Abstract
Vascular remodeling and smooth muscle cell proliferation are hallmark pathogenic features of pulmonary artery hypertension. MicroRNAs, endogenously expressed small noncoding RNAs, regulate gene expression at the posttranscriptional level. It has previously been shown that miR-17 overexpression in cultured human pulmonary artery smooth muscle cell (hPASMC) resulted in increased viable cell number. Previously, we have found that arginase II promotes hypoxia-induced proliferation in hPASMC. Therefore, we hypothesized that miR-17 would be upregulated by hypoxia in hPASMC and would result in greater arginase II expression. We found that levels of miR-17-5p and arginase II were significantly greater in cultured hPASMC exposed to 1% O2 for 48 h than in hPASMC exposed to 21% O2 for 48 h. Furthermore, inhibiting miR-17-5p expression decreased hypoxia-induced arginase II protein levels in hPASMC. Conversely, overexpressing miR-17-5p resulted in greater arginase II protein levels. Somewhat surprisingly, arginase II inhibition was associated with lower miR-17-5p expression in both normoxic and hypoxic hPASMC, whereas overexpressing arginase II resulted in greater miR-17-5p expression in hPASMC. These findings suggest that hypoxia-induced arginase II expression is not only regulated by miR-17-5p but also that there is a feedback loop between arginase II and miR-17-5p in hPASMC. We also found that the arginase II-mediated regulation of miR-17-5p was independent of either p53 or c-myc. We also found that l-arginine, the substrate for arginase II, and l-ornithine, the amino acid product of arginase II, were not involved in the regulation of miR-17-5p expression.
Keywords: hypoxia, pulmonary hypertension, pulmonary vascular remodeling
pulmonary hypertension (PH) is a life-threatening complication of chronic hypoxic lung diseases. PH is characterized by vasoconstriction, thrombosis, and vascular remodeling, the pathogenic hallmarks of PH involving all layers of the vessel wall, particularly the smooth muscle layer (10, 12). The smooth muscle layer plays an integral role in the pathogenesis of PH with extension of smooth muscle into smaller, normally nonmuscular pulmonary arteries (10, 19). In addition, pulmonary artery smooth muscle cells (PASMC) markedly proliferate, resulting in decreased luminal diameters and ultimately the obstruction of resistance-level pulmonary arteries (10, 23). This pathology can be triggered by seemingly disparate genetic and environmental stimuli.
MicroRNAs (miRs) are small noncoding RNAs that regulate gene expression at a posttranscriptional level (17). Recent studies describe a fundamental role of miRs in development and diseases, including PH (5, 8, 22, 26). Estimates from current data suggest that over 1,400 distinct miRs are predicted to be encoded by the human genome (7). Of these, the miR-17∼92 cluster is one of the most well-characterized miR families that have been described to play a role in controlling cell development, apoptosis, and proliferation in a variety of cellular and disease contexts (18). Indeed a series of recent papers have demonstrated essential roles for the miR-17∼92 cluster in the development of the heart, lung, immune system, and tumor formation (14, 25, 27). This cluster located on human chromosome 13 encodes seven individual miRs: miR-17-5p, miR-17–3p, miR-18a, miR-19a, miR-20a, miR-19b, and miR-92a. These seven miRs are transcribed as one common primary transcript. Overexpression of miR-17∼92 was reported to induce downregulation of BMPR2 (2), which has been repeatedly described as an important feature in the pathogenesis of PH. Recently, Pullamsetti et al. (24) found that inhibition of miR-17 in a mouse model of PH reduced right ventricular hypertrophy and decreased vascular remodeling, whereas overexpression of miR-17 in cultured human (h) PASMC resulted in increased proliferation.
Arginase, of which there are two described isoforms, arginase I and arginase II, has been shown to be important in the proliferation of a variety of cell types (13). Arginase I is a cytosolic enzyme highly expressed in the liver, whereas arginase II is an extrahepatic mitochondrial protein (6). Both arginase isoforms are expressed in the lung (20). Arginase II has been shown to have variety of functions, including promoting cell proliferation (4) and modulating NO production (15). Furthermore, arginase II inhibition also was found to improve coronary microvascular function and reduce ischemic infarct size in rats likely by its effect on NO production (9). We have previously shown that hypoxia induces arginase II expression in hPASMC and that the hypoxia-induced arginase II was necessary for hypoxia-induced proliferation in hPASMCs (4). However, little is known concerning the cellular mechanisms leading to hypoxia-induced arginase II expression in hPASMC.
Because both arginase II and miR-17-5p play important roles in proliferation of hPASMCs, we hypothesized that miR-17-5p would regulate hypoxia-induced arginase II expression in hPASMC. We used cultured hPASMC to study the effects of hypoxia on miR-17-5p expression. We also used antagomirs and overexpression of miR-17-5p to examine the role of miR-17-5p in regulating hypoxia-induced arginase II expression and viable cell numbers.
MATERIALS AND METHODS
Cell culture.
hPASMC (Lonza) were cultured as previously described (4). Briefly, hPASMC were grown in 21% O2-5% CO2-balance N2 at 37°C in smooth muscle growth media (SmGM; Lonza), which includes smooth muscle basal medium (Lonza), 5% FBS, 0.5 ng/ml human recombinant epidermal growth factor, 2 ng/ml human recombinant fibroblast growth factor, 5 μg/ml insulin, and 50 μg/ml gentamicin. The hPASMC were used in experiments between the fifth and eighth passages, throughout which no changes in cell morphology were noted. The cells were grown to ∼80–90% confluence and washed, fresh media was placed on the cells, and they were incubated in 21% O2-5% CO2-balance N2 (normoxia) or 1% O2-5% CO2-balance N2 (hypoxia) for 48 h. We have previously used 1% O2 as our hypoxic stimulus (4) because it provides consistent and reproducible changes in the hPASMC.
RNA isolation, reverse transcription, and quantitative real-time PCR.
RNA was isolated from hPASMCs and reverse transcribed, and quantitative real-time PCR performed as previously described (4, 20). Relative arginase I and II mRNA amounts were normalized to 18S mRNA expression using the ΔΔCT method (16). Samples were analyzed in triplicate.
miR isolation.
Total RNA was extracted from cell samples with the mirVana isolation kit (Applied Biosystems, Foster City, CA). The miR concentrations were quantified a by NanoDrop 1000 Spectrophotometer (NanoDrop Technologies, Waltham, MA).
Quantitative RT-PCR.
miR qRT-PCR was performed with the TaqMan microRNA assay kit using the manufacturer's protocol. Real-time PCR amplification was done for 40 cycles using the Applied Biosystems 7500 PCR System and miR specific probes. Experiments were done in triplicate. Expression was normalized to the small nucleolar RNA RNU48. Relative expression of miR was normalized to RNU48 expression using the ΔΔCT method. We found that the expression of RNU48 was unaffected by hypoxia in hPASMC (expression of RNU48 normalized to normoxia, 1.00 ± 0.01 normoxia and 1.01 ± 0.01 hypoxia).
Protein isolation and Western blot.
After treatment, hPASMCs were harvested for total protein as previously described (4, 20), and protein concentration was determined by the Bradford method (1). Protein was assayed for arginase II, c-myc, or p53 (1:500) (Santa Cruz Biotechnology) expression by Western blot analysis as previously described (4, 20). Protein bands were visualized using enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and quantified using densitometry (Sigma Gel; Jandel Scientific, San Rafael, CA). Expression of arginase II was normalized to β-actin (1:10,000) (Abcam, Cambridge, MA).
Transfection with miR-17-5p antagomir or overexpression of miR-17-5p.
To examine the effect of miR-17-5p on arginase II protein expression, hPASMC were transfected with miR-17-5p, antagomirs of miR-17-5p, or their respective negative controls. The miR-17-5p antagomir is single stranded with the following sequence: ACUACCUGCACUGUAAGCACUUUG. The miR-17-5p used for overexpression is double stranded with the following sequences: sense ACCUGCACUGUAAGCACUUUGTT and antisense CAAAGUGCUUACAGUGAGGUAG. hPASMCs were seeded in six-well plates at 70% confluence at the time of transfection. To inhibit miR-17-5p expression, cells were transfected with 80 nM of negative control or miR-17-5p antagomir overnight using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. To overexpress miR-17-5p, cells were transfected with 3 nM of negative control or miR-17-5p overnight using Lipofectamine RNAiMAX. The medium was then replaced with complete medium, and cells were incubated either in normoxia or hypoxia for another 48 h before protein or miRs were harvested.
Silencing arginase II by small-interfering RNA and adding l-arginine or l-ornithine into media.
To determine the effects of arginase II gene silencing on miR-17-5p expression, transient transfection of arginase II-small-interfering RNA (siRNA) was performed with DharmaFECT transfection reagent (Thermo Fisher Scientific, Lafayette, CO) as previously described (20). Briefly, in 1.5-ml centrifuge tubes, 100 μl of 2 μM arginase II-siRNA (Thermo Fisher Scientific) or nontargeting scramble siRNA (Thermo Fisher Scientific) were mixed with 100 μl of smooth muscle basal medium (Lonza), vortexed, and incubated at room temperature for 5 min. The DharmaFECT transfection reagent was diluted 1:100 (total volume of 200 μl) and incubated at room temperature for 5 min. The transfection reagent was then added to each sample of siRNA, mixed, and incubated at room temperature for 20 min. SmGM (600 μl) was then added to each tube for a total volume of 1 ml. For the vehicle-treated hPASMC, ddH2O was used in place of siRNA. hPASMC (Lonza), grown to 70% confluence in six-well plates, were washed with PBS. Fresh media (1 ml) and the siRNA-DharmaFECT reagent mixture (1 ml) were placed in each well of a six-well plate, and the hPASMC were incubated in normoxia for 24 h, then washed with PBS and incubated in complete medium. For investigating the effect of arginase II expression on miR-17-5p expression, different concentrations of l-arginine (3 or 10 mM) or l-ornithine (3 or 10 mM) were added to the media. Next, the hPASMC were incubated in either normoxia or hypoxia for another 48 h, and miRs were harvested for qRT-PCR analysis as described above.
Arginase II overexpression.
Recombinant adenoviral vectors carrying the human arginase II gene (AdArgII) under the control of a CMV promoter were used to overexpress arginase II in hPASMC. For transfection, cells were seeded and incubated at 37°C with 5% CO2 overnight and then transfected with AdArgII or a negative control adenoviral vector containing green fluorescent protein (GFP) under the control of a CMV promoter (AdGFP) at a multiplicity of infection of 20 overnight. The cells were washed with PBS and then were incubated in complete medium for 48 h before harvesting protein or miRs.
Statistical analysis.
Values are given as means ± SE. An unpaired t-test or a one-way ANOVA was used to compare groups. For the one-way ANOVA, differences were identified using Newman-Keuls post hoc testing (Prism; GraphPad Software, San Diego, CA). Differences were considered significant when P < 0.05.
RESULTS
Hypoxia and miR-17-5p expression.
To determine the effect of hypoxia on miR-17-5p expression, hPASMC were grown to ∼80–90% confluence and incubated in either normoxia or hypoxia for 48 h, and the expression of miR-17-5p was assessed by quantitative real-time PCR. There were significantly greater miR-17-5p expression levels in cultured hPASMC exposed to hypoxia for 48 h than in hPASMC exposed to normoxia for 48 h (Fig. 1A). Consist with previous results from our laboratory (4), both arginase II mRNA and protein expression was also greater in cultured hPASMC exposed to hypoxia for 48 h than in cells exposed to normoxia for 48 h (Fig. 1, B–D).
Fig. 1.
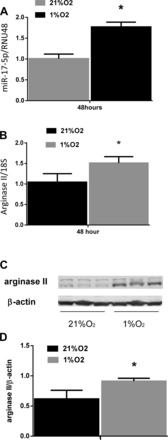
miR-17-5p and arginase II levels are increased by hypoxia. A: hypoxia increased miR-17-5p expression in human pulmonary artery smooth muscle cells (hPASMC). hPASMC were exposed to normoxia or hypoxia for 48 h (n = 3 for each group). miR-17-5p levels were analyzed by quantitative real-time PCR and normalized to RNU48 expression. Data are shown as means ± SE relative to respective normoxia controls at each time point. *Hypoxia different from normoxia controls, P < 0.05. B: hypoxia induces the increase in arginase II mRNA expression in hPASMC. hPASMC were exposed to normoxia or hypoxia for 48 h (n = 3 for each group). Arginase II mRNA levels were analyzed by quantitative real-time PCR and normalized to 18S expression using the ΔΔCT method. Data are shown as means ± SE relative to respective normoxia controls. *Hypoxia different from normoxia controls at same time point, P < 0.05. C: hypoxia induces an increase in arginase II protein expression in hPASMC. hPASMC were exposed to normoxia or hypoxia for 48 h (n = 3 for each group). Representative Western blots are shown for arginase II and β-actin. D: densitometric analysis of arginase II protein expression levels normalized to β-actin. Data are shown as means ± SE relative to β-actin. *Hypoxia different from normoxia controls at same time point, P < 0.05.
Inhibiting miR-17-5p prevented hypoxia-induced arginase II expression.
To determine the effect of miR-17-5p on hypoxia-induced arginase II protein expression, hPASMC were grown to ∼80–90% confluence, transfected with the miR-17-5p antagomir, and incubated in either 21% O2 or 1% O2 for 48 h. As expected, the miR-17-5p antagomir significantly attenuated miR-17-5p expression in both normoxia and hypoxia (Fig. 2A). Inhibiting miR-17-5p using the antagomir prevented hypoxia-induced arginase II protein expression in hPASMC exposed to 1% O2 for 48 h (Fig. 2, B and C).
Fig. 2.
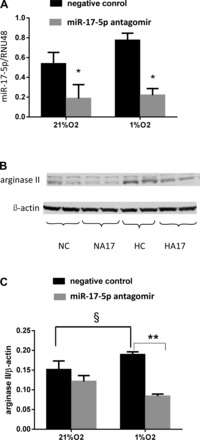
The miR-17-5p antagomir decreased arginase II protein levels. A: miR-17-5p antagomir decreased the miR-17-5p mRNA expression in hPASMC exposed to 21% O2 or 1% O2 for 48 h (n = 3 for each group). miR-17-5p levels were analyzed by quantitative real-time PCR and normalized to RNU48 expression using the ΔΔCT method. Data are shown as means ± SE relative to respective normoxia controls at each time point. *Different from negative control in normoxia and hypoxia, P < 0.05. B: arginase II protein levels are decreased by the miR-17-5p antagomir. hPASMC were exposed to 21% O2 or 1% O2 for 48 h. Representative Western blots for arginase II and β-actin. C: arginase II protein expression levels normalized to β-actin. Data are shown as means ± SE. §Negative control exposed to 21% O2 different from negative control exposed to 1% O2, P < 0.05. **During hypoxia, hPASMC transfected with the miR-17-5p antagomir different from negative controls, P < 0.01.
Overexpression of miR-17-5p augmented arginase II expression.
To determine the effect of overexpressing miR-17-5p on arginase II protein levels, hPASMC were transfected with miR-17-5p overnight. The hPASMC were then incubated in normoxia or hypoxia for 48 h. As expected, transfection of the miR-17-5p substantially increased miR-17-5p expression (Fig. 3A). Overexpression of miR-17-5p significantly increased arginase II protein levels both in normoxia and hypoxia (Fig. 3, B and C). To determine the effect of overexpression of miR-17-5p on viable cell numbers, hPASMC were transfected with miR-17-5p overnight and then incubated in normoxia for 120 h, and viable cell number was determined by trypan blue exclusion. Overexpression of miR-17-5p resulted in greater numbers of viable cells than in control hPASMC (Fig. 3D).
Fig. 3.
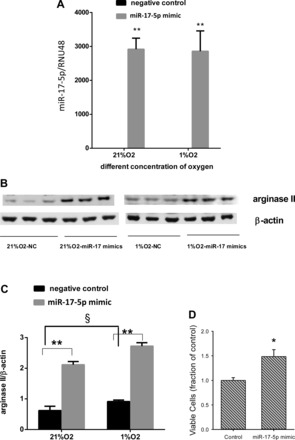
Overexpression of miR-17-5p increased arginase II protein expression. A: overexpression of miR-17-5p increased miR-17-5p expression in hPASMC exposed to normoxia or hypoxia for 48 h. miR-17-5p levels were analyzed by quantitative real-time PCR and normalized to RNU48 expression using the ΔΔCT method. Data are shown as means ± SE relative to respective normoxia controls at each time point. **miR-17-5p mimic treated different from negative control in both normoxia and hypoxia, P < 0.01. B: arginase II protein expression increased with miR-17-5p mimic in both normoxia and hypoxia for 48 h. Representative Western blots for arginase II and β-actin. C: densitometric data from Western blots for arginase II normalized to β-actin in hPASMC incubated in normoxia or hypoxia for 48 h. Data are shown as means ± SE relative to β-actin. §Negative control exposed to 21% O2 different from negative control exposed to 1% O2, P < 0.05. **hPASMC transfected with miR-17-5p mimic different from negative controls exposed to either 21% O2 or 1% O2, P < 0.01. D: overexpression of miR-17-5p resulted in greater number of viable cells. hPASMC were transfected with vehicle or miR-17-5p overnight, washed, and incubated for 120 h in normoxia, and viable cell numbers were counted by trypan blue exclusion. *Different from control, P < 0.01.
Knock down of arginase II prevents hypoxia-induced expression of miR-17-5p.
To determine the effects of knock down of arginase II on the expression of miR-17-5p, hPASMC were transfected with the siRNA against arginase II (Arg2-siRNA) and then incubated in either normoxia or hypoxia for 48 h. Arginase II protein levels were significantly decreased by transfection with Arg2-siRNA with little effect on c-myc or p53 protein levels (Fig. 4A). The expression of miR-17-5p was assessed by quantitative real-time PCR. The scramble siRNA had little effect on miR-17-5p expression in either normoxia or hypoxia (Fig. 4B). However, the Arg2-siRNA significantly decreased miR-17-5p expression levels in hPASMC incubated in either normoxia or hypoxia for 48 h (Fig. 4B).
Fig. 4.
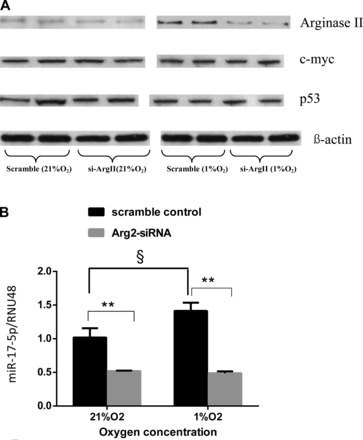
Knock down of arginase II decreased miR-17-5p expression. A: arginase II-small-interfering RNA (Arg2-siRNA) decreased arginase II protein expression in human PASMC exposed to either 21% O2 or 1% O2 for 48 h. Representative Western blots are shown for arginase II, c-myc, p53, and β-actin. B: relative miR-17-5p expression normalized to normoxia. §Scramble control exposed to normoxia different from scramble control exposed to hypoxia, P < 0.05. **hPASMC transfected with Arg2-siRNA different from scramble controls exposed to either 21% O2 or 1% O2, P < 0.01.
Arginase II overexpression increases miR-17-5p expression.
To determine the effects of arginase II overexpression on miR-17 expression levels, hPASMCs were transfected with recombinant adenoviral vectors carrying a GFP gene (AdGFP) or the human arginase II gene (AdArgII) and then incubated in either normoxia or hypoxia for 48 h. The AdGFP had little effect on arginase II protein levels, whereas the AdArgII substantially increased arginase II protein levels but not c-myc or p53 protein expression (Fig. 5A). The AdGFP had little effect on the expression of miR-17-5p, whereas AdArgII significantly increased miR-17-5p levels in hPASMC both in normoxia and hypoxia (Fig. 5B).
Fig. 5.
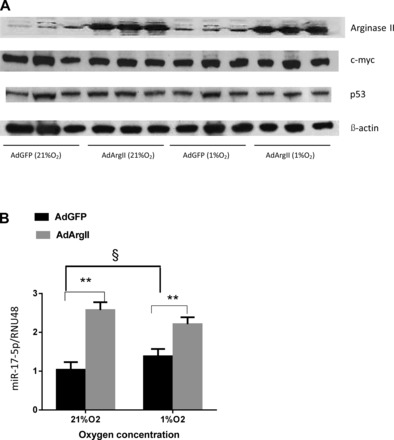
Arginase II overexpression increased miR-17-5p expression. hPASMC were transfected with recombinant adenoviral vectors carrying either green fluorescent protein (AdGFP) or the human arginase II gene (AdArgII) and then exposed to 21% O2 or 1% O2 for 48 h (n = 3 for each group). A: representative Western blots are shown for arginase II, c-myc, p53, and β-actin in hPASMC incubated in normoxia or hypoxia for 48 h. B: relative miR-17-5p expression normalized to normoxic controls. §hPASMC transfected with AdGFP exposed to 21% O2 different from those exposed to 1% O2, P < 0.05. **hPASMC transfected with AdArgII different from those transfected with AdGFP exposed to 21% O2 or 1% O2, P < 0.01.
The effect of l-arginine and l-ornithine on miR-17-5p expression.
To investigate the role of the substrate for, and the amino acid product of, arginase II on miR-17-5p expression independent of arginase II protein levels, hPASMC were transfected with an siRNA against arginase II (siArgII), and then treated with different concentrations of l-arginine or l-ornithine and incubated in either 21% O2 or 1% O2 for 48 h. The expression of miR-17-5p and arginase II was assessed by quantitative real-time PCR. Again Arg2-siRNA decreased expression of both miR-17-5p and arginase II in both normoxia and hypoxia (Fig. 6, A and B). Addition of l-arginine or l-ornithine to the media during normoxia resulted in an increase in miR-17-5p expression levels (Fig. 6A). However, neither l-arginine nor l-ornithine affected miR-17-5p expression levels during hypoxia (Fig. 6A). Addition of either l-arginine or l-ornithine following knock down of arginase II had little effect on arginase II mRNA levels in either normoxia or hypoxia (Fig. 6B). Interestingly, we found that the expression levels of miR-17-5p and arginase II mRNA were positively correlated in hPASMC (Fig. 6C). To determine if l-arginine would affect the expression of other miR17∼92 family members, we treated hPASMC with vehicle or 10 mM l-arginine and incubated the cells in normoxia for 48 h. We found no significant effect of 10 mM l-arginine on levels of miR-17-5p, miR-17–3p, miR-20a, or miR-92a.
Fig. 6.
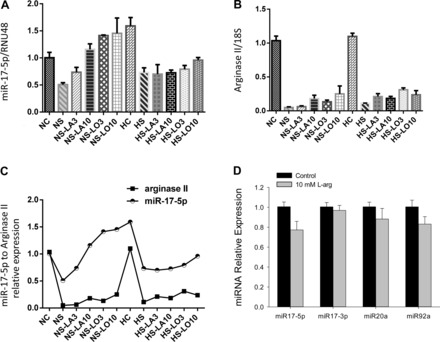
Arginine or ornithine did not affect hypoxic miR-17-5p or arginase II expression. hPASMC were transfected with arginase II-siRNA (Arg2-siRNA) or scramble siRNA, and different concentrations of l-arginine (3 or 10 mM) or l-ornithine (3 or 10 mM) were added to the media and then exposed to 21% O2 or 1% O2 for 48 h (n = 3 for each group). Transfected with scramble siRNA in 21% O2 (NC) or 1% O2 (HC); transfected with Arg2-siRNA in 21% O2 (NS) or 1% O2 (HS); transfected with Arg2-siRNA and l-arginine (3 or 10 mM) added to medium in 21% O2 (NS-LA3 and NS-LA10, respectively) or 1% O2 (HS-LA3 and HS-LA10, respectively); transfected with Arg2-siRNA and l-ornithine (3 or 10 mM) added to the medium in 21% O2 (NS-LO3 and NS-LO10, respectively) or 1% O2 (HS-LO3 and HS-LO10, respectively). A: relative miR-17-5p levels. B: arginase II mRNA levels. C: the relationship between changes in miR-17-5p levels and arginase II levels. D: l-arginine has little effect on the expression of miR-17∼92 family members in normoxia. hPASMC were incubated with 10 mM l-arginine in normoxia for 48 h, and levels of miR-17-5p, miR-17–3p, miR-20a, and miR-92a were determined.
DISCUSSION
The major findings of this study were that 1) miR-17-5p expression was significantly increased in hypoxic hPASMC, which was associated with an increase in arginase II mRNA and protein expression, 2) inhibition of miR-17-5p prevented hypoxia-induced arginase II expression, 3) overexpression of miR-17-5p augmented arginase II expression, 4) knock down of arginase II prevented hypoxia-induced expression of miR-17-5p, 5) arginase II overexpression increased miR-17-5p expression, and 6) arginase II regulated miR-17-5p expression independent of l-arginine or l-ornithine concentrations. These findings support our hypothesis that miR-17-5p is involved in hypoxia-induced arginase II protein expression. Interestingly, these results also demonstrate that arginase II regulates miR-17-5p expression.
A recent study looking at four of the miR17∼92 cluster members found that miR-17-5p, miR-19b, miR-92, and miR-20a were all increased in a monocrotaline model of PH in rats, whereas only miR-19b and miR-92 were increased in a hypoxia model of PH in rats (3). In another recent study, inhibition of miR-17 attenuated hypoxia-induced PH in mice as evidenced by reduced right ventricular hypertrophy and decreased pulmonary vascular remodeling (24). These findings are consistent with our findings in hPASMC that miR-17-5p expression was upregulated by hypoxia. Furthermore, in the present study, we found that miR-17-5p regulated arginase II expression and that inhibition of miR-17-5p prevented hypoxia-induced arginase II expression in hPASMC. Previously, we have demonstrated that arginase II expression was necessary for hypoxia-induced proliferation of hPASMC (4). Taken together, these results implicate miR-17-5p in the cellular mechanism of hypoxia-induced arginase II protein expression and the resultant proproliferative phenotype in hPASMC.
Of interest, this study also suggests for the first time that arginase II regulates miR-17-5p expression in hPASMC. The knock down of arginase II prevented hypoxia-induced miR-17-5p expression. Our studies demonstrate that arginase II may have a novel role in regulating miR-17-5p. To begin to examine how arginase II may regulate miR-17-5p expression, we considered our previous study that showed that the hypoxia-induced increase in arginase II expression resulted in an increase in arginase activity in hPASMC (4). An increase in arginase activity might affect levels of l-arginine and/or l-ornithine, the substrate and coproduct of arginase II metabolism, respectively. To determine if alterations in levels of substrate or product may be involved in the arginase II regulation of hypoxia-induced miR-17-5p expression, we knocked down arginase II expression and added l-arginine or l-ornithine to the media of the hPASMC. We found that adding l-arginine or l-ornithine resulted in greater miR-17 levels in normoxia but had little effect on miR-17-5p levels in hypoxia. The addition of either l-arginine or l-ornithine had little effect on arginase II expression levels in either normoxia or hypoxia. Thus, at least in terms of hypoxic regulation of miR-17-5p, neither the substrate nor the product of arginase II had any discernable effect on expression levels. To examine another potential pathway leading to arginase regulation of miR-17-5p expression, we turned to p53 and c-myc, which have been reported to be involved in modulating miR-17-5p expression in some cell types and conditions (21, 28). However, in our study, overexpressing or knock down of arginase II did not affect the expression of either p53 or c-myc. Thus, the effect of arginase II expression on miR-17-5p does not seem to be related to the enzymatic activity of arginase, or effects on p53 or c-myc. Further studies are needed to determine the exact mechanism by which arginase II expression regulates hypoxia-induced miR-17-5p expression in hypoxia.
In conclusion, we identified arginase II as a new target of miR-17-5p in hPASMC, with miR-17-5p involved in the hypoxic induction of arginase II in hPASMC. Interestingly, we also found evidence supporting a feedback loop between arginase II and miR-17-5p, such that arginase II regulates miR-17-5p expression in hPASMC. The effects of miR-17-5p on arginase II may underlie the role of miR-17-5p in experimental PH. We speculate that miR-17-5p and arginase II are potential therapeutic targets for PH.
DISCLOSURES
No conflicts of interest are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Youpeng Jin and Yi Jin performed experiments; Youpeng Jin and Yi Jin analyzed data; Youpeng Jin drafted manuscript; B.C. edited and revised manuscript; T.E.T. interpreted results of experiments; T.E.T. prepared figures; L.D.N. conception and design of research; L.D.N. approved final version of manuscript.
REFERENCES
- 1. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 2. Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res 104: 1184–1191, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 30: 716–723, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Chen B, Calvert AE, Cui H, Nelin LD. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am J Physiol Lung Cell Mol Physiol 297: L1151–L1159, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res 104: 724–732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34: 906–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet 12: 861–874, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med 60: 167–179, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Grönros J, Kiss A, Palmér M, Jung C, Berkowitz D, Pernow J. Arginase inhibition improves coronary microvascular function and reduces infarct size following ischaemia-reperfusion in a rat model. Acta Physiol (Oxf) 208: 172–179, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S–24S, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Ino Y, Yamazaki-Itoh R, Oguro S, Shimada K, Kosuge T, Zavada J, Kanai Y, Hiraoka N. Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One 8: e55146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis 45: 173–202, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Jenkinson CP, Grody WW, Kern Cederbaum SDRM. Comparative properties of arginases. Comp Biochem Physiol 114B: 107–132, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen KB, Cobb BS, Merkenschlager M, Rajewsky N, Rajewsky K. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell 132: 860–874, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Krause BJ, Prieto CP, Muñoz-Urrutia E, San Martín S, Sobrevia L, Casanello P. Role of arginase-2 and eNOS in the differential vascular reactivity and hypoxia-induced endothelial response in umbilical arteries and veins. Placenta 33: 360–366, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta deltac(t)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Lodish HF, Zhou B, Liu G, Chen CZ. Micromanagement of the immune system by microRNAs. Nat Rev Immunol 8: 120–130, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Mendell JT. miRiad roles for the miR-17–92 cluster in development and disease. Cell 133: 217–222, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy JD, Rabinovitch M, Goldstein JD, Reid LM. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr 98: 962–967, 1981 [DOI] [PubMed] [Google Scholar]
- 20. Nelin LD, Chicoine LG, Reber KM, English BK, Young TL, Liu Y. Cytokine-induced endothelial arginase expression is dependent on epidermal growth factor receptor. Am J Respir Cell Mol Biol 33: 394–401, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 435: 839–843, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Pasquinelli AE, Hunter S, Bracht J. MicroRNAs: a developing story. Curr Opin Genet Dev 15: 200–205, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder R. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 43: 25S–32S, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, Ghofrani HA, Weissmann N, Grimminger F, Bonauer A, Seeger W, Zeiher AM, Dimmeler S, Schermuly RT. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med 185: 409–419, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Ventura A, Young AG, Winslow MM, Linault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 132: 875–886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol 21: 461–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao C, Srinivasan L, Calao DP, Patterson HC, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17–92 expression in lymphocytes. Nature Immunology 9: 405–414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan HL, Xue G, Mei Q, Wang YZ, Ding FX, Liu MF, Lu MH, Tang Y, Yu HY, Sun SH. Repression of the miR-17–92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J 28: 2719–2732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]


