Abstract
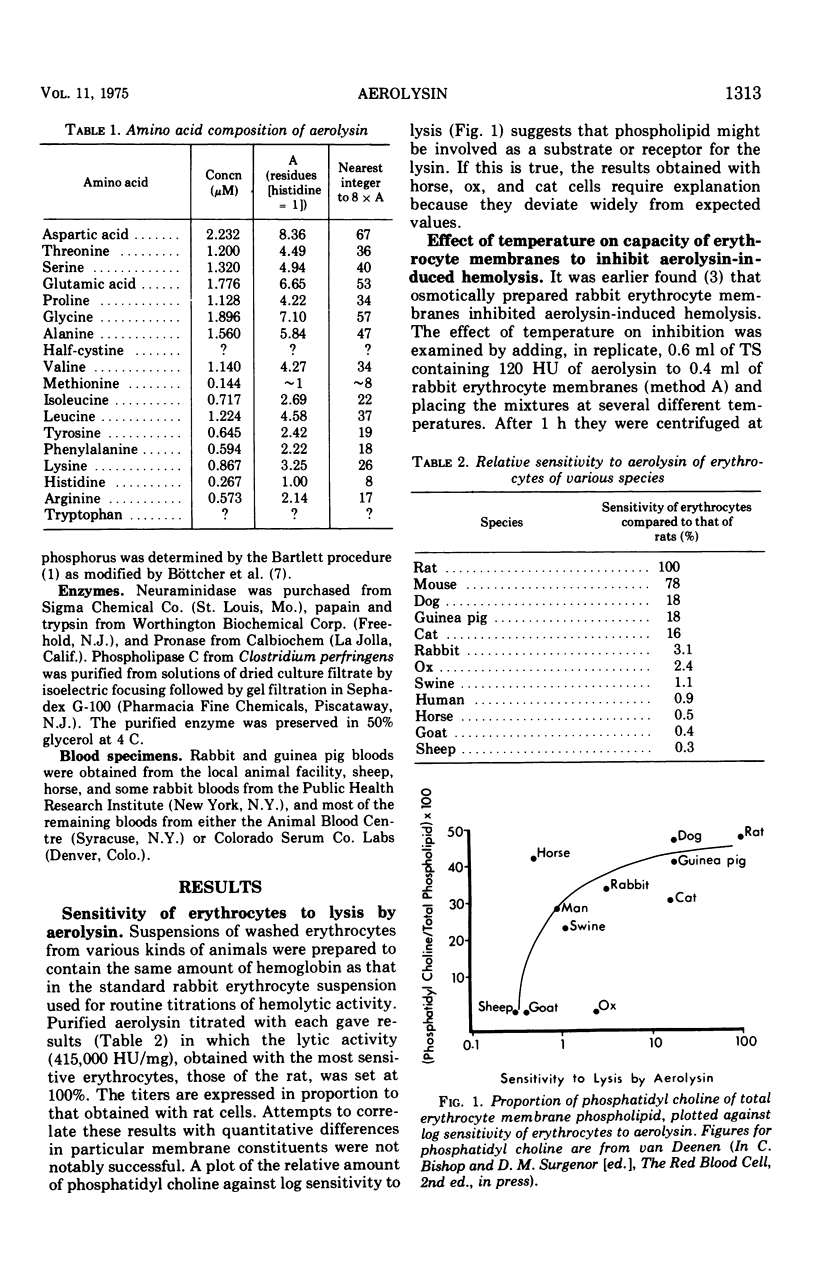
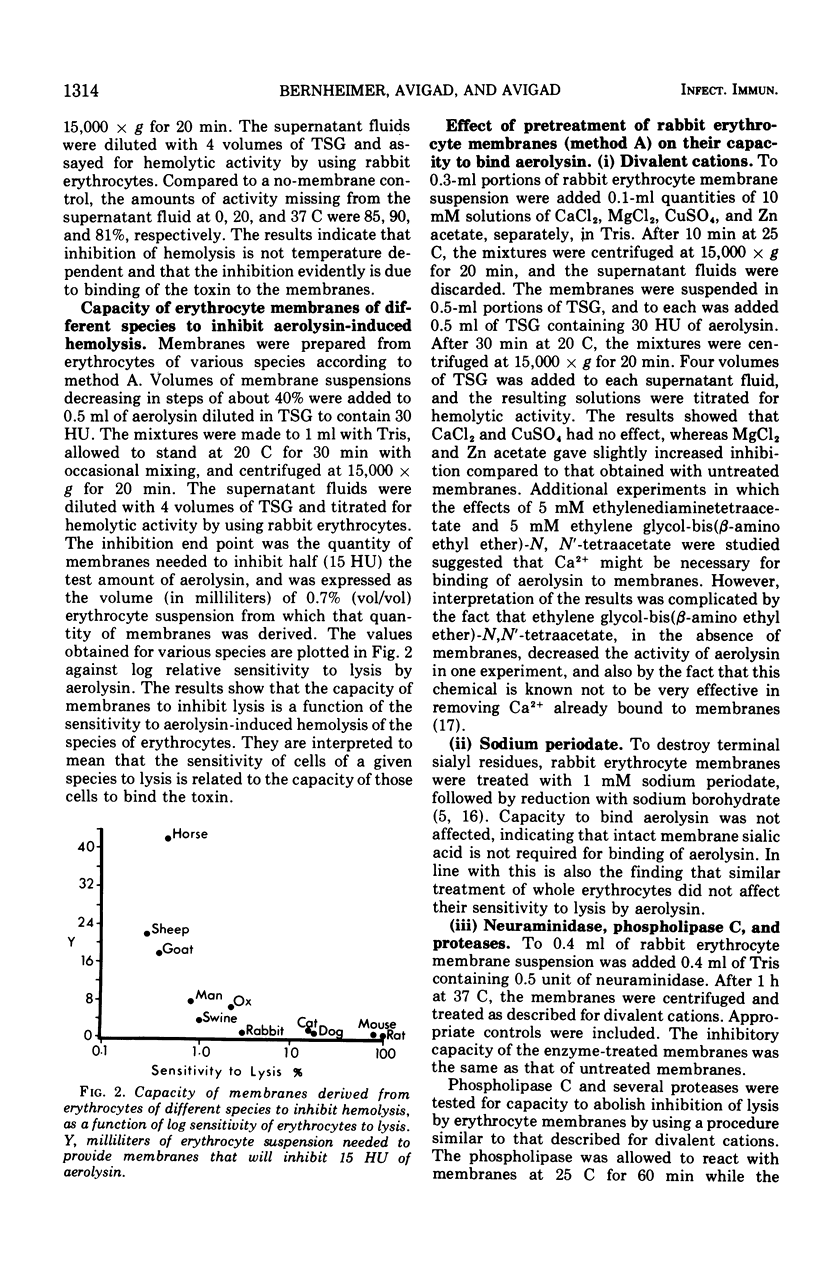
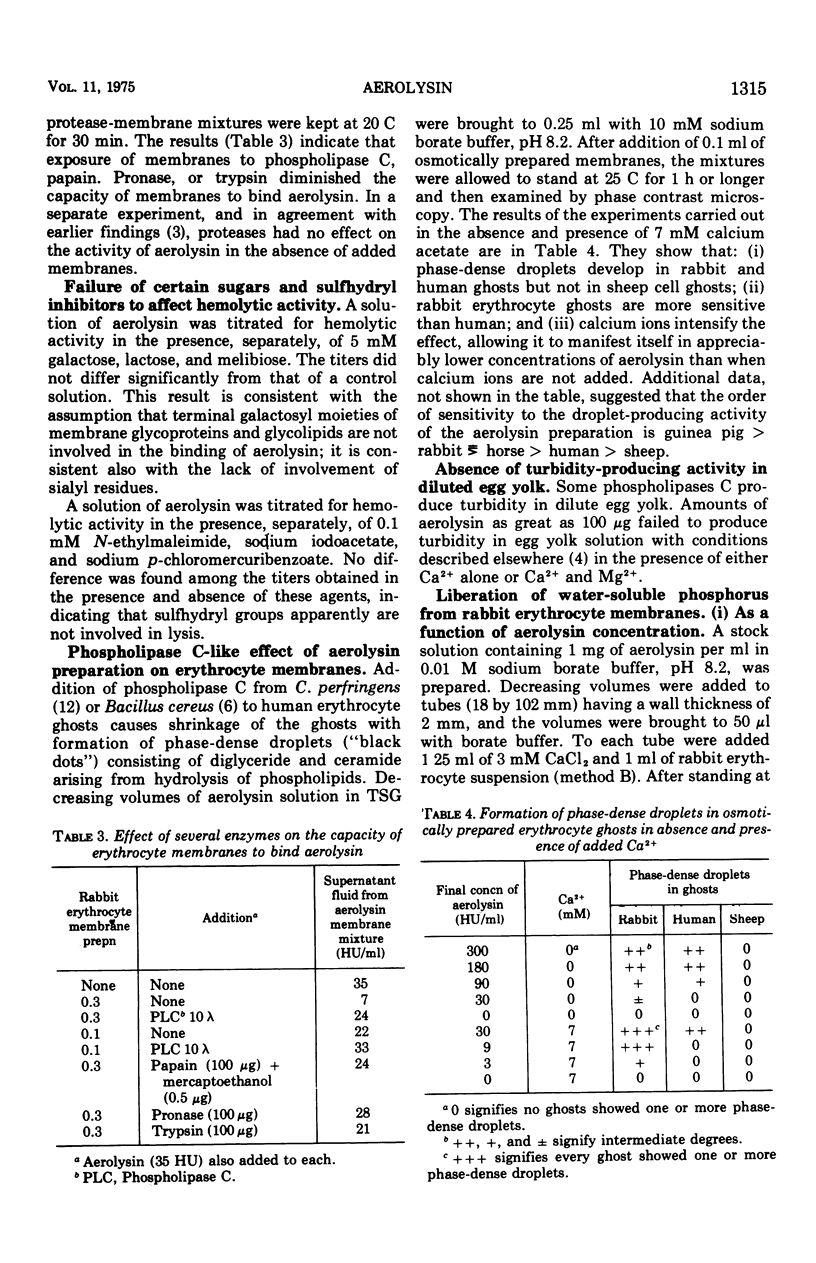
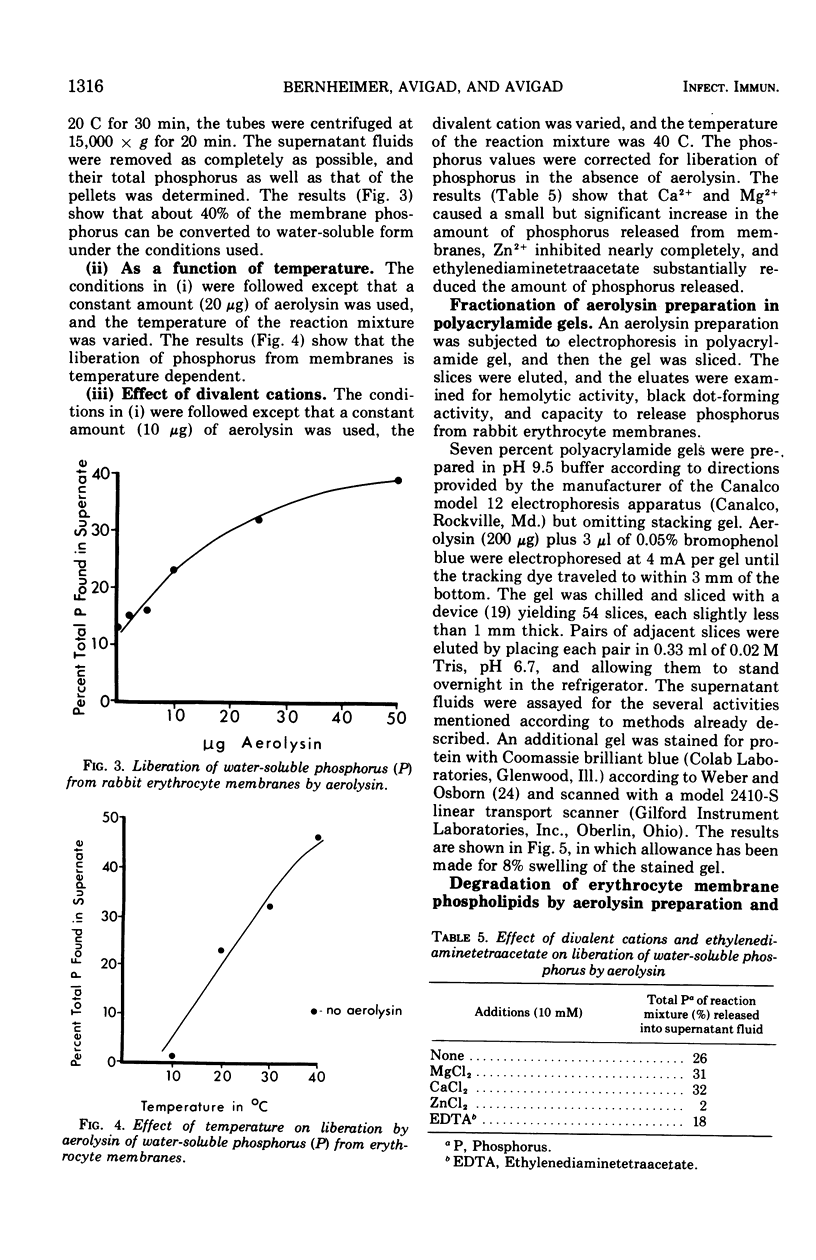
Aerolysin, a hemolytic and lethal exotoxin of Aeromonas hydrophila, was analyzed for amino acids. Assuming 8 histidine residues/mol, the purified toxic protein has, by summation, a molecular weight of 49,000, a value in agreement with earlier estimates by other methods. Erythrocytes from different animal species differ greatly in sensitivity to aerolysin's lytic action. There is some correlation between sensitivity and phosphatidyl choline content. Erythrocyte membranes of different species bind the toxin, and the efficiency of binding is a function of sensitivity to lysis. Binding is temperature independent, is not dependent upon membrane sialic acid, and is decreased by prior treatment with phospholipase C and proteases. Preparations of aerolysin convert substantial amounts of membrane phosphorus to water-soluble form; the conversion is concentration and temperature dependent. Most of the conversion is attributable to contaminating phospholipase(s) that is separable from the toxin. Aerolysin purified by electrophoresis in polyacrylamide gel retains some phospholipase activity, and this activity may or may not be a contaminant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S. Partial characterization of aerolysin, a lytic exotoxin from Aeromonas hydrophila. Infect Immun. 1974 Jun;9(6):1016–1021. doi: 10.1128/iai.9.6.1016-1021.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Grushoff P., Avigad L. S. Isoelectric analysis of cytolytic bacterial proteins. J Bacteriol. 1968 Jun;95(6):2439–2441. doi: 10.1128/jb.95.6.2439-2441.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld O. O., Gallop P. M., Liao T. H. Modification and introduction of a specific radioactive label into the erythrocyte membrane sialoglycoproteins. Biochem Biophys Res Commun. 1972 Jul 11;48(1):242–251. doi: 10.1016/0006-291x(72)90369-5. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim Biophys Acta. 1968 Mar 4;152(2):307–315. doi: 10.1016/0005-2760(68)90038-6. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M. Quantitative two-dimensional thin-layer chromatography of blood phospholipids. Clin Chim Acta. 1969 Mar;23(3):457–461. doi: 10.1016/0009-8981(69)90349-0. [DOI] [PubMed] [Google Scholar]
- CASELITZ F. H., GUENTHER R. [Hemolysin studies with Aeromonas strains]. Zentralbl Bakteriol. 1960 Sep;180:30–38. [PubMed] [Google Scholar]
- Coleman R., Finean J. B., Knutton S., Limbrick A. R. A structural study of the modification of erythrocyte ghosts by phospholipase C. Biochim Biophys Acta. 1970;219(1):81–92. doi: 10.1016/0005-2736(70)90063-5. [DOI] [PubMed] [Google Scholar]
- ESSELMANN M. T., LIU P. V. Lecithinase production by gramnegative bacteria. J Bacteriol. 1961 Jun;81:939–945. doi: 10.1128/jb.81.6.939-945.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka S., Liu P. V. Effect of the hemolysin of Pseudomonas aeruginosa on phosphatides and on phospholipase c activity. J Bacteriol. 1967 Feb;93(2):670–674. doi: 10.1128/jb.93.2.670-674.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T. H., Gallop P. M., Blumenfeld O. O. Modification of sialyl residues of sialoglycoprotein(s) of the human erythrocyte surface. J Biol Chem. 1973 Dec 10;248(23):8247–8253. [PubMed] [Google Scholar]
- Low D. K., Freer J. H., Arbuthnott J. P., Möllby R., Wadström T. Consequences of spingomyelin degradation in erythrocyte ghost membranes by staphylococcal beta-toxin (sphingomyelinase C). Toxicon. 1974 May;12(3):279–285. doi: 10.1016/0041-0101(74)90070-1. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Noda H. A simple device for transverse slicing of polyacrylamide gel rods. Anal Biochem. 1973 Dec;56(2):571–575. doi: 10.1016/0003-2697(73)90222-4. [DOI] [PubMed] [Google Scholar]
- Owens J. J. The egg yolk reaction produced by several species of bacteria. J Appl Bacteriol. 1974 Mar;37(1):137–148. doi: 10.1111/j.1365-2672.1974.tb00424.x. [DOI] [PubMed] [Google Scholar]
- REED C. F., SWISHER S. N., MARINETTI G. V., ENEN E. G. Studies of the lipids of the erythrocyte. I. Quantitative analysis of the lipids of normal human red blood cells. J Lab Clin Med. 1960 Aug;56:281–289. [PubMed] [Google Scholar]
- Scholz D., Scharmann W., Blobel H. Leucocidic substances for Aeromonas hydrophila. Zentralbl Bakteriol Orig A. 1974;228(3):312–316. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wretlind B., Hedén L., Wadström T. Formation of extracellular haemolysin by Aeromonas hydrophila in relation to protease and staphylolytic enzyme. J Gen Microbiol. 1973 Sep;78(1):57–65. doi: 10.1099/00221287-78-1-57. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Möllby R., Wadström T. Separation of two hemolysins from Aeromonas hydrophila by isoelectric focusing. Infect Immun. 1971 Oct;4(4):503–505. doi: 10.1128/iai.4.4.503-505.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



