Abstract
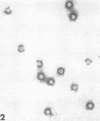
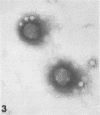
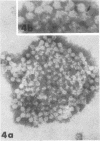
Although human infection with adenovirus-associated virus (AAV) has been demonstrated, there is no evidence that disease results from such infections. The proportion of adenovirus infections which are dual infections with AAV is virtually unknown, since special methods are required to demonstrate infection with AAV. To search for AAV, we re-examined a collection of specimens from 40 persons involved in an epidemic of pharyngoconjunctival fever associated with a swimming pool. Virological and serological studies indicated that the etiological agent was adenovirus type 3. When the 91 original eye, throat, and fecal specimens were re-examined, using methods suitable for detection of adenovirus and AAV, 37 strains of adenovirus type 3 and 35 strains of AAV type 3 (AAV3) were isolated. Surprisingly, 19 AAV3 but only 11 adenovirus isolates were found in eye specimens, whereas adenovirus isolates were equally distributed in all types of specimens. Four AAV3 strains were isolated from adults. Significant (fourfold or greater) rises in AAV3 complement-fixing antibody titers were seen in six of 14 persons shedding AAV3, whereas nine of 10 persons shedding adenovirus type 3 showed significant rises in adenovirus complement-fixing antibody. These results raise the question whether AAV persists better in eyes than adenovirus or that a possible association with conjunctivitis might be present. In contrast to the results in the specimens from the swimming pool epidemic, only one of 36 adenovirus strains isolated in other Seattle-based studies yielded AAV. Complement fixation tests on serial sets of sera collected from 60 children not involved in the swimming pool episode revealed nine AAV2 and 12 AAV3 infections during a 4-year period.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., Kapikian A. Z., Austin J. B., Rowe W. P. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol. 1968 Nov;88(3):368–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., Rowe W. P. Isolation of adenovirus-associated viruses from man. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1410–1415. doi: 10.1073/pnas.58.4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., Rowe W. P. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968 Feb;40(2):319–327. [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., Sereno M. S., Brandt C. D., Kim H. W., Parrott R. H., Chanock R. M. A seroepidemiologic study of adenovirus-associated virus infection in infants and children. Am J Epidemiol. 1971 Oct;94(4):359–366. doi: 10.1093/oxfordjournals.aje.a121331. [DOI] [PubMed] [Google Scholar]
- Casto B. C., Atchison R. W., Hammon W. M. Studies on the relationship between adeno-associated virus type I (AAV-1) and adenoviruses. I. Replication of AAV-1 in certain cell cultures and its effect on helper adenovirus. Virology. 1967 May;32(1):52–59. doi: 10.1016/0042-6822(67)90251-6. [DOI] [PubMed] [Google Scholar]
- Cooney M. K., Kenny G. E. Immunogenicity of rhinoviruses. Proc Soc Exp Biol Med. 1970 Feb;133(2):645–650. doi: 10.3181/00379727-133-34536. [DOI] [PubMed] [Google Scholar]
- Fox J. P., Hall C. E., Cooney M. K., Luce R. E., Kronmal R. A. The Seattle virus watch. II. Objectives, study population and its observation, data processing and summary of illnesses. Am J Epidemiol. 1972 Oct;96(4):270–285. doi: 10.1093/oxfordjournals.aje.a121458. [DOI] [PubMed] [Google Scholar]
- Foy H. M., Cooney M. K., Hatlen J. B. Adenovirus type 3 epidemic associated with intermittent chlorination of a swimming pool. Arch Environ Health. 1968 Nov;17(5):795–802. doi: 10.1080/00039896.1968.10665321. [DOI] [PubMed] [Google Scholar]
- Foy H. M., Cooney M. K., McMahan R., Bor E., Grayston J. T. Single-dose monovalent A 2 -Hong Kong influenza vaccine. Efficacy 14 months after immunization. JAMA. 1971 Aug 23;217(8):1067–1071. [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Kern J., Beddow T. G., Huebner R. J. Oncogenicity of mixtures of adeno-associated virus and adenovirus type 12. Nature. 1968 Jul 6;219(5149):80–81. doi: 10.1038/219080a0. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Kern J., Beddow T. G., Huebner R. J. Oncogenicity of mixtures of adeno-associated virus and adenovirus type 12. Nature. 1968 Dec 14;220(5172):1139–1139. doi: 10.1038/2201139b0. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. B., Ozer H. L., Hoggan M. D. Structural proteins of adenovirus-associated virus type 3. J Virol. 1971 Dec;8(6):860–863. doi: 10.1128/jvi.8.6.860-863.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschstein R. L., Smith K. O., Peters E. A. Inhibition of adenovirus 12 oncogenicity by adeno-associated virus. Proc Soc Exp Biol Med. 1968 Jul;128(3):670–673. doi: 10.3181/00379727-128-33095. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Houlditch G. S., Mumford D. M. Influence of adeno-associated satellite virus on adenovirus-induced tumours in hamsters. Nat New Biol. 1973 Jan 10;241(106):44–46. doi: 10.1038/newbio241044b0. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Ito M., Jordan L. E., Melnick L. Morphological studies on the replication of a defective satellite virus and its helper adenovirus. J Natl Cancer Inst. 1967 Jun;38(6):805–820. [PubMed] [Google Scholar]
- Parks W. P., Boucher D. W., Melnick J. L., Taber L. H., Yow M. D. Seroepidemiological and ecological studies of the adenovirus-associated satellite viruses. Infect Immun. 1970 Dec;2(6):716–722. doi: 10.1128/iai.2.6.716-722.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Casazza A. M., Alcott J., Melnick J. L. Adeno-associated satellite virus interference with the replication of its helper adenovirus. J Exp Med. 1968 Jan 1;127(1):91–108. doi: 10.1084/jem.127.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Melnick J. L., Rongey R., Mayor H. D. Physical assay and growth cycle studies of a defective adeno-satellite virus. J Virol. 1967 Feb;1(1):171–180. doi: 10.1128/jvi.1.1.171-180.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoza N. P., Atchinson R. W. Association of AAV-1 with simian adenoviruses. Nature. 1967 Sep 9;215(5106):1186–1187. doi: 10.1038/2151186a0. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M. J., Edwards E. A., Pierce W. E., Peckinpaugh R. O., Parks W. P., Melnick J. L. Serologic surveillance for adeno-associated satellite virus antibody in military recruits. J Immunol. 1971 Mar;106(3):711–720. [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Smith K. O., Gehle W. D., Thiel J. F. Properties of a small virus associated with adenovirus type 4. J Immunol. 1966 Dec;97(6):754–766. [PubMed] [Google Scholar]
- Sprecher-Goldberger S., Dekegel D., Otten J., Thiry L. Incidence of antibodies to adenovirus-associated viruses in patients with tumours or other diseases. Arch Gesamte Virusforsch. 1970;30(1):16–21. doi: 10.1007/BF01262578. [DOI] [PubMed] [Google Scholar]
- Sprecher-Goldberger S., Thiry L., Lefébvre N., Dekegel D., de Halleux F. Complement-fixation antibodies to adenovirus-associated viruses, cytomegaloviruses and herpes simplex viruses in patients with tumors and in control individuals. Am J Epidemiol. 1971 Oct;94(4):351–358. doi: 10.1093/oxfordjournals.aje.a121330. [DOI] [PubMed] [Google Scholar]