (P = 0.762; Fig. 6). We did observe a significant time × gender interaction (P = 0.039), indicating a differential serum VEGF response between men and women. Indeed, following the exercise bout, unadjusted serum VEGF concentrations were greater among men than women at all time points (mean difference: 0.052 log pg/ml). Additionally, we observed a trend toward a time × physical activity interaction for VEGF at T2 (P = 0.055). Similar to serum VEGF, muscle injury induced significant increases in intramuscular concentrations of VEGF (P = 0.035) and KDR (P = 0.023). However, the age × time interaction was not significant for either variable (VEGF, P = 0.757; KDR, P = 0.643). Likewise, a significant main effect for time was observed for CD31+ cells (P = 0.040), but despite a modest difference in the pattern of response, the age × time interaction was not significant (P = 0.315).
Fig. 6.
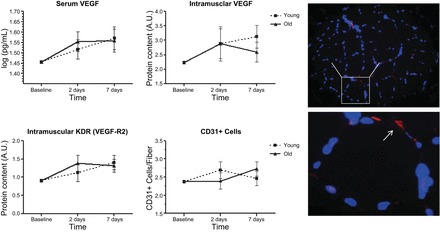
Serum and intramuscular indices of angiogenesis in young and older adults before and after exercise-induced skeletal muscle injury. Dashed lines represent values for young adults; solid lines represent values for older adults. Symbols represent means adjusted for gender, physical activity (steps/day), and baseline measure of the outcome; error bars represent SE. The age × time interaction was not statistically significant for any variable (P > 0.05). Adjusted main effect for time: serum VEGF receptor 2 (R2), P = 0.039; intramuscular VEGF, P = 0.035; intramuscular KDR, P = 0.023; CD31+ cells, P = 0.040. Right: representative muscle cross-section stained for DAPI (blue) and CD31 (red) to identify muscle capillaries. A.U., arbitrary units.
Finally, results of the multiple regression models indicated that myonuclear and capillary numbers, as well as habitual physical activity, were important predictors of the postinjury decline in and recovery of force production. Factors remaining in the final regression model to predict the change in force production at T2 (F = 6.490; P = 0.001) included number of steps/day (β = 0.405; t = 2.390), baseline strength (β = −0.600; t = −3.582), as well as the basal number of satellite cells (β = 0.751; t = 3.633) and myonuclei (β = −0.555; t = −2.685). At T3, steps/day (β = 0.285; t = 1.827), basal satellite cell number (β = 0.689; t = 3.360), and basal myonuclear number (β = −0.798; t = −3.478) remained in the final model (F = 5.822; P = 0.002), with the change in capillary number (β = 0.697; t = 4.116) and the change in myonuclear number (β = −0.467; t = −2.512) also contributing to the final model.
DISCUSSION
The findings of the present study indicate that compared with young adults, systemic and local skeletal muscle responses to acute muscle injury are not impaired among healthy older adults when accounting for important confounding factors. Notably, we evaluated postinjury changes in an array of biomarkers associated with muscle damage at 2 and 7 days postinjury. These parameters ranged from clinical indices of muscle injury (e.g., force production and CK) to bio- and histochemical markers of relevant regenerative processes, such as inflammation and myogenesis. We also used regression-based modeling to evaluate the relationship of these biological factors to the recovery of force production following injury. Across age groups, myogenic and angiogenic processes were strongly associated with the recovery of force production in the initial week following injury. However, in contrast to our a priori hypothesis, our results indicated consistently that active regeneration following skeletal muscle injury is not impaired by aging per se. This finding differs from prior reports in the literature—a disparity that may result, at least partially, from novel aspects of the present study, including the firmly controlled design, the comprehensive evaluation of myogenic and angiogenic responses to muscle injury, and the use of the gastrocnemius as the target muscle—an important distinction, given that the fiber-type composition in this muscle differs from the more commonly evaluated vastus lateralis.
Importantly for the interpretation of key study findings, clinical indicators of skeletal muscle injury did not differ between young and older adults. Whereas the eccentric exercise protocol was standardized and resulted in similar RPE ratings between groups, physiologic indicators of injury were also similar. Indeed, changes in peak torque, muscle soreness, and CK were similar between groups. The similarity between groups in these indicators of muscle injury was a critical factor in our ability to compare regenerative responses appropriately between groups. Similarly, changes in systemic concentrations of TNF-α and MCP1 were also similar between age groups. Proper interpretation of this finding is important, given the importance of inflammatory signaling to muscle-regenerative processes. Circulating concentrations of these analytes likely reflect a global responsiveness to injury, as well as metabolic activity of the muscle, rather than specific regenerative processes (38). As most human studies to date have evaluated regeneration in the first 24–48 h following injury, the purpose of the present study was to evaluate active muscle regeneration in the postacute inflammatory period. Accordingly, these data should not be interpreted to indicate local inflammatory responses to the eccentric exercise protocol. The local responses to muscle injury have been investigated previously, with ample evidence suggesting that the quantification of leukocyte cell-surface markers within the muscle itself is a more appropriate approach to such an inquiry (18, 37, 40).
The most significant finding of the present study is that we did not observe a significant effect of age on the responsiveness of satellite cells to contraction-induced muscle injury. These findings are in contrast to prior reports in the literature. Dreyer et al. (16) were among the first to report that increases in satellite cell number were significantly greater among young men than older men, 24 h following the exercise bout. Although with the use of an isotonic concentric-eccentric-resistance exercise protocol, Walker et al. (51) also recently reported a similar age-related disparity in Pax7+ cell responsiveness among men, 24 h postexercise. Several possible explanations exist for the disparate results observed between the findings of these studies and those of the present investigation. First, the present study design and analysis plan was tightly focused on controlling for confounding factors, such as co-morbidity and physical activity level. The lack of an observed effect of age could indicate that such factors are more important to “age-related” changes in regeneration than aging per se. For instance, control for habitual physical activity was important, given that skeletal muscle responses to contraction-induced injury are mitigated by training through adaptation to repeated bouts of exercise (6, 17, 29). Moreover, it should be noted that these studies reported differences only among men. Importantly, the present study observed a significant time × gender interaction, suggesting that satellite cell responsiveness to injury differs between men and women. Notably, this study is the first to report greater increases in satellite cell, as well as myonuclear, number among women at 7 days following eccentric exercise-induced muscle injury.
Other possibilities, including differences in biopsy timing and the muscle sampled, may also contribute to this apparent disparity in satellite cell responsiveness between this and prior studies. For example, the present study focused on the postacute recovery period, whereas these prior studies evaluated satellite cell responsiveness only within the initial 24 h following injury. New evidence from McKay et al. (32) suggests that the progression of satellite cells through the cell cycle in the first 48 h following resistance exercise may differ, at least modestly, between young and older men. Accordingly, it is possible that the age-related differences observed in prior studies are specific to the early postrecovery period. Additionally, these authors reported that increases in Pax7+ cells in type I fibers were similar among young and older men but that increases in type II fiber number were absent in older men in the 48 h following a bout of isotonic resistance exercise. These findings are in line with at least three studies that have reported type II fiber-specific declines in basal satellite cell number among older men (32, 48, 49). Because the gastrocnemius tends to have a higher proportion of type I fibers than the vastus lateralis (19), it is possible that this discrepancy in the muscle chosen may contribute to our findings. However, it is also possible that age-related differences existed within type II fibers that were not detected in our joint fiber-type analysis.
Similar to findings for Pax7+ cells, postinjury changes in skeletal muscle content of myogenic proteins and myonuclear (DAPI+) cells were similar between young and older adults. We did, however, observe a significant increase in total myonuclear cell number across groups—a finding that is in contrast to previous studies (16, 51). This finding may be explained by the significantly greater myonuclear cell response observed among women at T3. Indeed, the present study is the first to report greater increases in satellite cell and myonuclear number among women than men, 7 days following eccentric exercise-induced muscle injury. Previously, Bamman et al. (4) reported that muscle protein concentrations of the cyclin-dependent kinase inhibitor p27kip, an inhibitor of cell-cycle progression, were decreased to a greater degree in women than men following acute muscle loading. In particular, older women displayed the most dramatic decrease (27%) in p27kip expression. Later findings from this group also indicated that following loading, increases in mRNA expression of cyclin D1—a critical factor for cell-cycle progression—were also highest among older women compared with men and younger women (26). Thus it appears that irrespective of age, a disparity exists between genders in the regulation of the myogenic cell cycle in response to hypertrophic or injurious stimuli. As such, attention to the role of gender in recruitment and statistical analysis may be considered one of the major strengths of the present study.
Although we did not observe significant age × time interactions, we did, however, observe a differential pattern of expression between young and older adults for muscle concentrations of MyoD and cMET. As noted above, McKay et al. (32) reported differential regulation of the cell cycle between young and older men in the initial 48 h after exercise. These authors reported significant declines in whole muscle myostatin protein and mothers against decapentaplegic homolog 3 mRNA in older men between 24 and 48 h postexercise. These changes could signal a delayed, but not impaired, progression of the regenerative process in older adults. The present findings related to MyoD and cMET expression appear to support this possibility, as expression of each protein among younger adults peaked at T2 and decreased from T2 to T3. In contrast, expression of each protein was greatest at T3 among older adults. These findings re-emphasize the critical importance of biopsy timing for evaluating muscle-regenerative processes in humans. Accordingly, future studies may be warranted specifically to evaluate the timing of regenerative processes, with particular interest in identifying changes in the period following the completion of active regeneration.
Although indices of myogenesis provide the most direct marker of muscle-regenerative responses to injury, other contributory processes may provide key insights into the sequelae of muscle repair. Angiogenesis is one such process that has received relatively little attention in human studies of skeletal muscle repair. Indeed, the vascular system plays an important role in regulating muscle-regenerative capacity. In uninjured muscle, satellite cells lie near the endothelium and are closely associated with capillaries (13, 14). Moreover, the regulation of intramuscular VEGF levels has also been identified as a key factor in the regenerative process (36). In support of these findings, multiple regression modeling, used in the present study, identified the change in muscle capillary number as a significant contributor to the recovery of muscle-force production, 7 days following injury.
In line with this finding, we did observe robust angiogenic responses to the eccentric exercise protocol. Indeed, muscle injury stimulated significant increases across groups in serum and intramuscular VEGF, as well as intramuscular KDR and the number of CD31+ cells. However, we did not observe a significant effect of age on any of these parameters. This is perhaps not overly surprising, given the concordant findings for the myogenic markers and the closely related nature of the two processes. Moreover, as observed for myogenic cells, serum VEGF responses to the injury protocol differed between genders, as women demonstrated significantly greater postinjury increases than men. Although speculative, this elevation in systemic VEGF may at least contribute to the greater satellite and myonuclear cell responses observed among women than men at T3.
As with any study, the present investigation is not without limitations. Most notably, we did not perform fiber typing to evaluate differences in regenerative responses between type I and type II fibers. In light of recent findings indicating the type II fiber specificity of satellite cell responses of the vastus lateralis following eccentric exercise (12), it is possible that the differences existed within type II fibers, which we were unable to detect. However, future studies are needed to evaluate this hypothesis within the gastrocnemius, as the fiber-type mixture differs from that of the vastus lateralis. Another limitation of this study is the inability to evaluate passive muscle regeneration that continues to occur for several weeks after injury. It is certainly possible that aging per se may negatively impact extracellular matrix remodeling and instead, favor fibrotic processes. This possibility will be critical to investigate, given findings from animal models, suggesting the fibrotic conversion during repair as a central change to aging muscle (5).
Subsequent studies are needed to address these limitations, as well as other potential causes of disparity between this and prior investigations. For example, despite the significant merits of prior studies in this area, several of these studies represent models of adaptation rather than injury, as they are unlikely to induce the requisite tissue degeneration and inflammation necessary to evaluate active muscle regeneration accurately (3). Accordingly, eccentrically biased protocols are perhaps needed consistently, as they provide a stronger model to evaluate regeneration, given the extensive muscle damage created by repeated lengthening contractions. Furthermore, additional investigation is needed to evaluate the impact of age-related diseases on skeletal muscle regeneration. Notably, previous evidence has demonstrated that diabetes provides an unfavorable environment for regenerating muscle following injury (50). Thus studies of older adults with diabetes and other co-morbid conditions could provide critical information regarding the potential impact of muscle-regenerative processes on sarcopenia development and possibly in the development of therapeutic strategies if warranted.
In conclusion, the present study is one of the most comprehensive investigations to date to evaluate the impact of advanced age on active muscle regeneration following contraction-induced injury in humans. In contrast to prior human studies in this area, the present investigation is novel for investigating age-related differences in regenerative processes beyond the initial 24-h period following injury, as well as for evaluating these processes within the gastrocnemius muscle. Our findings are also bolstered by the fact that we were able to control for several important confounding factors, including habitual physical activity, gender, and co-morbid disease. These findings suggest that factors, such as decreased physical activity and increased co-morbidity, may contribute to age-related impairments in active skeletal muscle regeneration rather than aging per se.
GRANTS
Support for this study was provided by grants awarded to T. W. Buford from the National Center for Research Resources (NCRR; KL2TR000065) and from Merck (Investigator Initiated Study #37268). Additional support was also provided by the University of Florida Claude D. Pepper Older Americans Independence Center (National Institute on Aging P30AG028740) and Clinical and Translational Science Institute (NCRR UL1TR000064).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
Author contributions: T.W.B., M.P., and C.L. conception and design of research; T.W.B., R.G.M., M.D., and B.S. performed experiments; T.W.B., R.G.M., L.G.C., M.D., and T.M.M. analyzed data; T.W.B. and T.M.M. interpreted results of experiments; T.W.B. and M.D. prepared figures; T.W.B. drafted manuscript; T.W.B., R.G.M., L.G.C., M.D., B.S., M.P., T.M.M., and C.L. edited and revised manuscript; T.W.B., R.G.M., L.G.C., M.D., B.S., M.P., T.M.M., and C.L. approved final version of manuscript.
REFERENCES
- 1.Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol 56: 1668–1676, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker BA, Cutlip RG. Skeletal muscle injury versus adaptation with aging: novel insights on perplexing paradigms. Exerc Sport Sci Rev 38: 10–16, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol 97: 1329–1337, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Brooks SV, Opiteck JA, Faulkner JA. Conditioning of skeletal muscles in adult and old mice for protection from contraction-induced injury. J Gerontol A Biol Sci Med Sci 56: B163–B171, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Buford TW, Cooke MB, Manini TM, Leeuwenburgh C, Willoughby DS. Effects of age and sedentary lifestyle on skeletal muscle NF-kappaB signaling in men. J Gerontol A Biol Sci Med Sci 65: 532–537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buford TW, Cooke MB, Redd LL, Hudson GM, Shelmadine BD, Willoughby DS. Protease supplementation improves muscle function after eccentric exercise. Med Sci Sports Exerc 41: 1908–1914, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Buford TW, Cooke MB, Shelmadine BD, Hudson GM, Redd L, Willoughby DS. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl Physiol Nutr Metab 34: 745–753, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Buford TW, Lott DJ, Marzetti E, Wohlgemuth SE, Vandenborne K, Pahor M, Leeuwenburgh C, Manini TM. Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol 47: 38–44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carosio S, Berardinelli MG, Aucello M, Musaro A. Impact of ageing on muscle cell regeneration. Ageing Res Rev 10: 35–42, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, Gibala MJ, Van Loon LJ. Eccentric exercise increases satellite cell content in type II muscle fibers. Med Sci Sports Exerc 45: 230–237, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163: 1133–1143, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18: 1397–1409, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 81, Suppl 11: S52–S69, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33: 242–253, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Ebbeling CB, Clarkson PM. Exercise-induced muscle damage and adaptation. Sports Med 7: 207–234, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Hamada K, Vannier E, Sacheck JM, Witsell AL, Roubenoff R. Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB J 19: 264–266, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol 85: 1337–1341, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am 84-A: 822–832, 2002 [PubMed] [Google Scholar]
- 21.Hurme T, Kalimo H, Lehto M, Jarvinen M. Healing of skeletal muscle injury: an ultrastructural and immunohistochemical study. Med Sci Sports Exerc 23: 801–810, 1991 [PubMed] [Google Scholar]
- 22.Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med 33: 745–764, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, Aranda JM, Sandesara BD, Pahor M, Manini TM, Marzetti E, Leeuwenburgh C. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 11: 801–809, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaariainen M, Jarvinen T, Jarvinen M, Rantanen J, Kalimo H. Relation between myofibers and connective tissue during muscle injury repair. Scand J Med Sci Sports 10: 332–337, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 29: 120–127, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288: E1110–E1119, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Larkin KA, Macneil RG, Dirain M, Sandesara B, Manini TM, Buford TW. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med Sci Sports Exerc 44: 2077–2083, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord 9: 72–80, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Lynch GS, Faulkner JA, Brooks SV. Force deficits and breakage rates after single lengthening contractions of single fast fibers from unconditioned and conditioned muscles of young and old rats. Am J Physiol Cell Physiol 295: C249–C256, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackey DC, Manini TM, Schoeller DA, Koster A, Glynn NW, Goodpaster BH, Satterfield S, Newman AB, Harris TB, Cummings SR, Health, Aging, and Body Composition Study. Validation of an armband to measure daily energy expenditure in older adults. J Gerontol A Biol Sci Med Sci 66: 1108–1113, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marzetti E, Lees HA, Manini TM, Buford TW, Aranda JM, Jr, Calvani R, Capuani G, Marsiske M, Lott DJ, Vandenborne K, Bernabei R, Pahor M, Leeuwenburgh C, Wohlgemuth SE. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: an exploratory study. PLoS One 7: e32829, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J 26: 2509–2521, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut MF. Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 22: 724–732, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol 26: 535–542, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Muszalik M, Dijkstra A, Kedziora-Kornatowska K, Zielinska-Wieczkowska H, Kornatowski T. Independence of elderly patients with arterial hypertension in fulfilling their needs, in the aspect of functional assessment and quality of life (QoL). Arch Gerontol Geriatr 52: e204–e209, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, Shireman PK. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol 293: R651–R661, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Paulsen G, Egner I, Raastad T, Reinholt F, Owe S, Lauritzen F, Brorson SH, Koskinen S. Inflammatory markers CD11b, CD16, CD66b, CD68, myeloperoxidase and neutrophil elastase in eccentric exercised human skeletal muscles. Histochem Cell Biol. In press. [DOI] [PubMed] [Google Scholar]
- 38.Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev 18: 42–97, 2012 [PubMed] [Google Scholar]
- 39.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci 55: M691–M697, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Przybyla B, Gurley C, Harvey JF, Bearden E, Kortebein P, Evans WJ, Sullivan DH, Peterson CA, Dennis RA. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp Gerontol 41: 320–327, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Rader EP, Faulkner JA. Effect of aging on the recovery following contraction-induced injury in muscles of female mice. J Appl Physiol 101: 887–892, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Rader EP, Faulkner JA. Recovery from contraction-induced injury is impaired in weight-bearing muscles of old male mice. J Appl Physiol 100: 656–661, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell 1: 132–139, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Roth SM, Martel GF, Ivey FM, Lemmer JT, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell populations in healthy young and older men and women. Anat Rec 260: 351–358, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol 81: 775–785, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA 305: 50–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc 27: 1022–1032, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 64: 332–339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292: E151–E157, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Vignaud A, Ramond F, Hourde C, Keller A, Butler-Browne G, Ferry A. Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice. Pathobiology 74: 291–300, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Walker DK, Fry CS, Drummond MJ, Dickinson JM, Timmerman KL, Gundermann DM, Jennings K, Volpi E, Rasmussen BB. PAX7+ satellite cells in young and older adults following resistance exercise. Muscle Nerve 46: 51–59, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warren GL, Hulderman T, Mishra D, Gao X, Millecchia L, O'Farrell L, Kuziel WA, Simeonova PP. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J 19: 413–415, 2005 [DOI] [PubMed] [Google Scholar]


