Abstract
Defective apoptosis is a fundamental hallmark feature of CLL biology and is a major target of cancer therapy development. High levels of Bcl-2 family anti-apoptotic proteins are considered primarily responsible for inhibiting apoptosis in CLL cells. While several approaches were considered to selectively inhibit Bcl-2 family anti-apoptotic proteins, the discovery that gossypol binds and antagonizes anti-apoptotic effect of Bcl-2 family proteins was a major breakthrough in identifying specific Bcl-2 antagonists. The concept of mimicking BH3 domain emphasized the importance of Bcl-2 family-targeted therapy that can modulate the function of anti-apoptotic proteins. Although parent compound gossypol did not sustain in the clinic, its structural modifications led to the development of additional analogues that demonstrated improved efficacy and reduced toxicity in preclinical and clinical investigations. Proof of concept of this hypothesis was demonstrated by structure based BH3 mimetic ABT-737 that has shown greater cytotoxicity towards CLL cells both in pre-clinical models and clinical trials. Its oral compound ABT-263 has demonstrated the substantial susceptibility of chronic lymphocytic leukemia cells through Bcl-2 inhibition. Collectively, results of a Phase I Study of Navitoclax (ABT-263) in patients with relapsed or refractory disease warrants Bcl-2 as a valid therapeutic target in CLL. Importantly, molecules that mimic pro-apoptotic BH3 domains represent a direct approach to overcoming the protective effects of anti-apoptotic proteins such as Mcl-1, Bcl-2 and Bcl-XL.
Keywords: natural products, BH3 mimetics, Gossypol, CLL, apoptosis
Introduction
For several decades, plant products have been an excellent source of anti-cancer agents. They have been in use as a single agent or in combination with other chemotherapeutic drugs for the treatment of cancers, including liquid and solid tumors (as reviewed in [1]). Vinca alkaloids such as vinblastine and vincristine obtained from plant Madagascar periwinkle, Catharanthus roseus (formerly known as Vinca rosea, Apocynaceae) were the first natural products advanced into clinical use for the treatment of cancer [1]. The isolation of paclitaxel (Taxol) from the bark of the Pacific Yew tree, Taxusbrevifolia Nutt belonging to the family Taxaceae, is another evidence of the success in natural product drug discovery [2, 3]. Camptothecin, isolated from the Chinese ornamental tree Camptotheca acuminate Decne (Nyssaceae) known in China as tree of joy, was advanced to clinical trials by NCI, but was dropped because of severe bladder toxicity. Etoposide and teniposide are two semi-synthetic derivatives of epipodophyllotoxin, an isomer of podophyllotoxin isolated from the roots of Podophyllum species, Podophyllum peltatum Linnaeus and Podophyllumemodi Wallich (Berberidaceae) [4] and are used in the treatment of lymphomas and other cancers [5]. Homoharringtonine obtained from the Chinese tree Cephalotaxus harringtonia var. drupacea (Sieb and Zucc.) (Cephalotaxaceae), is another plant-derived product in clinical use [6]. A racemic mixture of harringtonine and homoharringtonine has been used successfully for the treatment of acute myelogenous leukemia (AML) and chronic myelogenous leukemia (CML) [7]. Flavopiridol is a synthetic flavone, derived from the plant alkaloid rohitukine, which was isolated from Dysoxylum binectariferum Hook. f. (Meliaceae)[8] and tested in phase I and II clinical trials against a broad range of tumors [9]. Synthetic agent roscovitine which is derived from natural product olomucine, originally isolated from Raphanus sativus L. (Brassicaceae), is in Phase II clinical trials in Europe [10]. Combretastatin A-4 isolated from the bark of the South African tree Combretum caffrum (Eckl. &Zeyh.) Kuntze (Combretaceae) [11], is active against solid and hematological malignancies. Together, natural products have proven useful by themselves as anti-cancer agents or have been a great source of synthetic or semisynthetic derivatives for preclinical investigations and/or clinical trials.
Cotton plant and gossypol
Gossypol is a polyphenolic aldehyde derived from the cotton plant (Gossypium hirsutum L. Family Malvaceae, Fig. 1). It was originally discovered by Longmore and was later structurally elucidated by Adams and Edwards [12, 13]. Chemically it is 2-2′ bis(formyl-1,6,7-trihydroxy-5-isopropyl-3-methyl)-naphathalene. Gossypol inherently displayed a broad spectrum of physiochemical and biological properties such as insecticidal activity, anti-oxidant property, anti-fertility property and anti-cancer activity [14–16]. It also exhibited cytotoxic effect against various carcinoma cell lines both in vitro and in vivo settings [17–20]. Extensive investigations on gossypol had revealed its diversified mechanisms of action, which include inhibitory role on enzyme LDH [21], protein kinase C activity [22], DNA synthesis inhibition [23], regulation on cell cycle proteins Rb and cyclin D1 [24], cellular proliferation [25], ROS independent mitochondrial pathway of apoptosis [26], execution of extrinsic cell death pathway through up-regulation of Fas/Fas ligand [27], Bax or Bax/Bak independent activation of apoptosis [28], suppression of NF-κB activity [29] and induction of autophagy [30, 31]. In early clinical trials, racemic gossypol administration to patients with various cancers demonstrated that gossypol was well tolerated with minimal clinical efficacy [32–34].
Figure 1.

Cotton plant photographs; obtained from online website.
Gossypol as a BH3 mimetic
Over-expression of anti-apoptotic B-cell lymphocyte/leukemia-2 (Bcl-2) family proteins is common in many human cancers and is a major target of cancer therapy development [35]. Besides all the investigations on gossypol, the discovery that gossypol binds and antagonizes anti-apoptotic effect of Bcl-2 family proteins and induces apoptosis in cancer cells was a major breakthrough in modulating the function of Bcl-2 [36]. On the basis of in vitro displacement assays with the fluorescein-labeled BH3 peptide, Kitada et al demonstrated that gossypol directly interacts with Bcl-XL and is able to displace BH3 peptides with an IC50 of 0.5 μM [36] (Fig. 2). Given that Bcl-XL is highly expressed in several hematological malignancies, gossypol was able to overcome the apoptotic resistance mediated by Bcl-XL in CML [37] as well as in CLL. In vitro study on primary CLL lymphocytes demonstrated that gossypol at micromolar levels induced caspase independent, AIF-mediated apoptosis in all samples tested irrespective of the disease stage or prognostic markers (Fig. 3A)[38]. Further investigations illustrated that gossypol is the only compound that inhibited major anti-apoptotic proteins such as Bcl-2, Bcl-XL, Mcl-1, Bcl-B with IC50 values of 0.28, 3.03, 1.75, 0.36 respectively, as well as Bcl-W (1.4 μM) whose prime function is to sustain developing sperm cells [39–43].
Figure 2.
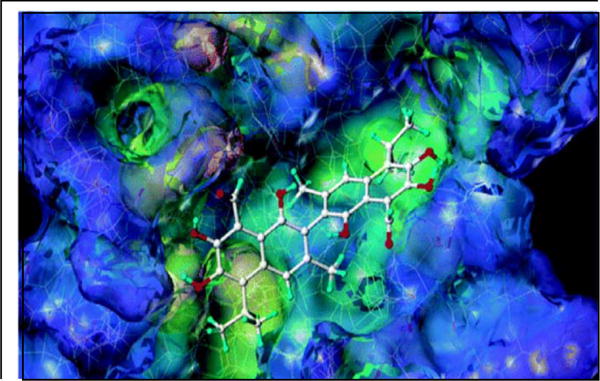
Surface representation of Bcl-xL with the docked structure of Gossypol obtained by FlexX. The surface is depicted according to cavity depth (blue, surface exposed; yellow, buried) representation. Reprinted (adapted) with permission from (J. Med. Chem., 2003, 46 (20), pp 4259–4264). Copyright (2003) American Chemical Society.
Figure 3.
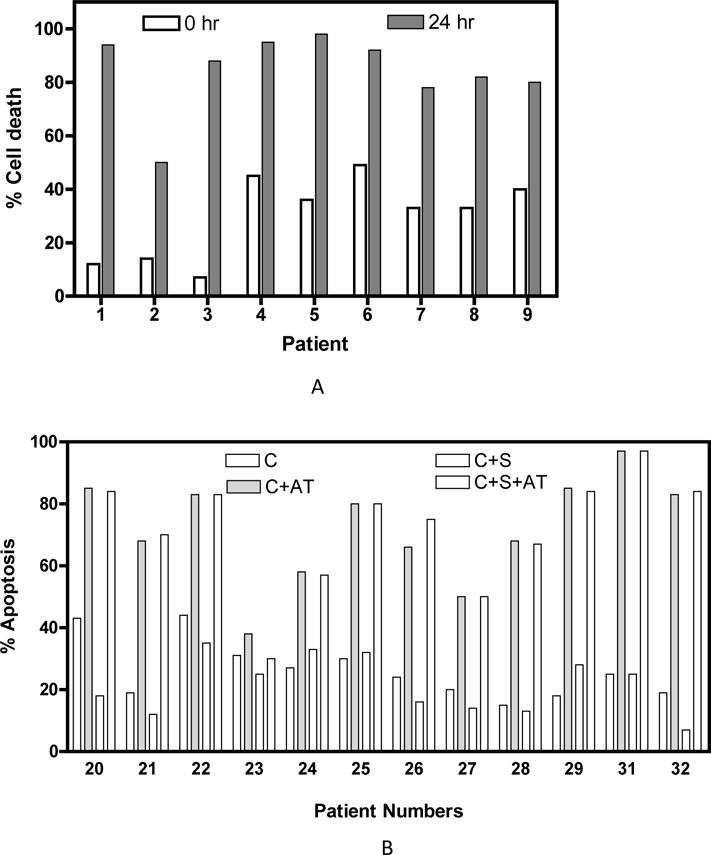
A. Percentage of cell death in CLL primary cells from 9 individual patients treated with gossypol for the indicated time. This research was originally published in Blood. Balakrishnan et al. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells, Blood, 2008; 112(5):1971–80.© The American Society of Hematology. B. AT-101 circumvents the stromal mediated CLL cell survival. CLL lymphocytes from patients (n = 12) were cultured either in suspension medium (C) in suspension medium with 20 μM AT-101 (C + AT), or with stromal cells in the absence (C + S) or presence of AT-101 (C + S + AT) and the apoptosis was measured after 24 hours (72 hours for samples from patients 31 and 32) by annexin-binding assay. This research was originally published in Blood. Balakrishnan et al. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood, 2009;113(1):149–53.© The American Society of Hematology.
Despite the broad spectrum of functionalities, toxic properties associated with hypokalemia and its effect on Bcl-W leading to male infertility limited gossypol’s application in clinical use. Though gossypol is unlikely candidate for clinic, it represented a lead compound for generation of a new class of antineoplastic agents [44]. Several efforts were undertaken to detoxify the compound by altering its structure to produce analogues with improved efficacy and/or reduced toxicity. Structural modification of gossypol guided by a model of multidimensional nuclear magnetic resonance based structural analysis led to the development of additional analogues of gossypol with improved efficacy. The rest of this review is devoted to discuss various gossypol derivatives and their efficacy in preclinical and clinical investigations.
AT-101
Gossypol naturally exists as a mixture of two enantiomers (+) and (−) that exhibit different biological activity. It was notable that (−)-gossypol also called AT-101, developed by Ascenta Pharmaceuticals, was approximately twice as active as racemic gossypol with added oral bio-availability. Similar to gossypol, AT-101 contains two reactive aldehyde groups and also binds to the BH3 motif of all major anti-apoptotic proteins, with better affinity (e.g., 0.32, 0.48, and 0.18 μM for Bcl-2, Bcl-XL, and Mcl-1, respectively) [45]. Studies evaluating the therapeutic response of AT-101 with respect to the effect of protective stromal cells demonstrated that AT-101 can completely overcome stroma mediated protectivity in CLL primary cells (Fig. 3B) [46].
There was a differential mechanism for survival advantage provided by two distinct microenvironments; enhanced cell survival was mediated by Mcl-1 protein induction in bone marrow microenvironment, while nurse like cells (a representative lymph node microenvironment) protected CLL cells via augmentation of Bfl-1 protein [46–49]. Targeting Bcl-2 family proteins with AT-101 markedly enhanced the therapeutic effects of several chemotherapeutic agents such as cyclophosphamide and rituximab both in vitro and in vivo models of B-cell lymphomas [50]. A recent report states that AT-101 not only triggers Bax activation but also induces mitochondrial SMAC release to enhance Bax-mediated cellular apoptosis [51]. In clinical study of AT-101 with topotecan in relapsed and refractory small-cell lung cancer [52] was not active but showed promise in the double blind placebo controlled randomized phase II study of AT-101 plus docetaxel in non-small cell lung carcinoma patients [53].
Apogossypol
Given that gossypol and AT-101 have toxicity problems likely due to two reactive aldehyde groups at 8, 8′-positions on the naphthalene rings, a semi-synthetic derivative apogossypol, was synthesized lacking two aldehyde groups, with enhanced activity and reduced toxicity [41, 54, 55]. Apogossypol and gossypol are shown to exhibit similar oral and intravenous pharmacokinetic profiles as well as in vitro stability, although apogossypol demonstrated a slower clearance rate, larger AUC (area under curve), and better microsomal stability [55, 56] suggesting favorable pharmacokinetics for this analogue.
Because gossypol enantiomers displayed differential pro-apoptotic activities, atropisomers of apogossypol were synthesized, evaluated and compared with racemic apogossypol for cellular activity [54]. 5, 5′ substituted ketone and amide apogossypol derivatives such as BI-79D10, compound 8r and BI-97C1 (sabutoclax) were synthesized. Each compound was subsequently tested for its ability to inhibit Bcl-XL in an in vitro fluorescence polarization competition assay as well as for its pro-apoptotic activity in human cancer cell lines. The potent compound BI-79D10 was shown to bind to Bcl-XL, Bcl-2, and Mcl-1 with IC50 values of 190, 360, and 520 nmol/L, respectively. It inhibited cell growth in the human lung cancer cell line with an EC50 value of 680 nmol/Land induced apoptosis in human lymphoma cell line. This compound had improved plasma and microsomal stability relative to apogossypol and showed little cytotoxicity against Bax/Bak-/- mouse embryonic fibroblast cells [57]. Compound 8r inhibited the binding of BH3 peptides to Bcl-XL, Bcl-2, Mcl-1, and Bfl-1 with IC50 values of 0.76, 0.32, 0.28, and 0.73 μM, respectively. This compound also potently inhibited cell growth of human lung cancer and human B-cell lymphoma cell lines in vitro and displayed efficacy in transgenic mice in which Bcl-2 is overexpressed in splenic B-cells. Similar to BI-79D10, 84 is also stable and appears to be a promising drug lead [58].
A new analogue, BI-97C1, an optically pure and most potent diastereoisomer of compound 8r, inhibited the binding of BH3 peptides to Bcl-XL, Bcl-2, Mcl-1, and Bfl-1 with IC50 values of 0.31, 0.32, 0.20, and 0.62 μM, respectively. The compound potently inhibited cell growth of human prostate cancer, lung cancer, and lymphoma cell lines with EC50 values of 0.13, 0.56, and 0.049 μM, respectively. This analog displayed in vivo efficacy in transgenic mice models and also demonstrated superior single-agent antitumor efficacy in a prostate cancer xenograft model. In prostate cancer cells, BI-97C1 (sabutoclax) sensitized MDA7/IL-24 mediated toxicity as well as exerted significantly improved therapeutic response in colorectal cancer patients in combination with AD5/3-MDA7 [59, 60]. Together, apogossypol and its analogues have demonstrated efficacy in preclinical studies, but they have not yet made to clinical trials.
Gossypolone and apogossypolone
Gossypolone, a major metabolite of AT-101, was synthesized without hydroxyl group upon oxidation reaction. It displayed similar cytotoxic effects as AT-101, with greater water solubility, lower toxicity with anti-proliferative activity against human cancer cell lines [61, 62]. Apogossypolone also known as ApoG2 is an analogue of gossypolone with three- to six-fold more potency than the parent compound (-)-gossypol. It was synthesized by the removal of hydroxyl and two reactive aldehyde groups to improve stability and reduce toxicity [63]. ApoG2 demonstrated a higher binding affinity to its targets (Ki, 35, 660, and 25 nM for Bcl-2, Bcl-XL, and Mcl-1, respectively) [64] suggesting to have high inhibitory constants for Mcl-1 and Bcl-2, but not for Bcl-XL and induced apoptosis in number of cancer cell lines by blocking binding of Bim and Bcl-2. Preclinical studies of apogossypolone in follicular lymphoma exhibited growth inhibitory effects in vitro and in vivo in SCID xenograft models through activation of intrinsic and extrinsic caspases and release of AIF [65, 66]. In CLL, Bax/Bak was required for apogossypolone induced cell death[67]. Head to head comparison of apogossypolone with gossypol revealed that ApoG2 was more stable and better tolerated by mice than was racemic gossypol, with no toxicity on peripheral blood lymphocytes [68]. ApoG2 has also been shown to potently disturb the proliferation of nasopharyngeal carcinoma cells by suppressing the c-Myc signaling pathway [69]. In vivo, it induced regression in several tumor xenograft models and its maximum tolerated dose (MTD) was comparatively higher than the MTD of AT-101. Given that 5,5′ substituted apogossypol derivatives displayed improved in vitro and in vivo activities compared to apogossypol, derivatives of 5,5′-substituted apogossypolone were synthesized by replacing their isopropyl groups with alkyl, ketone and amide groups, which resulted in compounds with improved biological activities [70].
Concept - to - clinic
The mechanistic approach of identifying novel agents to induce pan-inhibition of anti-apoptotic proteins opened a new avenue of successfully treating diseases that inherit high levels of Bcl-2 family proteins. Although natural product gossypol and its derivatives were not effective by themselves in the clinic, the concept of employing BH3 mimetics to modulate the function of Bcl-2 has been quite successful. Two novel Bcl-2 inhibitors obatoclax and ABT-737 have represented the proof-of- principle of this approach in clinic.
Obatoclax
Obatoclax (GX015-070; Geminx) is a hydrophobic molecule that was specifically designed to inhibit all relevant anti-apoptotic members of the Bcl-2 family (IC50 of Bcl-2, Bcl-XL, Mcl-1, was 1.11, 4.69, 2.9 μM respectively)[39]. Preclinical studies with single agent obatoclax demonstrated that GX015-070 antagonizes Mcl-1 and overcomes Mcl-1-mediated resistance to apoptosis [71]. Further studies revealed that obatoclax demonstrate a promising role in apoptosis induction in a variety of hematological malignancies such as multiple myeloma [72], AML [73], CLL [74, 75], MCL [76] and solid tumors like breast [77] and pancreatic cancers [78]. The mechanism of action of obatoclax was not limited to apoptosis as it was effective in inducing autophagy in several cancer cells [79, 80]. Since it is a pan Bcl-2 family inhibitor it has the advantage to be rationally combined with other targeted anti-cancer agents such as ABT-737 [73], HDAC inhibitors [79], sorafinib [81], TRAIL [82] and proteasome inhibitor, bortezomib.
Obatoclax is first in its class of BH3 mimetics to enter clinical trials. Multiple phase I and phase II trials evaluating safety profile and MTD in patients with refractory leukemia and myelodysplasia demonstrated tolerability of obatoclax by IV infusion [83]. Phase I study of single agent obatoclax in 26 heavily pretreated CLL patients at doses ranging from 3.5–14 mg/m2 showed modest clinical activity through Bax mediated mechanism of apoptosis [84]. A phase II study of obatoclax in combination with topotecan in patients with relapsed SCLC demonstrated that this combination was not better in response rate than topotecan alone [85]. Clinical investigations in relapsed or refractory classical Hodgkin’s lymphoma demonstrated that obatoclax has limited clinical activity [86]. Together these results imply that a need for less toxic and better targeted Bcl-2 antagonists in the clinic is pressing.
ABT-737
ABT-737 is a cell permeant small molecule synthesized to bind to the hydrophobic BH3 binding groove of anti-apoptotic proteins with higher affinity (Ki ≤ 1 nM) to Bcl-XL, Bcl-2 and Bcl-W, but not to proteins Bcl-B, Mcl-1 and Bfl-1 (Ki= 0.46 ± 0.11 μM, >1 μM and >1 μM, respectively). With plasma protein binding, this compound had lower affinity to Bcl-2 and Bcl-XL (100 nM and 35 nM respectively) [87, 88]. Of all the putative BH3 mimetics in the field, ABT-737 was the only agent that was shown to specifically target Bcl-2 proteins by directly activating the cell death machinery via Bax/Bak activation [89]. ABT-737 reactivated program cell death in vitro and in vivo (xenograft models) both in solid tumors as well as hematological malignancies such as MCL [90], CML [91], AML [92], CLL [93, 94], MM [95, 96] and ALL [97, 98]; all of them express high levels of Bcl-2 family anti-apoptotic proteins. Overall, this compound predominantly induces apoptosis via intrinsic pathway by disrupting Bcl-2/Bax association [92] or dissociating Bim from Bcl-2 [99], thus typically representing the function of a BH3 only protein either by activating pro-apoptotic Bcl-2 proteins (Bak/Bax) or by inhibiting anti-apoptotic protein functions (Bcl-2/Bcl-XL).
However, as described below, ABT-737 has some limitations. Unlike other pan Bcl-2 inhibitors, ABT-737 does not bind to other members of Bcl-2 family, such as Mcl-1 or Bfl-1 [100]. Seed analysis of off-target siRNAs revealed an essential role of Mcl-1 in resistance to ABT-737 [101]. Several mechanistic studies showed that targeting proteins that critically stabilizes Mcl-1 via inhibiting ubiqitination enhanced the sensitivity of ABT-737 [102, 103]. Accordingly, multiple strategies were employed to neutralize Mcl-1 and potentiate ABT-737 to cells for apoptosis such as combinations with obtaoclax, a pan Bcl-2 inhibitor, sorafinib, bortezomib [104], HDAC inhibitor [105] and numerous-other chemotherapeutic agents [106]. Combination with homoharringtonine, a protein translation inhibitor that reduces Mcl-1 protein [107], or co-administration of roscovitine [108], a CDK inhibitor that decreases Mcl-1 transcript and protein levels-demonstrated synergy with ABT-737.
Extensive studies on CLL primary cells showed nM efficacy of ABT-737 (EC50 of 4.5 ± 2.2 nM) in displacing Bim from Bcl-2 to induce cell death suggesting Bcl-2 complexed to Bim is the critical target for ABT-737 in CLL [93]. Even though it was speculated that BH3 mimetics should function without p53, CLL primary cells with p53 deletion or dysfunction showed decreased sensitivity to ABT-737, while combination with nutlin showed synergy implying the significance of p53 in ABT-induced apoptosis [109]. Furthermore, ABT-737 also was shown to overcome the lymph node-mediated (CD40-stimulated) CLL cell survival via balancing the NOXA/Mcl1 axis [110]. Studies using gene-targeted mouse strains demonstrated that while ABT-737 avidly bind to Bcl-2, Bcl-XL and Bcl-W in vitro, it was found that only Bcl-2 is its critical target in vivo suggesting that tumors exclusively over-expressing Bcl-2 are most likely to benefit [111].
ABT-263
A major limitation of ABT-737 is that it is not orally bioavailable. ABT-263 (Navitoclax) was synthesized with oral properties and first tested in xenograft model and was reported to disrupt Bcl-2/Bcl-XL interactions with pro-death proteins (e.g., Bim), leading to the initiation of apoptosis within 2 hours post treatment [112]. Human cancer cells and a panel of SCLC xenograft models were tested for dose and schedule evaluation and combined with standard cytotoxic agents for induction of apoptosis [113, 114]. Phase I study of ABT-263 in patients with SCLC and other solid tumors demonstrated that navitoclax is safe and well tolerated, with dose-dependent thrombocytopenia as the major adverse effect [115]. Patients with relapsed or refractory lymphoid malignancies (n=55) were enrolled in a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and anti-tumor activity of ABT-263, demonstrating that Navitoclax has a novel mechanism of peripheral thrombocytopenia and T-cell lymphopenia, attributable to high-affinity inhibition of Bcl-XL and Bcl-2, respectively[116]. A phase II study of single agentABT-263 to evaluate safety and toxicity, response rate, progression free and overall survival revealed that the baseline levels of biomarkers correlated with clinical benefit in SCLC patients [117]. Recent report obtained from clinical trials conducted in patients with relapsed or refractory CLL revealed that lymphocytosis was reduced by more than 50%. Importantly, clinical activity was indeed observed in patients with fludarabine-refractory disease, bulky adenopathy, and (17p) del subset of CLL. Consistent to preclinical data, low Mcl1 expression and high Bim: Mcl1or Bcl-2 ratios correlated with clinical response [118]. The data so far obtained from all clinical studies with ABT-263 have demonstrated favorable anti-leukemic activity in many cancers except it induces a rapid but reversible thrombocytopenia.
ABT-199
Navitoclax is a potent Bcl-XL inhibitor, circulating platelet survival is dependent on Bcl-XL, inhibiting Bcl-XL also destroyed platelets leading to thrombocytopenia, a dose limiting side effect. So the goal was to develop an agent that inhibits mostly Bcl-2, a critical survival factor for cancer cells, while sparing Bcl-XL that is important for survival of circulating platelets. ABT-199 is a reverse engineered version of ABT-263 designed to have high affinity for Bcl-2, but low affinity for Bcl-XL. Given that Bcl-2 is a validated drug target for CLL, this provided a solid scientific rationale for using it in clinical trials for CLL. A Phase 1, open-label, multicenter study evaluating the safety and pharmacokinetics profile of ABT-199 in relapsed or refractory chronic lymphocytic leukemia (CLL) and non-Hodgkin’s lymphoma (NHL) is ongoing[119].
Conclusion and Future Approaches
For several years the hallmark of cancer treatment has been the use of traditional chemotherapy. These cytotoxic agents target rapidly dividing cells including certain normal cells thus lacking specificity and/or selectively. Although conventional chemotherapy remains the treatment of choice for many malignancies, targeted therapies are now a component of treatment for many types of cancers. In the past decade, of the new anticancer drugs approved by FDA, 15 have been targeted therapies, compared with only five traditional chemotherapeutic agents[120].
Given that CLL is replicationally quiescent disease and its prognosis exclusively depends upon the expression of Bcl-2 family anti-apoptotic proteins, agents that neutralize the anti-apoptotic properties of molecular targets could be a more defined choice of treatment for this disease. There have been several agents synthesized and tested for inhibition of Bcl-2 family anti-apoptotic proteins. Maritoclax was specifically designed to inhibit the protein Mcl-1, the major anti-apoptotic target of CLL [121]. ABT-199 was synthesized to have more inhibitory effect on Bcl-2 in comparison to Bcl-XL. Bad-like BH3 mimetic ABT-263 or ABT-737 that lack ability to bind to Mcl1 is confirmed to be a true BH3 mimetic that induce Bax/Bak dependent apoptosis. Several putative BH3 mimetics such as GX15-070, TW37, gossypol and analogues were tested and disclosed as pan Bcl-2 family anti-apoptotic protein inhibitors (Table 1). Since Bcl-2 protein also interacts with autophagic protein such as BECLIN1, pharmacological BH3 mimetics competitively disrupt the inhibitory interaction between Beclin-1 and Bcl-2 or Bcl-XL, thereby stimulating autophagy. Thus, BH3 mimetics have the ability to trigger both apoptotic and autophagic response machineries. Additionally, combining BH3 mimetic with chemotherapeutic agents sensitized cancer cells better than single agent alone. These statements provide strong evidence upon the notion that targeted therapeutics; particularly Bcl-2 antagonists have optimistic future in the clinic. It could add more strength to the therapeutic index if the agent is a derivative of natural product, which in turn spares normal cells. Promoting further investigations on combination with B-cell receptor kinase inhibitors that exhibit promising activity in preclinical and clinical trials (CAL-101, R406, BTK inhibitions) should infer additional insights on the mechanism of actions of these agents.
Table 1.
overview of Bcl-2 family antagonists
| BH3 mimetic | TARGETS | PRECLINICAL | CLINICAL |
|---|---|---|---|
| Gossypol[20] | Bcl-2, XL, Mcl-1, Bcl-w, Bcl-b, BFL1 | Yes | Yes |
| AT-101[52] | Bcl-2, XL, Mcl-1, Bcl-w, Bcl-b, BFL1 | Yes | Yes |
| Apogossypol | Bcl-2, XL, Mcl-1, Bclw, Bclb | Yes | No |
| BI-79D10[57] | Bcl-2, XL, Mcl-1 | Yes | No |
| Compound r[58] | Bcl-2, XL, Mcl-1, BFL-1 | Yes = | No |
| BI-97C1[120] Sabutoclax | Bcl-2, XL, Mcl-1, BFL-1 | Yes | No |
| Gossypolone[61] | Bcl-2, XL, Mcl-1, Bcl-w, Bcl-b | Yes | No |
| Apogossypolone (ApoG2)[65] | Bcl-2, XL, Mcl-1 | Yes | No |
| Obatoclax[83] | Bcl-2, XL, Mcl-1, Bcl-w, Bcl-b, BFL-1 | Yes | Yes |
| ABT-737[92] | Bcl-2, XL, Bcl-W | Yes | No |
| ABT-263[121] Navitoclax | Bcl-2, XL, Bcl-W | Yes | Yes |
| ABT-199 | Bcl-2 | Yes | Yes |
| Maritoclax[119] | Mcl-1 | Yes | No |
| Bims2A [122] | Mcl-1 | No | No |
| Mcl-1 SAHB[123] | Mcl-1 | No | No |
| TW 37[45] | Bcl-2, XL, Mcl-1 | Yes | No |
Footnotes
Conflict of interest: This work is supported in part by a CLL Consortium grant CA81534 from NCI and a CLL Global Research Foundation grant.
Conflict-of-interest disclosure: The authors declare that they have no conflict of interest.
References
- 1.Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–9. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Rowinsky EK, Onetto N, Canetta RM, Arbuck SG. Taxol: the first of the taxanes, an important new class of antitumor agents. Semin Oncol. 1992;19:646–62. [PubMed] [Google Scholar]
- 3.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–7. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 4.Stahelin H. Activity of a new glycosidic lignan derivative (VP 16-213) related to podophyllotoxin in experimental tumors. Eur J Cancer. 1973;9:215–21. doi: 10.1016/s0014-2964(73)80021-0. [DOI] [PubMed] [Google Scholar]
- 5.Harvey AL. Medicines from nature: are natural products still relevant to drug discovery? Trends Pharmacol Sci. 1999;20:196–8. doi: 10.1016/s0165-6147(99)01346-2. [DOI] [PubMed] [Google Scholar]
- 6.Powell RG, Weisleder D, Smith CR, Jr, Rohwedder WK. Structures of harringtonine, isoharringtonine, and homoharringtonine. Tetrahedron Lett. 1970:815–8. doi: 10.1016/s0040-4039(01)97839-6. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, O’Brien S, Anderlini P, Talpaz M. Treatment of myelogenous leukemia: current status and investigational options. Blood. 1996;87:3069–81. [PubMed] [Google Scholar]
- 8.Kelland LR. Flavopiridol, the first cyclin-dependent kinase inhibitor to enter the clinic: current status. Expert Opin Investig Drugs. 2000;9:2903–11. doi: 10.1517/13543784.9.12.2903. [DOI] [PubMed] [Google Scholar]
- 9.Christian MC, Pluda JM, Ho PT, Arbuck SG, Murgo AJ, Sausville EA. Promising new agents under development by the Division of Cancer Treatment, Diagnosis, and Centers of the National Cancer Institute. Semin Oncol. 1997;24:219–40. [PubMed] [Google Scholar]
- 10.Meijer L, Raymond E. Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Acc Chem Res. 2003;36:417–25. doi: 10.1021/ar0201198. [DOI] [PubMed] [Google Scholar]
- 11.Pettit GR, Singh SB, Niven ML, Hamel E, Schmidt JM. Isolation, structure, and synthesis of combretastatins A-1 and B-1, potent new inhibitors of microtubule assembly, derived from Combretum caffrum. J Nat Prod. 1987;50:119–31. doi: 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- 12.JD E. Total synthesis of gossypol. J Am Chem Soc. 1958;80:3798–3799. [Google Scholar]
- 13.Adams RMR, Geissman TA, Butterbaugh DJ, Kirkpatrick EC. Structure of gossypol. An interpretation of its reaction. J Am Chem Soc. 1938;60:2193–2203. [Google Scholar]
- 14.Wu D. An overview of the clinical pharmacology and therapeutic potential of gossypol as a male contraceptive agent and in gynaecological disease. Drugs. 1989;38:333–41. doi: 10.2165/00003495-198938030-00001. [DOI] [PubMed] [Google Scholar]
- 15.Waites GM, Wang C, Griffin PD. Gossypol: reasons for its failure to be accepted as a safe, reversible male antifertility drug. Int J Androl. 1998;21:8–12. doi: 10.1046/j.1365-2605.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 16.Coutinho EM. Gossypol: a contraceptive for men. Contraception. 2002;65:259–63. doi: 10.1016/s0010-7824(02)00294-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, et al. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochemical pharmacology. 2003;66:93–103. doi: 10.1016/s0006-2952(03)00248-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Liu H, Tian Z, Griffith BN, Ji M, Li QQ. Gossypol induces apoptosis in human PC-3 prostate cancer cells by modulating caspase-dependent and caspase-independent cell death pathways. Life sciences. 2007;80:767–74. doi: 10.1016/j.lfs.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Oliver CL, Bauer JA, Wolter KG, Ubell ML, Narayan A, O’Connell KM, et al. In vitro effects of the BH3 mimetic, (-)-gossypol, on head and neck squamous cell carcinoma cells. Clin Cancer Res. 2004;10:7757–63. doi: 10.1158/1078-0432.CCR-04-0551. [DOI] [PubMed] [Google Scholar]
- 20.Van Poznak C, Seidman AD, Reidenberg MM, Moasser MM, Sklarin N, Van Zee K, et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat. 2001;66:239–48. doi: 10.1023/a:1010686204736. [DOI] [PubMed] [Google Scholar]
- 21.Tso WW, Lee CS. Lactate dehydrogenase-X: an isozyme particularly sensitive to gossypol inhibition. Int J Androl. 1982;5:205–9. doi: 10.1111/j.1365-2605.1982.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 22.Teng CS. Gossypol-induced apoptotic DNA fragmentation correlates with inhibited protein kinase C activity in spermatocytes. Contraception. 1995;52:389–95. doi: 10.1016/0010-7824(95)00227-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Rao PN. Effect of gossypol on DNA synthesis and cell cycle progression of mammalian cells in vitro. Cancer research. 1984;44:35–8. [PubMed] [Google Scholar]
- 24.Ligueros M, Jeoung D, Tang B, Hochhauser D, Reidenberg MM, Sonenberg M. Gossypol inhibition of mitosis, cyclin D1 and Rb protein in human mammary cancer cells and cyclin-D1 transfected human fibrosarcoma cells. Br J Cancer. 1997;76:21–8. doi: 10.1038/bjc.1997.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shidaifat F, Canatan H, Kulp SK, Sugimoto Y, Zhang Y, Brueggemeier RW, et al. Gossypol arrests human benign prostatic hyperplastic cell growth at G0/G1 phase of the cell cycle. Anticancer Res. 1997;17:1003–9. [PubMed] [Google Scholar]
- 26.Hou DX, Uto T, Tong X, Takeshita T, Tanigawa S, Imamura I, et al. Involvement of reactive oxygen species-independent mitochondrial pathway in gossypol-induced apoptosis. Archives of biochemistry and biophysics. 2004;428:179–87. doi: 10.1016/j.abb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Chang JS, Hsu YL, Kuo PL, Chiang LC, Lin CC. Upregulation of Fas/Fas ligand-mediated apoptosis by gossypol in an immortalized human alveolar lung cancer cell line. Clinical and experimental pharmacology & physiology. 2004;31:716–22. doi: 10.1111/j.1440-1681.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- 28.Lei X, Chen Y, Du G, Yu W, Wang X, Qu H, et al. Gossypol induces Bax/Bak-independent activation of apoptosis and cytochrome c release via a conformational change in Bcl-2. Faseb J. 2006;20:2147–9. doi: 10.1096/fj.05-5665fje. [DOI] [PubMed] [Google Scholar]
- 29.Moon DO, Kim MO, Lee JD, Kim GY. Gossypol suppresses NF-kappaB activity and NF-kappaB-related gene expression in human leukemia U937 cells. Cancer letters. 2008;264:192–200. doi: 10.1016/j.canlet.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Lian J, Wu X, He F, Karnak D, Tang W, Meng Y, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ. 2011;18:60–71. doi: 10.1038/cdd.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao P, Bauvy C, Souquere S, Tonelli G, Liu L, Zhu Y, et al. The Bcl-2 homology domain 3 mimetic gossypol induces both Beclin 1-dependent and Beclin 1-independent cytoprotective autophagy in cancer cells. J Biol Chem. 2010;285:25570–81. doi: 10.1074/jbc.M110.118125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flack MR, Pyle RG, Mullen NM, Lorenzo B, Wu YW, Knazek RA, et al. Oral gossypol in the treatment of metastatic adrenal cancer. J Clin Endocrinol Metab. 1993;76:1019–24. doi: 10.1210/jcem.76.4.8473376. [DOI] [PubMed] [Google Scholar]
- 33.Stein RC, Joseph AE, Matlin SA, Cunningham DC, Ford HT, Coombes RC. A preliminary clinical study of gossypol in advanced human cancer. Cancer Chemother Pharmacol. 1992;30:480–2. doi: 10.1007/BF00685601. [DOI] [PubMed] [Google Scholar]
- 34.Bushunow P, Reidenberg MM, Wasenko J, Winfield J, Lorenzo B, Lemke S, et al. Gossypol treatment of recurrent adult malignant gliomas. J Neurooncol. 1999;43:79–86. doi: 10.1023/a:1006267902186. [DOI] [PubMed] [Google Scholar]
- 35.Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–18. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 36.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–64. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 37.Meng Y, Li Y, Li J, Li H, Fu J, Liu Y, et al. (-)Gossypol and its combination with imatinib induce apoptosis in human chronic myeloid leukemic cells. Leukemia & lymphoma. 2007;48:2204–12. doi: 10.1080/10428190701583991. [DOI] [PubMed] [Google Scholar]
- 38.Balakrishnan K, Wierda WG, Keating MJ, Gandhi V. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2008;112:1971–80. doi: 10.1182/blood-2007-12-126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–21. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 40.Oliver CL, Miranda MB, Shangary S, Land S, Wang S, Johnson DE. (-)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistance. Mol Cancer Ther. 2005;4:23–31. [PubMed] [Google Scholar]
- 41.Becattini B, Kitada S, Leone M, Monosov E, Chandler S, Zhai D, et al. Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-X(L) Chem Biol. 2004;11:389–95. doi: 10.1016/j.chembiol.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, et al. Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–6. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- 43.Johnsen O, Mas Diaz J, Eliasson R. Gossypol; a potent inhibitor of human sperm acrosomal proteinase. Int J Androl. 1982;5:636–40. doi: 10.1111/j.1365-2605.1982.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 44.Qiu J, Levin LR, Buck J, Reidenberg MM. Different pathways of cell killing by gossypol enantiomers. Exp Biol Med (Maywood) 2002;227:398–401. doi: 10.1177/153537020222700605. [DOI] [PubMed] [Google Scholar]
- 45.Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, Guo J, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–42. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 46.Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113:149–53. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–13. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 48.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–63. [PubMed] [Google Scholar]
- 49.Pedersen IM, Kitada S, Leoni LM, Zapata JM, Karras JG, Tsukada N, et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–801. [PubMed] [Google Scholar]
- 50.Paoluzzi L, Gonen M, Gardner JR, Mastrella J, Yang D, Holmlund J, et al. Targeting Bcl-2 family members with the BH3 mimetic AT-101 markedly enhances the therapeutic effects of chemotherapeutic agents in in vitro and in vivo models of B-cell lymphoma. Blood. 2008;111:5350–8. doi: 10.1182/blood-2007-12-129833. [DOI] [PubMed] [Google Scholar]
- 51.Hu W, Wang F, Tang J, Liu X, Yuan Z, Nie C, et al. Proapoptotic protein Smac mediates apoptosis in cisplatin-resistant ovarian cancer cells when treated with the anti-tumor agent AT101. J Biol Chem. 2012;287:68–80. doi: 10.1074/jbc.M111.271205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, et al. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J Thorac Oncol. 2011;6:1757–60. doi: 10.1097/JTO.0b013e31822e2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ready N, Karaseva NA, Orlov SV, Luft AV, Popovych O, Holmlund JT, et al. Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. J Thorac Oncol. 2011;6:781–5. doi: 10.1097/JTO.0b013e31820a0ea6. [DOI] [PubMed] [Google Scholar]
- 54.Wei J, Rega MF, Kitada S, Yuan H, Zhai D, Risbood P, et al. Synthesis and evaluation of Apogossypol atropisomers as potential Bcl-xL antagonists. Cancer Lett. 2009;273:107–13. doi: 10.1016/j.canlet.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitada S, Kress CL, Krajewska M, Jia L, Pellecchia M, Reed JC. Bcl-2 antagonist apogossypol (NSC736630) displays single-agent activity in Bcl-2-transgenic mice and has superior efficacy with less toxicity compared with gossypol (NSC19048) Blood. 2008;111:3211–9. doi: 10.1182/blood-2007-09-113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jia L, Coward LC, Kerstner-Wood CD, Cork RL, Gorman GS, Noker PE, et al. Comparison of pharmacokinetic and metabolic profiling among gossypol, apogossypol and apogossypol hexaacetate. Cancer Chemother Pharmacol. 2008;61:63–73. doi: 10.1007/s00280-007-0446-3. [DOI] [PubMed] [Google Scholar]
- 57.Wei J, Kitada S, Rega MF, Emdadi A, Yuan H, Cellitti J, et al. Apogossypol derivatives as antagonists of antiapoptotic Bcl-2 family proteins. Mol Cancer Ther. 2009;8:904–13. doi: 10.1158/1535-7163.MCT-08-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei J, Kitada S, Rega MF, Stebbins JL, Zhai D, Cellitti J, et al. Apogossypol derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2009;52:4511–23. doi: 10.1021/jm900472s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci U S A. 2011;108:8785–90. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azab B, Dash R, Das SK, Bhutia SK, Shen XN, Quinn BA, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. J Cell Physiol. 2012;227:2145–53. doi: 10.1002/jcp.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilbert NE, O’Reilly JE, Chang CJ, Lin YC, Brueggemeier RW. Antiproliferative activity of gossypol and gossypolone on human breast cancer cells. Life Sci. 1995;57:61–7. doi: 10.1016/0024-3205(95)00243-y. [DOI] [PubMed] [Google Scholar]
- 62.Dao VT, Dowd MK, Martin MT, Gaspard C, Mayer M, Michelot RJ. Cytotoxicity of enantiomers of gossypol Schiff’s bases and optical stability of gossypolone. Eur J Med Chem. 2004;39:619–24. doi: 10.1016/j.ejmech.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Zhan Y, Jia G, Wu D, Xu Y, Xu L. Design and synthesis of a gossypol derivative with improved antitumor activities. Arch Pharm (Weinheim) 2009;342:223–9. doi: 10.1002/ardp.200800185. [DOI] [PubMed] [Google Scholar]
- 64.Mohammad RMYD, Chen B, Aboukameel A, Chen J, Nikolovska-Coleska Z, Al-Katib A, Wang S. ApoG2, a potent, non-toxic small-molecule inhibitor of Bcl-2 family: A preclinical trial in lymphoma. Proc Amer Assoc Cancer Res. 2006 Abstract #1335. [Google Scholar]
- 65.Arnold AA, Aboukameel A, Chen J, Yang D, Wang S, Al-Katib A, et al. Preclinical studies of Apogossypolone: a new nonpeptidic pan small-molecule inhibitor of Bcl-2, Bcl-XL and Mcl-1 proteins in Follicular Small Cleaved Cell Lymphoma model. Mol Cancer. 2008;7:20. doi: 10.1186/1476-4598-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun J, Li ZM, Hu ZY, Lin XB, Zhou NN, Xian LJ, et al. ApoG2 inhibits antiapoptotic Bcl-2 family proteins and induces mitochondria-dependent apoptosis in human lymphoma U937 cells. Anticancer Drugs. 2008;19:967–74. doi: 10.1097/CAD.0b013e32831087e8. [DOI] [PubMed] [Google Scholar]
- 67.Balakrishnan K, Aggarwal S, Wierda W, Gandhi V. Bax and Bak are required for apogossypolone, a BH3-mimetic, induced apoptosis in chronic lymphocytic leukemia cells. Leuk Lymphoma. 2013;54:1097–100. doi: 10.3109/10428194.2012.718344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Y, Wu J, Aboukameel A, Banerjee S, Arnold AA, Chen J, et al. Apogossypolone, a nonpeptidic small molecule inhibitor targeting Bcl-2 family proteins, effectively inhibits growth of diffuse large cell lymphoma cells in vitro and in vivo. Cancer Biol Ther. 2008;7:1418–26. doi: 10.4161/cbt.7.9.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu ZY, Sun J, Zhu XF, Yang D, Zeng YX. ApoG2 induces cell cycle arrest of nasopharyngeal carcinoma cells by suppressing the c-Myc signaling pathway. J Transl Med. 2009;7:74. doi: 10.1186/1479-5876-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei J, Kitada S, Stebbins JL, Placzek W, Zhai D, Wu B, et al. Synthesis and biological evaluation of Apogossypolone derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2010;53:8000–11. doi: 10.1021/jm100746q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–7. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–8. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 73.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68:3413–20. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campas C, Cosialls AM, Barragan M, Iglesias-Serret D, Santidrian AF, Coll-Mulet L, et al. Bcl-2 inhibitors induce apoptosis in chronic lymphocytic leukemia cells. Exp Hematol. 2006;34:1663–9. doi: 10.1016/j.exphem.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Perez-Galan P, Roue G, Lopez-Guerra M, Nguyen M, Villamor N, Montserrat E, et al. BCL-2 phosphorylation modulates sensitivity to the BH3 mimetic GX15-070 (Obatoclax) and reduces its synergistic interaction with bortezomib in chronic lymphocytic leukemia cells. Leukemia. 2008;22:1712–20. doi: 10.1038/leu.2008.175. [DOI] [PubMed] [Google Scholar]
- 76.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–9. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 77.Mitchell C, Yacoub A, Hossein H, Martin AP, Bareford MD, Eulitt P, et al. Inhibition of MCL-1 in breast cancer cells promotes cell death in vitro and in vivo. Cancer Biol Ther. 2010;10:903–17. doi: 10.4161/cbt.10.9.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009;15:150–9. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei Y, Kadia T, Tong W, Zhang M, Jia Y, Yang H, et al. The combination of a histone deacetylase inhibitor with the BH3-mimetic GX15-070 has synergistic antileukemia activity by activating both apoptosis and autophagy. Autophagy. 2010;6:976–8. doi: 10.4161/auto.6.7.13117. [DOI] [PubMed] [Google Scholar]
- 80.Martin AP, Park MA, Mitchell C, Walker T, Rahmani M, Thorburn A, et al. BCL-2 family inhibitors enhance histone deacetylase inhibitor and sorafenib lethality via autophagy and overcome blockade of the extrinsic pathway to facilitate killing. Mol Pharmacol. 2009;76:327–41. doi: 10.1124/mol.109.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rahmani M, Aust MM, Attkisson E, Williams DC, Jr, Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 anti-apoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood. 2012 doi: 10.1182/blood-2011-09-378141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinez-Paniagua MA, Baritaki S, Huerta-Yepez S, Ortiz-Navarrete VF, Gonzalez-Bonilla C, Bonavida B, et al. Mcl-1 and YY1 inhibition and induction of DR5 by the BH3-mimetic Obatoclax (GX15-070) contribute in the sensitization of B-NHL cells to TRAIL apoptosis. Cell Cycle. 2011;10:2792–805. doi: 10.4161/cc.10.16.16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schimmer AD, O’Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:8295–301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 84.O’Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paik PK, Rudin CM, Pietanza MC, Brown A, Rizvi NA, Takebe N, et al. A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer. 2011;74:481–5. doi: 10.1016/j.lungcan.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oki Y, Copeland A, Hagemeister F, Fayad LE, Fanale M, Romaguera J, et al. Experience with obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood. 2012;119:2171–2. doi: 10.1182/blood-2011-11-391037. [DOI] [PubMed] [Google Scholar]
- 87.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 88.Vogler M, Furdas SD, Jung M, Kuwana T, Dyer MJ, Cohen GM. Diminished sensitivity of chronic lymphocytic leukemia cells to ABT-737 and ABT-263 due to albumin binding in blood. Clin Cancer Res. 2010;16:4217–25. doi: 10.1158/1078-0432.CCR-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–9. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 90.Touzeau C, Dousset C, Bodet L, Gomez-Bougie P, Bonnaud S, Moreau A, et al. ABT-737 induces apoptosis in mantle cell lymphoma cells with a Bcl-2high/Mcl-1low profile and synergizes with other antineoplastic agents. Clin Cancer Res. 2011;17:5973–81. doi: 10.1158/1078-0432.CCR-11-0955. [DOI] [PubMed] [Google Scholar]
- 91.Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci U S A. 2006;103:14907–12. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 93.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vogler M, Dinsdale D, Sun XM, Young KW, Butterworth M, Nicotera P, et al. A novel paradigm for rapid ABT-737-induced apoptosis involving outer mitochondrial membrane rupture in primary leukemia and lymphoma cells. Cell Death Differ. 2008;15:820–30. doi: 10.1038/cdd.2008.25. [DOI] [PubMed] [Google Scholar]
- 95.Kline MP, Rajkumar SV, Timm MM, Kimlinger TK, Haug JL, Lust JA, et al. ABT-737, an inhibitor of Bcl-2 family proteins, is a potent inducer of apoptosis in multiple myeloma cells. Leukemia. 2007;21:1549–60. doi: 10.1038/sj.leu.2404719. [DOI] [PubMed] [Google Scholar]
- 96.Bodet L, Gomez-Bougie P, Touzeau C, Dousset C, Descamps G, Maiga S, et al. ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood. 2011;118:3901–10. doi: 10.1182/blood-2010-11-317438. [DOI] [PubMed] [Google Scholar]
- 97.Kang MH, Kang YH, Szymanska B, Wilczynska-Kalak U, Sheard MA, Harned TM, et al. Activity of vincristine, L-ASP, and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo. Blood. 2007;110:2057–66. doi: 10.1182/blood-2007-03-080325. [DOI] [PubMed] [Google Scholar]
- 98.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–9. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen S, Dai Y, Pei XY, Grant S. Bim Up-regulation by Histone Deacetylase Inhibitors Mediates Interactions with the Bcl-2 Antagonist ABT-737: Evidence for Distinct Roles for Bcl-2, Bcl-xL and Mcl-1. Molecular and cellular biology. 2009 doi: 10.1128/MCB.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, et al. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–9. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 102.Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–9. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–7. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 104.Paoluzzi L, Gonen M, Bhagat G, Furman RR, Gardner JR, Scotto L, et al. The BH3-only mimetic ABT-737 synergizes the antineoplastic activity of proteasome inhibitors in lymphoid malignancies. Blood. 2008;112:2906–16. doi: 10.1182/blood-2007-12-130781. [DOI] [PubMed] [Google Scholar]
- 105.Chen S, Dai Y, Pei XY, Grant S. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009;29:6149–69. doi: 10.1128/MCB.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mason KD, Khaw SL, Rayeroux KC, Chew E, Lee EF, Fairlie WD, et al. The BH3 mimetic compound, ABT-737, synergizes with a range of cytotoxic chemotherapy agents in chronic lymphocytic leukemia. Leukemia. 2009;23:2034–41. doi: 10.1038/leu.2009.151. [DOI] [PubMed] [Google Scholar]
- 107.Kuroda J, Kimura S, Andreeff M, Ashihara E, Kamitsuji Y, Yokota A, et al. ABT-737 is a useful component of combinatory chemotherapies for chronic myeloid leukaemias with diverse drug-resistance mechanisms. Br J Haematol. 2008;140:181–90. doi: 10.1111/j.1365-2141.2007.06899.x. [DOI] [PubMed] [Google Scholar]
- 108.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 109.Kojima K, Duvvuri S, Ruvolo V, Samaniego F, Younes A, Andreeff M. Decreased sensitivity of 17p-deleted chronic lymphocytic leukemia cells to a small molecule BCL-2 antagonist ABT-737. Cancer. 2012;118:1023–31. doi: 10.1002/cncr.26360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tromp JM, Geest CR, Breij EC, Elias JA, van Laar J, Luijks DM, et al. Tipping the Noxa/Mcl-1 balance overcomes ABT-737 resistance in chronic lymphocytic leukemia. Clin Cancer Res. 2012;18:487–98. doi: 10.1158/1078-0432.CCR-11-1440. [DOI] [PubMed] [Google Scholar]
- 111.Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-xL and Bcl-w are not equivalent targets of ABT-737 and Navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012 doi: 10.1182/blood-2011-12-400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 113.Shoemaker AR, Mitten MJ, Adickes J, Ackler S, Refici M, Ferguson D, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–77. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 114.Ackler S, Xiao Y, Mitten MJ, Foster K, Oleksijew A, Refici M, et al. ABT-263 and rapamycin act cooperatively to kill lymphoma cells in vitro and in vivo. Mol Cancer Ther. 2008;7:3265–74. doi: 10.1158/1535-7163.MCT-08-0268. [DOI] [PubMed] [Google Scholar]
- 115.Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–16. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilson WH, O’Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–59. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II Study of Single-Agent Navitoclax (ABT-263) and Biomarker Correlates in Patients with Relapsed Small Cell Lung Cancer. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–96. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 120.Centerwatch. Drugs approved by the FDA (2007) http://www.centerwatch.com/patient/drugs/druglist.html. Accessed May 8.
- 121.Doi K, Li R, Sung SS, Wu H, Liu Y, Manieri W, et al. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. J Biol Chem. 2012;287:10224–35. doi: 10.1074/jbc.M111.334532. [DOI] [PMC free article] [PubMed] [Google Scholar]


