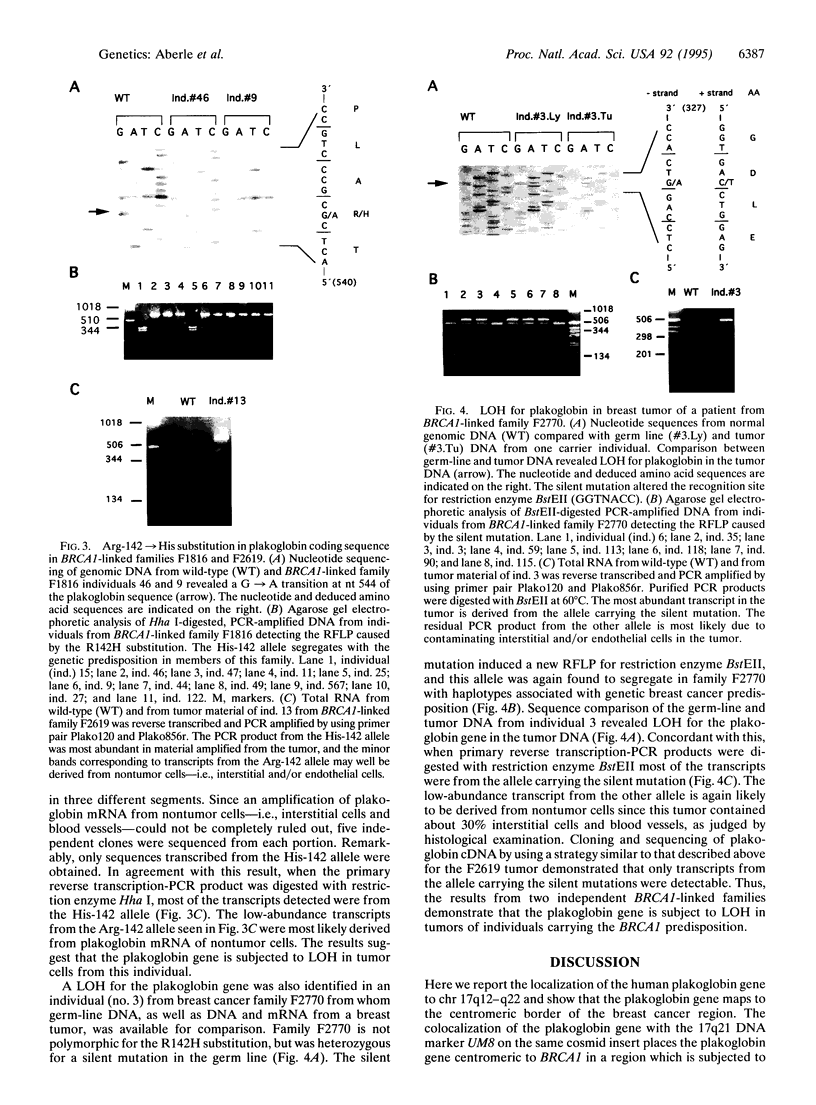
Abstract
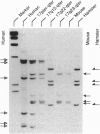
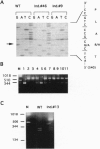
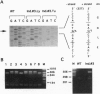
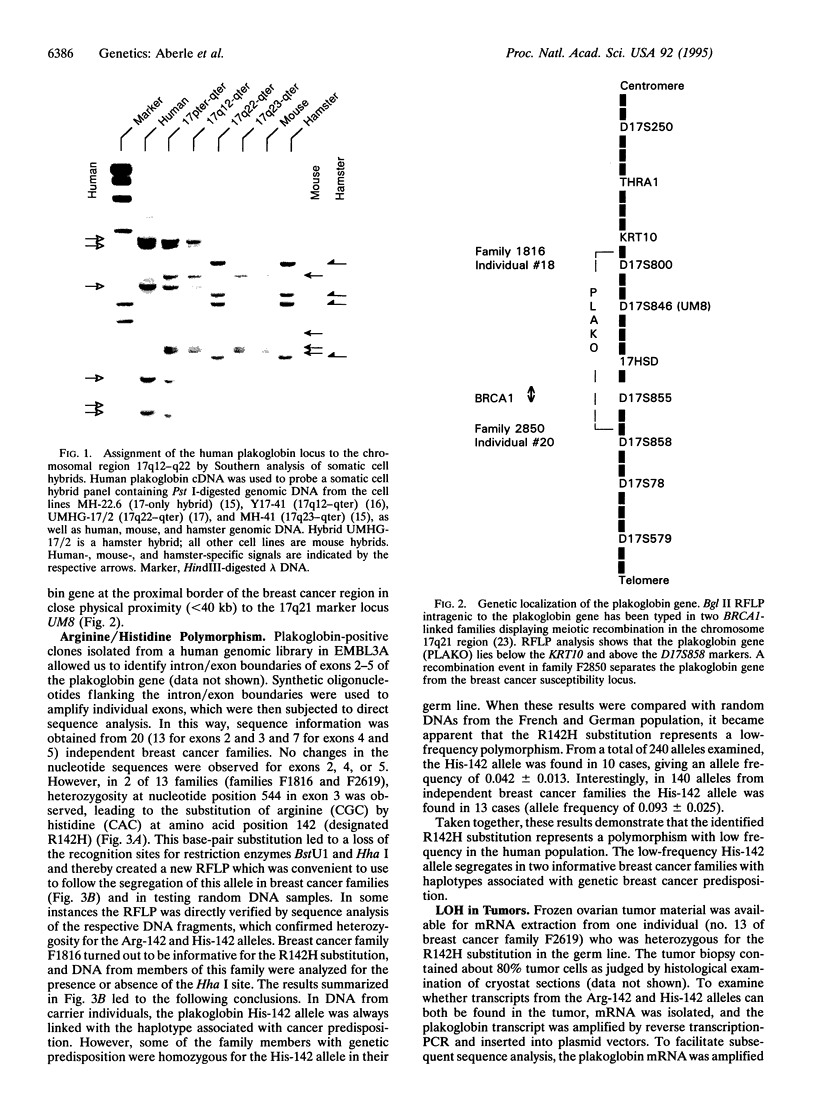
The gene encoding human plakoglobin was mapped to chromosome 17q12-q22. An intragenic restriction fragment length polymorphism was used to localize the plakoglobin gene distal to locus KRT10 and proximal to the marker D17S858. The plakoglobin gene colocalizes with the polymorphic 17q21 marker UM8 on the same cosmid insert. This subregion of chromosome 17 is known to be particularly subjected to genetic alterations in sporadic breast and ovarian tumors. We show loss of heterozygosity of the plakoglobin gene in breast and ovarian tumors. We have identified a low-frequency polymorphism in the plakoglobin coding sequence which results in an arginine to histidine substitution at amino acid position 142 of the protein, as well as a silent mutation at nucleotide position 332 of the coding sequence. This polymorphism allowed us to demonstrate an allelic association of plakoglobin with predisposition to familial breast and ovarian cancers. Our results, together with the present knowledge about the biological function of plakoglobin, suggest that plakoglobin might represent a putative tumor suppressor gene for breast and ovarian cancers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsen H. M., Smith S. A., Mazoyer S., Fujimoto E., Stevens J., Williams B., Rodriguez P., Cropp C. S., Slijepcevic P., Carlson M. A physical map and candidate genes in the BRCA1 region on chromosome 17q12-21. Nat Genet. 1994 Aug;7(4):472–479. doi: 10.1038/ng0894-472. [DOI] [PubMed] [Google Scholar]
- Butz S., Kemler R. Distinct cadherin-catenin complexes in Ca(2+)-dependent cell-cell adhesion. FEBS Lett. 1994 Nov 28;355(2):195–200. doi: 10.1016/0014-5793(94)01205-9. [DOI] [PubMed] [Google Scholar]
- Butz S., Stappert J., Weissig H., Kemler R. Plakoglobin and beta-catenin: distinct but closely related. Science. 1992 Aug 21;257(5073):1142–1144. doi: 10.1126/science.257.5073.1142-a. [DOI] [PubMed] [Google Scholar]
- Cowin P., Kapprell H. P., Franke W. W., Tamkun J., Hynes R. O. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986 Sep 26;46(7):1063–1073. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- Cropp C. S., Nevanlinna H. A., Pyrhönen S., Stenman U. H., Salmikangas P., Albertsen H., White R., Callahan R. Evidence for involvement of BRCA1 in sporadic breast carcinomas. Cancer Res. 1994 May 15;54(10):2548–2551. [PubMed] [Google Scholar]
- Fain P. R., Solomon E., Ledbetter D. H. Second international workshop on human chromosome 17. Cytogenet Cell Genet. 1991;57(2-3):66–77. [PubMed] [Google Scholar]
- Flejter W. L., Kukowska-Latallo J. F., Kiousis S., Chandrasekharappa S. C., King S. E., Chamberlain J. S. Tetranucleotide repeat polymorphism at D17S846 maps within 40 kb of GAS at 17q12-q22. Hum Mol Genet. 1993 Jul;2(7):1080–1080. doi: 10.1093/hmg/2.7.1080. [DOI] [PubMed] [Google Scholar]
- Flejter W. L., Watkins M., Abel K. J., Chandrasekharappa S. C., Weber B. L., Collins F. S., Glover T. W. Isolation and characterization of somatic cell hybrids with breakpoints spanning 17q22-->q24. Cytogenet Cell Genet. 1993;64(3-4):222–223. doi: 10.1159/000133581. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Goldschmidt M. D., Zimbelmann R., Mueller H. M., Schiller D. L., Cowin P. Molecular cloning and amino acid sequence of human plakoglobin, the common junctional plaque protein. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4027–4031. doi: 10.1073/pnas.86.11.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y., Nakatsuru S., Nagafuchi A., Tsukita S., Muto T., Nakamura Y., Horii A. Structure, expression and chromosome assignment of the human catenin (cadherin-associated protein) alpha 1 gene (CTNNA1). Cytogenet Cell Genet. 1994;65(1-2):74–78. doi: 10.1159/000133603. [DOI] [PubMed] [Google Scholar]
- Futreal P. A., Liu Q., Shattuck-Eidens D., Cochran C., Harshman K., Tavtigian S., Bennett L. M., Haugen-Strano A., Swensen J., Miki Y. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994 Oct 7;266(5182):120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- Hinck L., Näthke I. S., Papkoff J., Nelson W. J. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994 Jun;125(6):1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoschuetzky H., Aberle H., Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994 Dec;127(5):1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993 Sep;9(9):317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Knudsen K. A., Wheelock M. J. Plakoglobin, or an 83-kD homologue distinct from beta-catenin, interacts with E-cadherin and N-cadherin. J Cell Biol. 1992 Aug;118(3):671–679. doi: 10.1083/jcb.118.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C., Liehr T., Hülsken J., Behrens J., Birchmeier W., Grzeschik K. H., Ballhausen W. G. Localization of the human beta-catenin gene (CTNNB1) to 3p21: a region implicated in tumor development. Genomics. 1994 Sep 1;23(1):272–274. doi: 10.1006/geno.1994.1493. [DOI] [PubMed] [Google Scholar]
- Mathur M., Goodwin L., Cowin P. Interactions of the cytoplasmic domain of the desmosomal cadherin Dsg1 with plakoglobin. J Biol Chem. 1994 May 13;269(19):14075–14080. [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Neuhausen S. L., Swensen J., Miki Y., Liu Q., Tavtigian S., Shattuck-Eidens D., Kamb A., Hobbs M. R., Gingrich J., Shizuya H. A P1-based physical map of the region from D17S776 to D17S78 containing the breast cancer susceptibility gene BRCA1. Hum Mol Genet. 1994 Nov;3(11):1919–1926. doi: 10.1093/hmg/3.11.1919. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard J., Feunteun J., Lenoir G., Tonin P., Normand T., Luu The V., Vivier A., Lasko D., Morgan K., Rouleau G. A. Genetic mapping of the breast-ovarian cancer syndrome to a small interval on chromosome 17q12-21: exclusion of candidate genes EDH17B2 and RARA. Hum Mol Genet. 1993 Aug;2(8):1193–1199. doi: 10.1093/hmg/2.8.1193. [DOI] [PubMed] [Google Scholar]
- Su L. K., Vogelstein B., Kinzler K. W. Association of the APC tumor suppressor protein with catenins. Science. 1993 Dec 10;262(5140):1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- van Tuinen P., Rich D. C., Summers K. M., Ledbetter D. H. Regional mapping panel for human chromosome 17: application to neurofibromatosis type 1. Genomics. 1987 Dec;1(4):374–381. doi: 10.1016/0888-7543(87)90042-5. [DOI] [PubMed] [Google Scholar]