Abstract
A growing number of minimally invasive surgical and diagnostic procedures require the insertion of an optical, mechanical, or electronic device in narrow spaces inside a human body. In such procedures, precise motion control is essential to avoid damage to the patient’s tissues and/or the device itself. A typical example is the insertion of a cochlear implant which should ideally be done with minimum physical contact between the moving device and the cochlear canal walls or the basilar membrane. Because optical monitoring is not possible, alternative techniques for sub millimeter-scale distance control can be very useful for such procedures. The first requirement for distance control is distance sensing. We developed a novel approach to distance sensing based on the principles of scanning electrochemical microscopy (SECM). The SECM signal, i.e., the diffusion current to a microelectrode, is very sensitive to the distance between the probe surface and any electrically insulating object present in its proximity. With several amperometric microprobes fabricated on the surface of an insertable device, one can monitor the distances between different parts of the moving implant and the surrounding tissues. Unlike typical SECM experiments, in which a disk-shaped tip approaches a relatively smooth sample, complex geometries of the mobile device and its surroundings make distance sensing challenging. Additional issues include the possibility of electrode surface contamination in biological fluids and the requirement for a biologically compatible redox mediator.
Index Terms: Cochlear implants, inverse problems, noninvasive treatments, scanning probe microscopy, sensor systems
I. Introduction
Cochlear implants (CIs) are devices to evoke hearing sensation by electrical stimulation in patients with severe, profound, or total hearing loss. The surgical procedure involves manual drilling of the temporal bone to access the inner ear, either through the round window or via a cochleostomy. The CI electrode is inserted into the spirally coiled inner ear space where noble metal electrodes stimulate spiral ganglion neurons. The inner ear is compartmentalized into three spaces [scala tympani (ST), scala media, and scala vestibuli (SV)] by two membranes that extend longitudinally through the cochlea: the basilar membrane and Reissner’s membrane. The ST is the ideal canal in which a CI can be inserted minimally invasively to preserve most of the intricate inner ear structure, especially the basilar membrane in which auditory sensory receptors reside. However, during the insertion, the CI can be dislocated from the ST into the SV breaking the basilar membrane and resulting in complete loss of any residual acoustic hearing.
Recently, as implantation criteria have expanded to the patients with more residual hearing, studies have shown the potential of residual hearing preservation. Increased hearing preservation may help improve hearing performance by both electrical and acoustic stimulation using the combination of a CI and a hearing aid in the same ear [1]. One difficulty is that basilar membrane penetration during the insertion of a CI electrode is regarded as significant physical trauma which is likely to affect preservation of residual hearing [2], [3]. One of the most successful strategies to avoid basilar membrane penetration is the use of a short CI which does not reach the first turn of the cochlear canal. This approach can provide “electroacoustic” stimulation to the implanted ear by providing electrical stimulation to neurons close to the base of the cochlea and acoustic stimulation to neurons closer to the apex of the cochlea [1]. The success of hearing preservation surgery has stimulated further study to evaluate the positive correlation between electroacoustic stimulation and the improvement of hearing performance [4].
Two important factors make it difficult to achieve atraumatic insertion of CIs. First, surgeons have to insert a CI into a tightly curled canal without any visual nor positional information. Optical endoscopy is too invasive and fluoroscopy requires more improvement on the temporal/spatial resolution for intraoperative guidance [5]–[9]. Currently, CI designers aim at optimizing the mechanical properties of CIs for atraumatic insertion to be as easy as possible for surgeons [10]. Second, the narrowly coiled ST space imposes a strict constraint on the design of CIs when the engineers and researchers keep improving the mechanical properties and, more dramatically, try to invent intelligent and active CIs with the incorporation of actuators or sensors [11]–[13]. A high-resolution sensor with miniaturization capability can be a breakthrough toward the complete atraumatic insertion of CIs.
Here, we propose the implementation of the scanning electrochemical microscope (SECM) as a proximity sensor to detect ST walls and basilar membrane without physical contact. The implementation of SECM features real-time, compact size, and high sensitivity. One of the ultimate goals is to estimate and/or control the trajectory of the CI to avoid any contact of the CI to the ST walls.
II. Application of SECM to CI
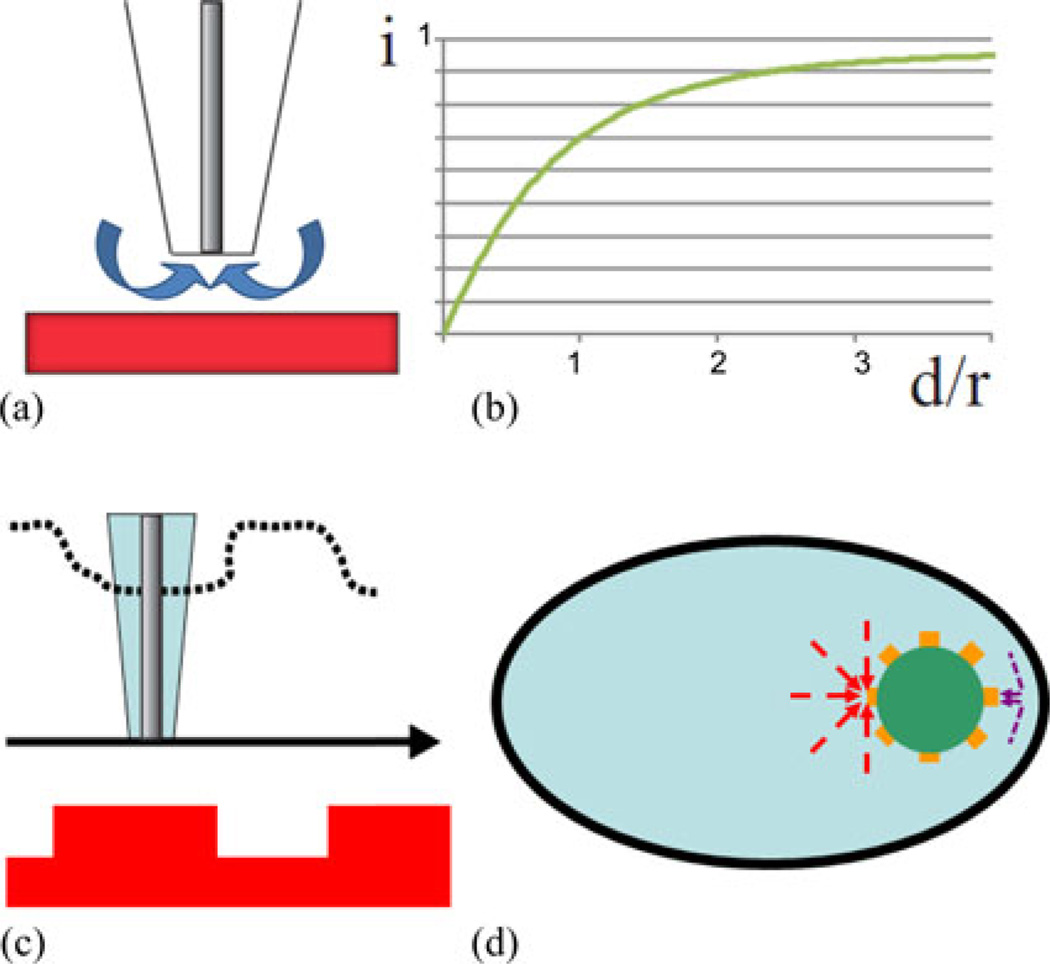
SECM is an electrochemical scanning probe microscopic technique, which has been widely used to observe local physical/chemical processes in solution and on various surfaces [14]. The tip potential is controlled versus the reference electrode, and the current flowing at the tip is determined by the rate of the diffusion of redox species to its surface. When the tip is brought close (i.e., within a few tip radii) to an insulating substrate the current decreases because the insulator blocks the diffusion of redox species to the microelectrode surface (negative feedback). The negative feedback mode of the SECM operation can be used to amperometrically image the topography of an insulative substrate by scanning a microelectrode (“tip”) above its surface in an aqueous solution containing a redox mediator (see Fig. 1).
Fig. 1.
Schematic diagram of the SECM feedback mode with a disk-shaped tip electrode. (a) The redox mediator diffusion is shown by arrows. An approach curve, (i: current, d: distance, and r: radius,) is shown schematically in (b) and topographical scan (c). (d) A simplified image of a CI with an eight-channel proximity sensor array in a ST. Arrows indicate the diffusion of redox mediators in confined (purple) or open (red) space.
Similarly, if a CI is equipped with microelectrodes, the insulative biological tissues such as bone and basilar membrane [15] can be detected by change in the measured current [see Fig. 1(d)]. Because the microdisk electrode is made of a noble metal, it is essentially no different from a CI stimulation electrode except for its size, shape, and voltage application regimen for a specific redox mediator.
To apply SECM to atraumatic CI insertion, the size of a noble metal disk electrode and a suitable redox mediator must be selected. The size of the electrode determines the proximity range, i.e., the maximum distance at which the proximity sensor can detect an object. A typical current/distance characteristic (approach curve) for SECM with a disk-shaped tip approaching a flat insulating sample [see Fig. 1(a)] is shown schematically in Fig. 1(b). The current shows clear decrease when the distance becomes less than ~10 tip radii. The radius of about 50 µm is suitable to have an appropriate range to detect the proximity of the ST wall whose cross-sectional size is less than 3 mm. The redox mediator has to be present in the body abundantly and the electrochemical reaction must be safe for body tissues.
Oxygen (O2) is an endogenous key molecule involved in the metabolism to sustain a life. Since the discovery in 1897, electrochemical reduction of O2 has been used to measure the concentration of O2 in a solution using platinum (Pt) electrode via the reactions [16], [17],
O2 + 2H2O + 4e− → 4OH− and O2 + 4H+ + 4e− → 2H2O.
Bare type electrodes directly immersed in bodily fluid have been used widely in acute experiments which have aimed at determining the oxygen tension in the brain of freely moving animals, or in the perilymph solution of guinea pigs [18]–[21]. In these studies including histology, the oxygen sensor performance degraded before emergence of any issues with biocompatibility. For the chronic sensing of O2 reduction up to one year, research efforts have been dedicated to overcome the loss of accessing the dissolved O2 cased by the adsorption of impurities such as proteins on the electrode surface [22], [23]. Since 1953, Clark electrode, which employs an oxygen permeable coating encapsulating the Pt electrode and isolated operating solution from the body tissues has been used to measure blood oxygen tension [24]. One of the Clark type electrodes is Licox®, which is a human implant to monitor the brain oxygen tension in traumatic brain injury [25]–[27].
The history of the development of oxygen sensors in the human or animal body suggests that the primary challenge is that the duration necessary for the Pt electrode to operate to detect the wall of ST has to be longer than the time which allows the Pt electrode surface to foul. The secondary issue is the biocompatibility of the reduction of oxygen. The pH elevation from the hydroxide formation and hydronium ion consumption should be considered from the amplitude and duration of the reduction current.
In this paper, we present a feasibility study of implementation of SECM as a proximity sensor for atraumatic CI insertion; specifically, 1) a design of a novel distance sensor electrode based on the SECM feedback mode and current CI designs, 2) use of O2 as an endogenous distance sensing mediator, 3) demonstration of proximity sensing within the geometry of the human inner ear, 4) estimation of a possible trajectory based on the analysis and the interpretation of the proximity sensing using SECM, 5) time-dependent effects on proximity sensing, and 6) an indication of the biocompatibility of the distance sensor.
III. Materials and Methods
A. Distance Sensor Design and Fabrication
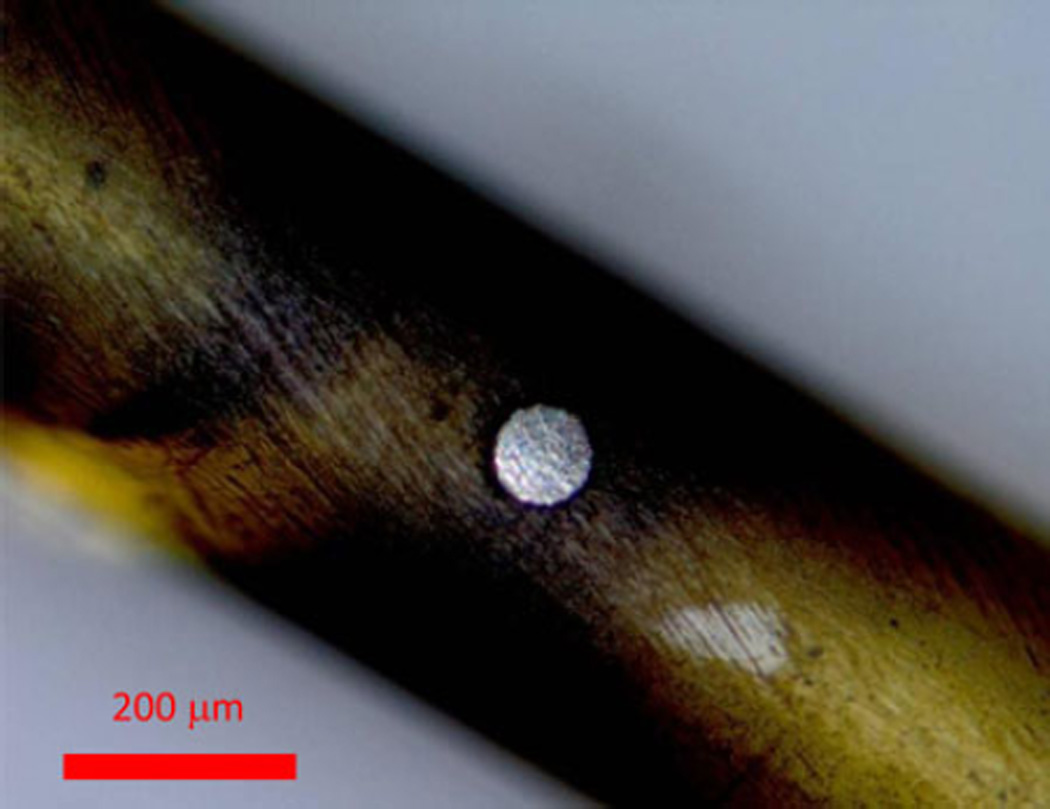
A polyimide tube was selected as an electrode carrier to reduce vibration during proximity sensing experiments and to facilitate fabrication (see Fig. 2). A 75-µm diameter Pt wire (A-M Systems, Carlsborg, WA, USA) was selected to allow for a proximity range of ~100 µm (see Fig. 3). The wire was embedded in a polyimide tube carrier, which was designed so that the proposed technology could be easily employed in current CI designs by replacing its Pt stimulation electrode with the electrode presented here.
Fig. 2.
Polyimide carrier with a proximity sensor used in the experiment is shown. From the right, the yellow tube is a 4-cm long 400-micron diameter polyimide tube. The Pt disk electrode is located 1 mm from the tip of the polyimide tube. The black paint from 1 cm from the tip indicates the direction which the electrode faces. The platinum wire was structurally fixed and electrically insulated with epoxy glue to the polyimide tube from the tip located at the right and the wire was electrically connected with a fuse wire inserted from the left tip. The left tip of the polyimide tube and the fuse wire was also fixed and secured with enough amount of epoxy to stabilize the structural and electrical connection. The direction of the disk face is also painted on the epoxy glue. The wire was soldered to a pin on the leftmost side.
Fig. 3.
Platinum wire (center circle) is embedded in a polyimide tube. This picture was taken with an optical microscope in Brookhaven National Laboratory. The disk electrode surface is flush with the surrounding polyimide surface.
Fig. 4 shows the schematic diagram of the fabrication of a Pt microelectrode in a polyimide tube. A 28-gauge polyimide tube was purchased from Small parts (Logansport, IN). An 80-µm-diameter hole was created on the wall of the polyimide tube by a YAG laser. The Pt wire was inserted through the 80-µm hole and pulled toward one end. From the other end, epoxy glue (Devcon 2 Ton Epoxy) was introduced to fix the wire to the tube and seal the hole. The Pt wire was cut roughly and polished with a drill followed by a cotton applicator immersed with distilled water and 50-nm alumina under a binocular microscope. The Pt wire was polished until the wire surface and the polyimide tube surface were continuous. Before use in SECM, the electrode was polished again with alumina and washed by distilled water.
Fig. 4.
Schematic diagram of the fabrication of a proximity sensor. The hole in the wall of a polyimide tube was made with a YAG laser to insert a single Pt wire. The Pt wire was fixed with epoxy glue, introduced from the tip of the tube and electrically connected to a fuse wire. The tip of the Pt wire was grinded roughly and polished to make the surface continuous.
B. Electrochemical Characterization
To characterize the electrochemical behavior of the prototyped electrode, linear ramp cyclic voltammetry (CV) was carried out in ferrocenemethanol (FeMeOH) solution as a control and phosphate buffer solution (PBS) as a biological standard. All chemicals used were of analytical grade unless mentioned otherwise, and deionized water was used (Milli-Q, Millipore Co.) to prepare solutions. FeMeOH, selected here as a redox mediator to calibrate a prototyped electrode has been widely used in SECM experiments because its one-electron oxidation/reduction yields nearly perfect electrochemical response [28]. PBS was prepared to contain an ionic composition equivalent to the perilymph solution in human ST [29]. The pH buffer was modified from carbonate to phosphate. The contents were NaCl 137 mM, KCl 2.7 mM, NaH2PO4-H2 O 8.1 mM, Na2 HPO4 1.76 mM pH 7.4. KNO3 0.1 M, and KCl 0.1 M (≥99%, Aldrich) were used as supporting electrolytes for FeMeOH 1 mM. To remove O2 in the saline solution, the solutions were purged with high-purity nitrogen before and during the experiments as a negative control. Either an EI-400 bipotentiostat (Ensman Instruments, Bloomington, IN, USA) or a BAS-100 B electrochemical analyzer (Bioanalytical Systems, West Lafayette, IN, USA) was used to obtain CVs. All experiments were performed at room temperature (23 ± 2 °C) inside a Faraday cage. The scan speed was 50 mV/sec. The voltage windows to characterize FeMeOH oxidation and O2 reduction were from –100 to 500 mV and from 1100 to −700 mV, respectively. A two-electrode configuration was employed using a 0.3-mm diameter Ag wire coated with AgCl as a reference electrode. More than 10 mm of the wire was always immersed in the solution to make the surface area of the reference electrode always thousand times bigger than that of the working electrode. The concentration of O2 under atmospheric pressure (Pat) with the oxygen partial pressure (PO2) of 159 mm Hg is 0.262 mM.
C. Proximity Sensing Demonstration
The demonstration of the feasibility of proximity sensing using SECM was performed with the previously described homebuilt SECM instrument [30]. As a control experiment to analyze the voltage effect in reducing oxygen in the artificial perilymph solution during proximity sensing, a standard SECM was performed with a Pt disk electrode (50 µm in diameter) embedded in a glass capillary as a working electrode such that the difference from this experiment and the following experiment is the geometry of the working electrode and the scanned substrate. Approach curves with varied voltage (0~−800 mV, 50-mV interval) were obtained. The electrode tip was lowered to a glass plate until the electrode made contact. The contact point was confirmed by an abrupt change in the approach curve. Current versus distance relationships were recorded by departing the glass capillary electrode from the contact point at the speed of 4 µm/sec.
An acrylic ST model (Advanced Bionics, Valencia, CA, USA) was used as a substrate which approximated the physical size of the human ST [31]. The polyimide carrier was inserted into the canal filled with FeMeOH solution or PBS. These solutions were instilled from the apex end of the canal that was located on the back of the ST model. Before PBS instillation, the solution was exposed to air for enough time to attain equilibrium under Pat. This procedure ensured the saturation of the solution with O2 and the elimination of bubbles that can be formed in the canal during the proximity sensing experiments. The FeMeOH solution was first instilled and then replaced with PBS in the canal without changing the position of the ST model, in order to preserve the spatial conditions between the scans using FeMeOH solution and PBS. After the FeMeOH solution removal, the canal was flushed with the PBS three times. This protocol enabled us to compare proximity sensing with two different mediators, i.e., FeMeOH and O2.
The polyimide carrier was inserted into and retracted from the ST model by a piezoelectric actuator. The relative angle between the electrode insertion path and the acrylic ST model was adjusted with a stage angle adjustment so that the carrier almost contacted the wall during the course of the movements. The inward and outward movements were repeated six times at a speed of 20 µm/s. The sampling rate was 40 Hz, the displacement in each direction was 6 mm, and number of data points was 12 000 at each inward and outward movement. The voltage of either 400 mV or −700 mV versus Ag/AgCl was applied to the proximity sensor in the FeMeOH solution and PBS, respectively.
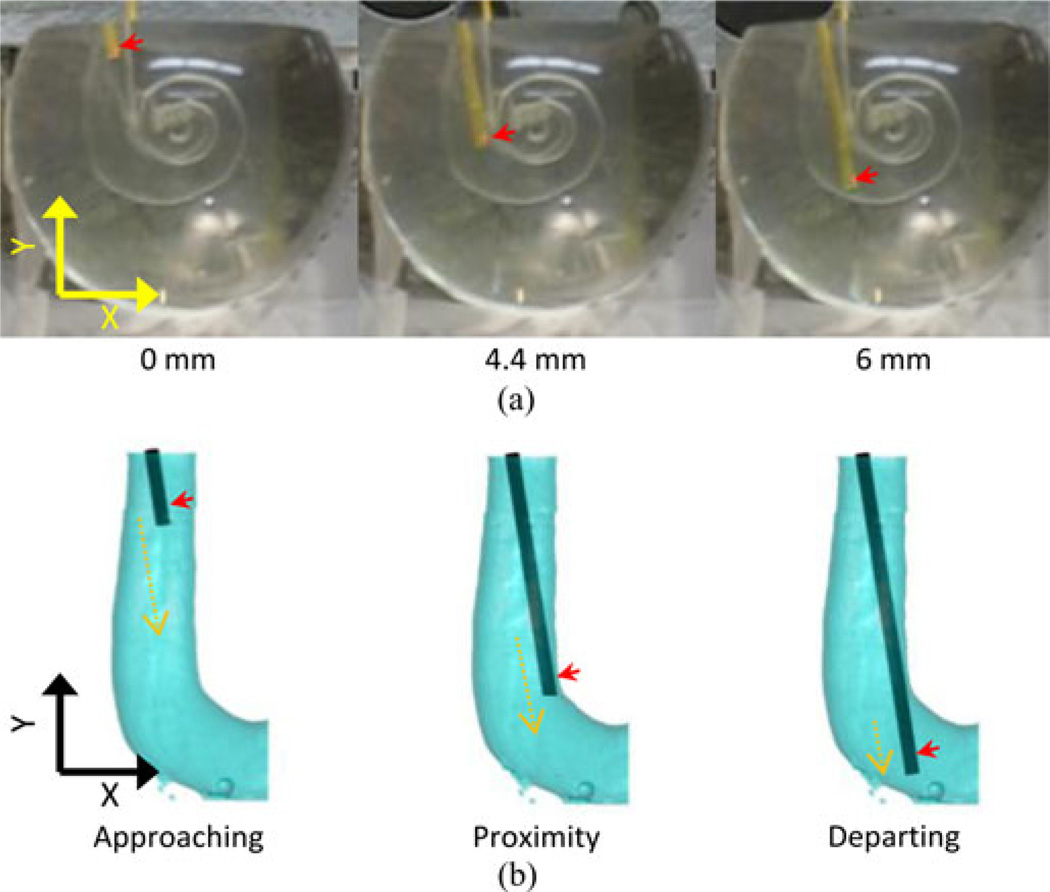
The movements were recorded using a digital camera (Power Shot SD800 IS, Cannon, Japan) [see Fig. 5(a)] at 30 frames/sec. The beginning and the end of the scan were identified in the recording with the start and the cessation of the clicking sound produced by the movement of the piezoelectric actuator. In each frame, the tip of the polyimide tube was located in the pixel map using a custom-made program written in MATLAB. The polyimide carrier was confirmed to be in the proximity of the inner curvature of the canal wall at the first turn of the ST model.
Fig. 5.
Polyimide carrier insertion into a ST model. Figure (a) shows video frames of the insertion of the electrode into the canal of the acrylic model. Red arrows indicate the position of the microdisk electrode. Their insertion depths are indicated below. Figure (b) shows three locations of the reconstructed electrode insertion model based on the optical recording. Red arrows indicate the position of the disc electrode. Orange broken arrows represent the length of the movement in each one of the three sections indicated below. (a) Optical recording. (b) Reconstructed insertion.
D. MATLAB Insertion Model for Virtual Visualization and Three-Dimensional (3-D) Numerical Analysis
A virtual insertion model was developed numerically using MATLAB to visualize the insertion of the polyimide carrier into the acrylic ST model in 3-D and analyze the spatial relationship among the polyimide carrier, proximity sensor and ST acrylic canal in 3-D.
In the MATLAB model, the 3-D geometry of the ST canal was reconstructed from the geometric measurement of the acrylic ST model using micro computed tomography (µCT) (Scanco medical 40, Switzerland) at the NYU College of Dentistry Department of Biomaterials and Biomimetics. The voxel size was 12.5 µm. To enhance the radioactive signal, 1 mM of radiocontrast agent: sodium diatrizoate hydrate (≥99%, Sigma-Aldrich) was instilled into the ST acrylic canal. The slice data were converted to DICOM format for further processing to reconstruct the canal structure in the MATLAB model.
The position of the polyimide carrier in a 2-D coordinate plane (i.e., the xy plane in Figs. 5 and 6) was confirmed approximately based on the digital camera images and the other orthogonal coordinate (i.e., z-coordinate) was set in the MATLAB model arbitrarily. The image data (see Fig. 5 top) was not used directly because of the image distortion by the lens effect of the spherical acrylic ST model. The trajectories of the electrode were assumed to be straight and without any contact to the wall.
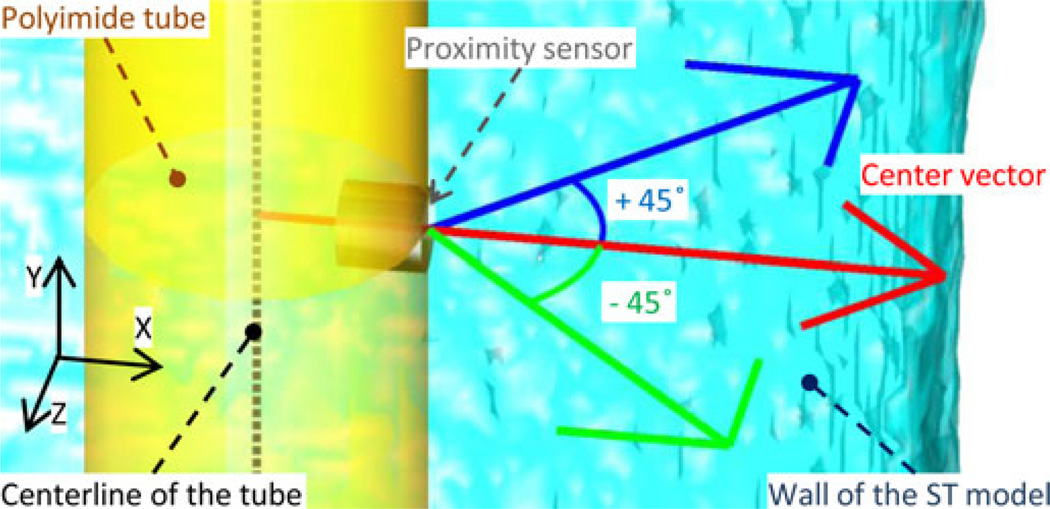
Fig. 6.
Magnified view of the MATLAB reconstruction image. The three colored arrows show the vectors used to calculate the distance between the center of the sensor to the wall of the ST model. All three vectors are in the plane perpendicular to the polyimide tube. The center (red) vector is perpendicular to the electrode surface and to the center line of the tube (dashed black line). The other two vectors are 45° away from the center vector.
The position of the proximity sensor was determined relative to the polyimide carrier in the MATLAB model. The direction of the proximity sensor was confirmed to face the inner curvature of the first turn of the ST model before the experiment and always laid within the xy plane. The distance of the center of the disk Pt electrode from the tip of the polyimide tube was 1 mm.
E. Inverse Problem for Trajectory Estimation
Given the current measured with the SECM proximity sensing, estimation of the position of a CI carrier is qualitatively equivalent to a challenging nonlinear inverse problem, which this study ultimately aims at solving. In this paper, the experiment was designed to simplify the trajectory of the proximity sensor in 2-D to detect the first turn, and, the single channel proximity sensor was intended to provide enough information, in addition to the insertion depth recorded with a piezoelectric actuator, to determine the solution uniquely within the 2-D space that was confirmed with the digital camera images. To explain any potential disagreements between the SECM data (see Fig. 9) and the digital camera images, we consider the effects of the 3-D structure of the ST acrylic model, the lack of the third orthognal dimensional information in the digital camera images and the near-isotropic sensitivity of the proximity sensor because the characteristics depend on the diffusion of redox mediators.
Fig. 9.
Detection of the proximity of the canal wall with SECM. Left and right columns show the average of 6 inward and outward scans respectively. The error bars are shown at the reduced interval of 100 µm for clarity. Top and bottom rows show the scans with FeMeOH and O2, respectively. The broken green and blue lines in vertical axis indicate the approaching and departing sections. The broken lines in horizontal axis indicate the deviation from the average of the each sections.
We hypothesized that the proximity sensor could be found close to the wall of the ST acrylic model in the departing section when we see it in an xz plane. Because the proximity sensor contains 3-D information, the experimental data were used as constraints to estimate the position of the proximity sensor in the z-coordinate, in addition to the constraints determined from the digital camera images. By estimating the z-coordinate in the 3-D trajectry of the proximity sensor using the constraints, we intended to explain the disagreement with the existence of one trajectory which agrees with both digital camera images and the SECM current data.
Thus, using the MATLAB insertion model as a 3-D function whose input is the trajectory of the polyimide carrier and whose output is the distance between the proximity sensor and the wall of ST canal, a numerical analysis was conducted to estimate one possible trajectory of the polyimide carrier by comparing the output, i.e., distances, and the SECM current data. Variable trajectories were used to calculate the distances iteratively until the experimental SECM current data agreed well with the output distance.
First, the 2-D images taken by the digital camera determined the trajectory of the polyimide carrier in an xy plane and the distance in the center vector (see Fig. 6) which is in the perpendicular direction from the proximity sensor and always in an xy plane. The constraints were 1) in the approaching section, the distance is far, 2) in the proximity section, the proximity sensor should be close to or almost touching the wall, and 3) in the departing section, the proximity sensor should be far from the wall in the direction of the center vector.
The output distances were compared with the SECM data as follows. From the analysis of the SECM data presented in Fig. 9, the following two constraints were added; 1) in the departing section, the wall was far in all directions, and 2), in the departing section, the proximity sensor must be close to the wall in a direction other than in the direction of the center vector.
In addition to the center vector, two other vectors shown in Fig. 6 were used to calculate the distances between the proximity sensor and the wall of ST acrylic model in the MATLAB insertion model. The distances in three directions from the proximity sensor to the wall of the ST model were calculated iteratively until these distances satisfied the constraints described previously to solve the inverse problem to estimate the trajectory in the physically conducted experiment. By confirming the existence of a trajectory that meets all the constraints, the disagreement in the experimental data can be explained.
IV. Results
A. Cyclic Voltammetry
Fig. 7 shows typical CVs obtained with the Pt electrode immersed in the FeMeOH solution and the artificial perilymph solution before the proximity sensing. The plateau current of the FeMeOH oxidation [10.5 nA in Fig. 7(a)] is the diffusion limiting current in the bulk solution. Fig. 7(b) shows the CV obtained in the artificial perilymph solution under Pat (red line) and the same solution purged with nitrogen (green line). The red line shows a typical O2 reduction wave and the green line represents the background current at the Pt electrode in PBS without O2. The red line shows a plateau from −500 to −700 mV indicating the diffusion limited current. Abrupt increase of current caused by hydrogen gas evolution was observed at more negative voltages (data not shown). In the CV [see Fig. 7(b)], the voltage window did not include the range of the gas evolution to keep the electrode in a pristine condition for the proximity sensing. The relatively rectangular shape of the green line indicates that the background current was consumed mostly to charge double-layer capacitance. By background subtraction of the capacitative current, the maximum diffusion limiting current of O2 reduction was determined to be −18.7 nA (82% of the total current) at −700 mV.
Fig. 7.
CVs obtained with the prototyped electrode. (a) The CV of FeMeOH solution. (b) CV of the artificial perilymph solution indicating O2 reduction under atmospheric pressure (red line). As a negative control, the solution was purged from O2 with nitrogen flow (green line).
CV of FeMeOH was used to check the cleanliness of the electrode surface. When the electrode surface was contaminated, the plateau current exhibited an upward tilt. Typically, insufficient cure of the epoxy and the presence of other impurities affected the CV shape unless the surface was well polished using alumina.
B. Distance Sensing Demonstration
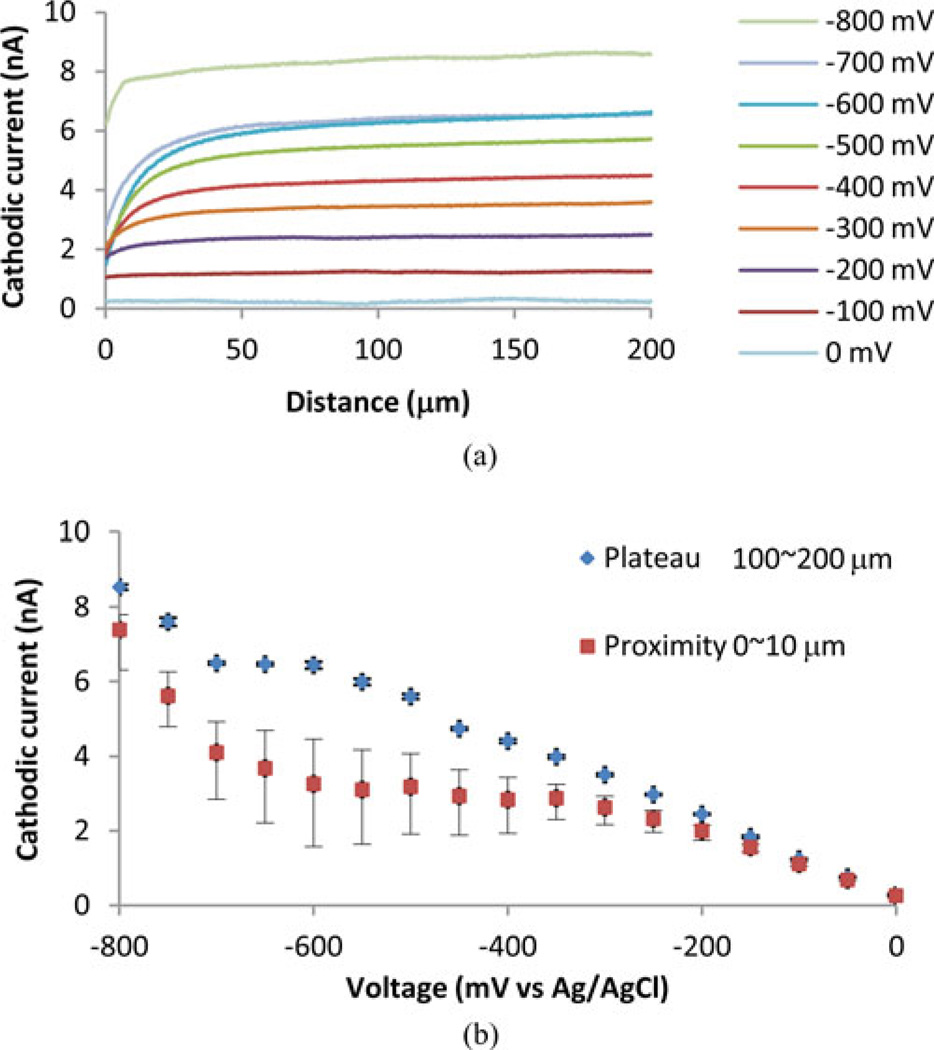
Fig. 8(a) shows the approach curves obtained with varied voltages (0 to −800 mV at 100 mV interval). The approach curves denoted (−200 to −700 mV) shows clear increase of the cathodic currents from 0 to 50 µm. From 100 to 200 µm, all the curves show plateaus with slight increase. These increases were not present in cases that the electrode was being lowered toward the glass substrate instead of being departed (data not shown). Fig. 8(b) shows the average, minimum, and maximum cathodic currents from 0 to 10 µm (proximity). The average currents from 100 to 200 µm (plateau) are also shown. Standard deviations of the plateau currents were less than 2% except for that of 0 mV.
Fig. 8.
Approach curves obtained with a Pt disk electrode embedded in a glass capillary. (a) The relationship between cathodic currents and distance obtained with voltages from 0 to −800 mV. Curves below −200 mV show clear distance dependence. −800 mV line shows large bias current. (b) The plateau and proximity currents are shown to indicate the dynamic ranges of the proximity sensing at varied voltages. The averages and standard deviations of the cathodic currents from 100 to 200 µm are shown as plateau currents. The average cathodic currents from 0 to 10 µm are shown as proximity currents. Minimum and maximum currents of the proximity currents are also shown.
The plateau currents at −600, −650, and −700 mV did not show significant differences indicating that the electrochemical reactions were diffusion limited. Together with the CV which showed a nearly plateau current below −500 mV, these results suggest that SECM using oxygen as a redox mediator should be performed within this voltage range. The approach curves obtained with the voltages above −500 up to −200 mV also showed enough distance dependence, i.e., the difference between the plateau and proximity currents, indicating that this voltage range can be also used for the proximity sensing. Below −750 mV, both the plateau and proximity currents increased due to the hydrogen evolution current and the dynamic range was compromised.
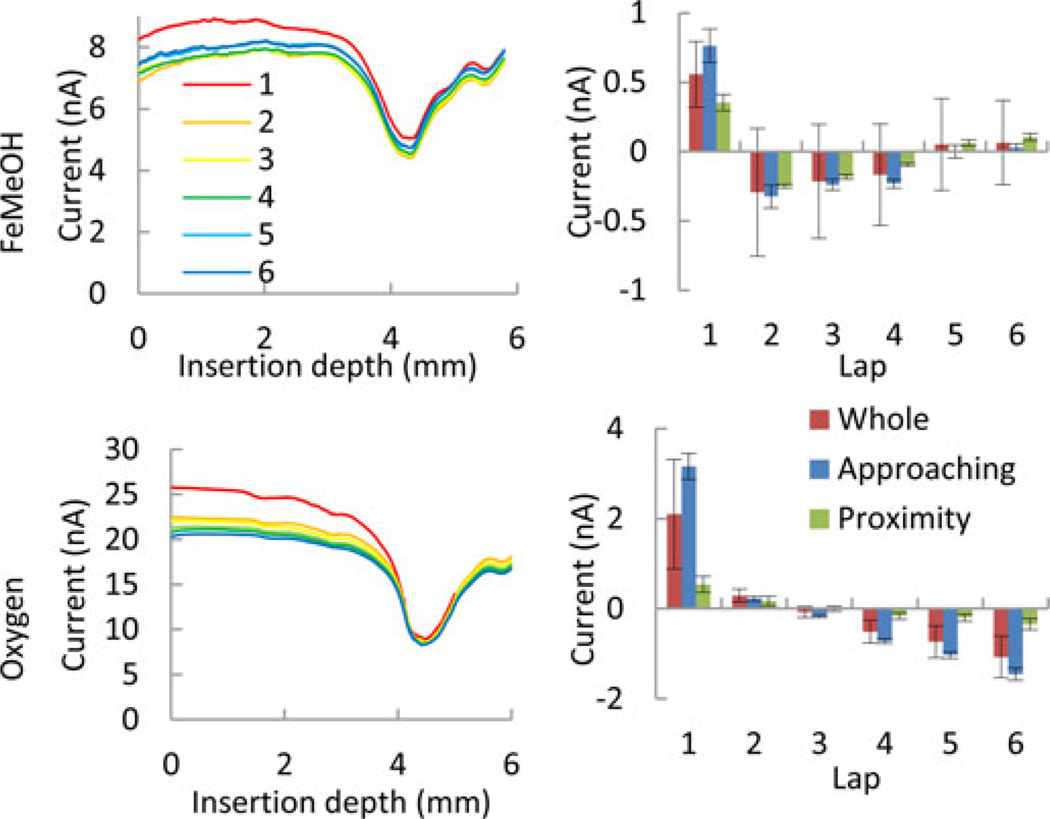
Fig. 9 shows the average and standard deviation of the current amplitudes at each insertion depth in six laps. The mediators were FeMeOH (upper row) and O2 (lower row) respectively. The directions of the movements were inward in the left column and outward in the right column.
The trajectory of the tube was divided into three sections, approaching, proximity, and departing [see Figs. 5(b) and 9]. In the approaching section from 0 mm to 3.0 mm in depth, when the electrode is not close enough to the wall, the currents in both mediators are relatively stable close to the maximum current of 7.97 and 20.6 nA, respectively. In the proximity section around a depth of 4.5 mm, when the electrode is almost touching the wall, both types of scans showed clear and significant decrease of the current down to 3.32 to 8.51 nA, respectively. In the departing section, from 5.0 mm to the end of the scan, after the electrode passed the first turn of the canal, the current increased and reached a local maximal value at 5.6 mm deep. However, the green and blue lines in all four graphs indicated that theses current values were significantly smaller than the corresponding ones in the bulk solution.
Scan-to-scan comparison shows that the variation in the current value at each insertion depth among six scans is attributable to time-dependent changes, and the detection of the wall was not affected significantly (see Fig. 10, left). The first inward scans with FeMeOH and O2 mediators produced significantly larger current than other scans did (see Fig. 10, right). Furthermore, FeMeOH scans showed slight increases of the average current after the second scan and O2 scans showed that current kept decreasing steadily.
Fig. 10.
Six consecutive inward SECM scans (left columns). Obtained with FcMeOH (top) and oxygen (bottom) redox mediators. The scan numbers for both FeMeOH and O2 are color-coded according to the inset in the top-left graph. The right column show the deviation from the average of six laps for FeMeOH (top) and O2 (bottom) scans, respectively. The deviation from the average in the whole, approaching, and proximity sections are shown as red, blue, and green bars respectively.
C. Numerical Analysis Using Virtual Insertion Model
A series of images in Fig. 5 (bottom) show a reconstructed insertion of the polyimide carrier and proximity sensor into the ST canal in the MATLAB insertion model.
Fig. 11 shows the comparison of the results of the three calculated distance versus insertion depth (solid color lines) and the normalized current (broken line) from the inward O2 scan. All constraints were satisfied accordingly. As the polyimide carrier was being inserted down to the depth of 4.5 mm, the distances decreased in the center direction (red line) and other directions, 45° from the center (blue and green). In two directions (red and green lines), after the local minimum, the distance increased steeply. However, in the blue line, the distance decreased again almost to zero at the insertion depth of 6 mm. These distances calculated from a trajectory are in good accordance with the data obtained from the SECM measurement. In turn, this trajectory can be used as an estimate of the polyimide carrier and the proximity sensor positions.
Fig. 11.
Dependences of the distance between the sensor and the wall of ST model in three directions from Fig. 6 on the insertion depth (solid color lines; left axis) along one of the trajectories reconstructed using MATLAB insertion model. These distances were estimated using the MATLAB insertion model, as the polyimide tube was inserted 6 mm into the model following a straight line. The black dashed line shows the corresponding normalized current at each moment in time (right axis).
V. Discussion
A. Design and Fabrication
The use of a noble metal microdisk electrode demonstrates that a proximity sensor using SECM negative feedback mode can be easily implemented in current CIs. The current CI electrodes for stimulation of neurons are band electrodes made of Pt as the interface exposed and delivering electrical current to the perilymph. In both stimulation electrodes and proximity sensors, Pt is selected to minimize any unnecessary electrochemical reaction and to maximize biocompatibility. Therefore, the proximity sensor proposed in this study satisfies some of the most important requirements of CIs for biocompatibility.
The mechanical property of the polyimide tube and the wire inside increased the bending stiffness to be high enough to reduce the amplitude of the vibration during insertion and also reduce noise. However, the high bending stiffness prevents insertion deeper than the first turn of the ST model. Current CI carriers are made of PDMS and wires are designed to reduce the bending stiffness to be able to be bent to fit the tight ST canal with sufficient depth. The use of a PDMS carrier is appropriate to reduce the bending stiffness for a deeper insertion. Possible challenges with a PDMS carrier are effects of the vibration caused by the insertion traction force and the contact between the carrier and the wall.
B. O2 as an Endogenous Redox Species
O2 is an endogenous reducible mediator abundantly found in the human perilymph solution supplied from the middle ear [19]. The average PO2 is 55 mm Hg in the inner ear, which is one third of that under Pat. Therefore, we can expect that the current level will be lower than the value obtained in this study. The decrease in the signal level is not likely to compromise the signal to noise ratio to the level at which feasibility of the method might come into question. The values found in the literature vary, but the report on spatial and temporal variation of the PO2 in in vivo guinea pig is encouraging. The PO2 near the basilar membrane (10–44 mm Hg) is significantly smaller than the average (55 mm Hg). This spatial variation is encouraging to our demonstrated technology. Because the PO2 was measured using an electrochemical O2 sensor, the smaller current can be caused by 1) actual PO2 level, 2) hindered O2 diffusion, or 3) both of them. In any of these cases, the detection of the basilar membrane will be possible. The temporal variation in PO2 is expected to be within a few percent (close to 10%) over a 60 min interval. Such a slow change in the overall ST PO2 would not affect detection of the cochlear walls.
C. O2 Reduction in Perilymph Solution with Pt Disk Electrodes
CV indicated that the voltage should be between −500 and −700 mV to perform SECM with the prototyped electrode using O2 as redox mediator. Approach curves obtained with a glass capillary electrode indicated that −600 to −700 mV should be better to ensure the diffusion current in the bulk solution. The dynamic range of the proximity sensing reached maximum at −600 mV. While the plateau current did not change from −600 to −700 mV, the increase in proximity current reduced the dynamic range from 49.5% to 36.9%. Since the proximity sensing demonstration in the acrylic cochlea model was performed at the voltage of −700 mV, the current in the proximity section could be smaller if the demonstration was performed at −600 mV. To determine the optimal voltage for the proximity sensing via reduction of oxygen, it is necessary to investigate the physical and electrochemical variables of the system. The physical variables are the geometries of the electrode and the substrate, the direction and speed of the electrode movement, and the time-sensitive factors such as capacitance current and mass-transport. Electrochemical variables are the presence or absence of oxygen or biochemical redox mediators, electrode materials, and pH.
D. Proximity Sensor Performance
Detecting the proximity of the electrode to the ST model was repeatable and robust for at least two hours regardless of the utilized redox mediator, the direction of the movement, and time-dependent changes in the electrochemical response. The difference in the current profiles between the two mediators are most likely due to slight changes in the actual trajectory of the polyimide carrier after the solution was replaced. The steady-state concentration profiles should be similar for FeMeOH and O2 despite their different diffusion coefficients [14], [20]. The effect of the direction of movement was also small, suggesting that the speed employed in this demonstration is appropriate. At the speed of 20 µm/sec, the duration of the full insertion of a standard CI (approximately 20 mm) is 1000 seconds. This insertion speed is much slower than that of the manual insertion performed by a surgeon [32], [33]. This slow insertion speed should not be a problem but may be beneficial because 1) improving the duration of the entire surgery (more than 2 hours) is considered to be more beneficial than that of the insertion alone, and 2) slower insertion speed (250 µm/sec versus 1 mm/sec) may be preferred for the preservation of residual hearing [34].
The total duration of the experiment was more than two hours and the current change in the proximity section was always distinguishable. This result suggests that the time-dependent effects should not be a problem during the implantation surgery, which would take 16 min for the full insertion of a CI at the speed of 20 µm/sec. In this study, the observed decrease in the oxygen current was caused by oxidation of the Pt surface [35]. This effect was clearly significant for the slow, inner-sphere oxygen reduction process but not for the rapid outer-sphere oxidation of FeMeOH [27]. On the other hand, the increase of the current observed during FeMeOH scan laps was most likely caused by the evaporation and consequent increase of FeMeOH condensation. In case of O2 scans, the concentration of O2 was constant despite the evaporation of water because the solution was saturated with oxygen under atmospheric pressure. Further experiments are desirable to determine the effect of the adsorption of biological molecules in a solution containing amino acids or in the perilymph of animals.
E. Biocompatibility
The oxygen reduction reaction states that pH elevation is the primary biocompatibility concern. In the human inner ear, this pH elevation can be deduced not to be a problem as follows. The flux and the total dosage of hydroxide or hydronium ions can be estimated from the cathodic current (20 nA). The CV indicates that more than 80% of the cathodic current was consumed by the oxygen reduction ( = 16 nA). Here, the production of hydrogen peroxide on Pt electrode can be ignored [36]. Because the PO2 levels in the inner ear is one third of that under Pat, the oxygen reduction current is expected to be 5.3 nA [19], [20]. From the Faraday constant, the flux of the monovalent hydroxide ions is 55 fmol/sec. If a full CI insertion (20 mm) is performed at the speed of 20 µm/sec, 55 pmole of hydroxide ions will be released into the perilymph solution in 1,000 sec. Since the source of hydroxide ions will be continuously moving along the insertion of the CI carrier, we can assume that hydroxide ions will be homogenously distributed in the human ST. The volume of the human ST is about 150 µl. Thus, the increase of hydroxide ions in concentration can be estimated as 0.34 mM. The human perilymph solution contains a carbonate buffer and the speed of a carbonate buffer is on the order of a few seconds. Using Henderson–Hasselbalch equation, the perilymph pH increase can be calculated from 7.31 to 7.44 [37]. The inner ear neurons exhibits amazing capability to resist perilymphatic alkalosis (up to pH 10) for 30 min [38]–[41]. Therefore, macroscopic pH increase can be ignored.
The local and transient pH elevation within the vicinity of the proximity sensor is theoretically high but the inner ear is equipped with a swift defense mechanism against alkalosis. Experimentally, the local pH elevation should be quantified using antimony or iridium oxide electrode. The transient pH elevation cannot be alleviated by the slow carbonic buffer system [42]–[44]. However, tissues in the inner ear including hair cells produce carbonic anhydrate which catalyzes the buffer reaction at the speed up to 106. This speed is likely to protect the hair cells from alkalosis effectively [45], [46]. Finally, the anodic reaction will not cause any problems because the anode can be placed outside of the ST to isolate the reaction at the Ag/AgCl electrode from the ST solution.
Via neural prostheses such as CI, the net dc current has to be minimized or eliminated completely from the amperostatic electrical stimulation because the excessive net dc current is harmful. First of all, the dc current of 100 nA was observed in an inner ear as a byproduct of routine stimulation via a commercially available and FDA-approved CI [47]. Again, the oxygen reduction has been widely applied as chronic electrochemical oxygen sensors implanted in human brains, hearts and in vivo animals. Therefore, the maximum current of 20 nA observed in this report is encouraging regarding the biocompatibility of the SECM in the inner ear.
Furthermore, there is a huge scientific difference between the amperostatic electrical stimulation via standard neural prostheses and the potentiostatic electrochemical oxygen reduction. Amperostatic charging of electrons on the capacitive neural membrane is adequate to control the membrane potentials of neurons, to raise them above the threshold potentials constantly, and to elicit action potentials robustly [48]. However, the current to elicit neural responses (>1 mA) requires the voltage across the electrode-solution interface to be about 10 V because of the resistance between the implanted electrodes (~10 kΩ), which surpasses by far any electrolysis potentials, [47], [49]. Since dc is the sum of any Faradic reactions, Lily et al introduced biphasic charge balanced electrical stimulation to cancel out the net-dc and demonstrated the biocompatibility [50]. A caveat is that the biphasic stimulation can minimize the Faradic reaction so long as the anodic and cathodic reactions are reversible with each other. To remove Faradic reactions completely from the amperostatic biphasic stimulation, it is effective to restrict the charge transfer within the realm of electrical double layer formation at the electrode solution interface or submonolayer electrodeposition of H+ at the electrode-solution interface [51], [52]. Since capacitive accumulation of ions precedes any Faradic reaction, limiting the pulse duration and current density can remove the nonspecific Faradic reaction. Conversely, the oxygen sensor utilizes potentiostatic voltage application. The Nernst equation states that any Faradic reactions harmful against body tissues grow exponentially as a function of voltage. Potentiostatic voltage control is suitable to control the Faradic reactions. The anodic reaction on the electrode surface is controlled directly such that the causality of the biocompatibility is clearer. Therefore, the criteria as to the biocompatibility have to be determined differently for these two technology types.
F. Toward Trajectory Estimation/Implementation
The MATLAB insertion model demonstrated that the interpretation of the SECM results can be well explained in a 3-D analysis and used as a predictor of the trajectory of the proximity sensor by taking advantage of the isotropic proximity sensor characteristics. To develop a system to guide CI insertion in order to avoid contact to ST tissues without any other visualization method during insertion, this MATLAB insertion model can be further developed.
The SECM proximity sensing system should be expanded from single- to multichannel to near-uniquely determine the estimated trajectory. The isotropic characteristic of the proximity sensor does not guarantee the direction in which the wall is close except for the case in which the sensor is almost touching the wall. Two additional proximity sensors placed on the both sides of one proximity sensor will be able to determine the direction of the wall in 3-D. The density of the proximity sensor array necessary in one CI carrier can be estimated and designed using the MATLAB model.
More quantitative characterization and demonstration will complement the study. Ideally, trajectory validation should be performed by simultaneous monitoring using two optical methods with a cochlea model without light distortion or with light pathway calibration. In case of in vitro or in vivo experiments, the position of the electrode has to be visualized fluoroscopically. We have gained some confidence in the detectability of biological tissues such as bone and basilar membrane using the proposed methods in the other preliminary studies. Another possible line of analysis to further quantitatively characterize the relation between SECM data and trajectory is to use the MATLAB 3-D model for a numerical modeling of diffusion and convection.
Currently, preoperative CT scan is becoming a standard procedure in clinics. Robotic insertion of the CI is also on the horizon. By combining these technologies, electrode trajectory estimation during CI surgery is an exciting goal.
VI. Conclusion
We demonstrated the feasibility to apply SECM as a novel proximity sensor to help achieve atraumatic insertion of a CI electrode. The design and fabrication of the Pt proximity sensor is similar to those of the CI stimulation electrode, so the proximity sensor can be easily implemented. O2 was a desirable choice as an endogenous and biocompatible redox mediator. The proximity sensing was successfully demonstrated using the prototyped Pt microelectrode and O2 mediator in an acrylic scala tympani model under atmospheric pressure. By developing a numerical insertion model in conjunction with the µCT scan of the ST model, we showed that the proximity sensor can be useful for estimating the trajectory of the CI carrier. The dc current measured in the proximity sensor was well below those flowing in existing clinical devices. Taken together, these conclusions strongly support further investigation of the proposed technology.
Acknowledgments
This work was supported in part by the Cornell NanoScale Facility, a member of the National Nanotechnology Infrastructure Network, which is supported by the National Science Foundation under Grant ECS-0335765, and in part by the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract DE-AC02–98CH10886. The work of MVM was supported by the National Science Foundation under Grant CHE-0957313.
Contributor Information
H. Watanabe, Email: hw2420@columbia.edu, NYU Langone Medical Center, New York University School of Medicine, New York, NY 10016 USA.
J. Velmurugan, Email: vjeyvel@yahoo.com, Queens College, Flushing, NY 11367 USA.
M. V. Mirkin, Email: mmirkin@qc.cuny.edu, Queens College, Flushing, NY 11367 USA.
M. A. Svirsky, Email: Mario.Svirsky@NYUMC.ORG, New York University School of Medicine, New York, NY 10016 USA.
A. K. Lalwani, Email: akl2144@cumc.columbia.edu, Department of Otolaryngology, Columbia University Medical Center, New York, NY 10032, USA.
R. R. Llinas, Email: rodolfo.llinas@nyumc.org, NYU Langone Medical Center, New York University School of Medicine, New York, NY 10016 USA.
References
- 1.Woodson EA, Reiss LA, Turner CW, Gfeller K, Gantz BJ. The hybrid cochlear implant: A review. Adv. Otorhinolaryngol. 2010;67:125–134. doi: 10.1159/000262604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedland DR, Runge-Samuelson C. Soft cochlear implantation: Rationale for the surgical approach. Trends Amplif. 2009;13:124–138. doi: 10.1177/1084713809336422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briggs RJ, Tykocinski M, Saunders E, Hellier W, Dahm M, Pyman B, Clark GM. Surgical implications of perimodiolar cochlear implant electrode design: Avoiding intracochlear damage and scala vestibuli insertion. Cochlear. Implants Int. 2001;2:135–149. doi: 10.1179/cim.2001.2.2.135. [DOI] [PubMed] [Google Scholar]
- 4.Turner CW, Reiss LA, Gantz BJ. Combined acoustic and electric hearing: Preserving residual acoustic hearing. Hear Res. 2008;242:164–171. doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Xu SA, Cohen LT, Clark GM. Cochlear view: Postoperative radiography for cochlear implantation. Am. J. Otol. 2000;21:49–56. [PubMed] [Google Scholar]
- 6.Kong WJ, Cheng HM, Ma H, Wang YJ, Han P. Evaluation of the implanted cochlear implant electrode by CT scanning with three-dimensional reconstruction. Acta. Otolaryngol. 2012;132:116–122. doi: 10.3109/00016489.2011.626794. [DOI] [PubMed] [Google Scholar]
- 7.Campbell AP, Suberman TA, Buchman CA, Fitzpatrick DC, Adunka OF. Flexible cochlear microendoscopy in the gerbil. Laryngoscope. 2010;120:1619–1624. doi: 10.1002/lary.20979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carelsen B, Grolman W, Tange R, Streekstra GJ, van Kemenade P, Jansen RJ, Freling NJ, White M, Maat B, Fokkens WJ. Cochlear implant electrode array insertion monitoring with intraoperative 3D rotational X-ray. Clin. Otolaryngol. 2007;32:46–50. doi: 10.1111/j.1365-2273.2007.01319.x. [DOI] [PubMed] [Google Scholar]
- 9.Fishman AJ, Roland JT, Jr, Alexiades G, Mierzwinski J, Cohen NL. Fluoroscopically assisted cochlear implantation. Otol. Neurotol. 2003;24:882–886. doi: 10.1097/00129492-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Bruce IA, Bates JE, Melling C, Mawman D, Green KM. Hearing preservation via a cochleostomy approach and deep insertion of a standard length cochlear implant electrode. Otol. Neurotol. 2011;32:1444–1447. doi: 10.1097/MAO.0b013e3182355824. [DOI] [PubMed] [Google Scholar]
- 11.Jianbai W, Wise KD. A thin-film cochlear electrode array with integrated position sensing. J. Microelectromech. Syst. 2009;18:385–395. [Google Scholar]
- 12.Schurzig D, Labadie RF, Hussong A, Rau TS, Webster RJ. A force sensing automated insertion tool for cochlear electrode implantation; Proc. IEEE Int. Conf. Robot. Autom; 2010. pp. 3674–3679. [Google Scholar]
- 13.Zhang J, Xu K, Simaan N, Manolidis S. A pilot study of robot-assisted cochlear implant surgery using steerable electrode arrays. Med. Image Comput. Comput.-Assisted Intervention - Miccai 2006, Pt 1. 2006;4190:33–40. doi: 10.1007/11866565_5. [DOI] [PubMed] [Google Scholar]
- 14.Sun P, Laforge FO, Mirkin MV. Scanning electrochemical microscopy in the 21st century. Phys. Chem. Chem. Phys. 2007;9:802–823. doi: 10.1039/b612259k. [DOI] [PubMed] [Google Scholar]
- 15.Goldwyn JH, Bierer SM, Bierer JA. Modeling the electrode-neuron interface of cochlear implants: Effects of neural survival, electrode placement, and the partial tripolar configuration. Hear Res. 2010;268:93–104. doi: 10.1016/j.heares.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danneel HL. Uber den durch diffundierende gase hervorgerufenen restrom. Zeitschrift Elektrochem. 1897;4:227–242. [Google Scholar]
- 17.Schmickler W, Santos E. Interfacial Electrochemistry. Berlin, Germany Springer: 2010. Inner sphere and ion-transfer reactions; pp. 145–162. [Google Scholar]
- 18.McHugh SB, Fillenz M, Lowry JP, Rawlins JN, Bannerman DM. Brain tissue oxygen amperometry in behaving rats demonstrates functional dissociation of dorsal and ventral hippocampus during spatial processing and anxiety. Eur. J. Neurosci. 2011;33:322–337. doi: 10.1111/j.1460-9568.2010.07497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haupt H, Scheibe F, Ludwig C, Petzold D. Measurements of perilymphatic oxygen tension in guinea pigs exposed to loud sound. Eur. Arch. Otorhinolaryngol. 1991;248:413–416. doi: 10.1007/BF01463566. [DOI] [PubMed] [Google Scholar]
- 20.Scheibe F, Haupt H, Ludwig C. Intensity-dependent changes in oxygenation of cochlear perilymph during acoustic exposure. Hear Res. 1992;63:19–25. doi: 10.1016/0378-5955(92)90069-y. [DOI] [PubMed] [Google Scholar]
- 21.Bazzu G, Puggioni GG, Dedola S, Calia G, Rocchitta G, Migheli R, Desole MS, Lowry JP, O’Neill RD, Serra PA. Real-time monitoring of brain tissue oxygen using a miniaturized biotelemetric device implanted in freely moving rats. Anal. Chem. 2009;81:2235–2241. doi: 10.1021/ac802390f. [DOI] [PubMed] [Google Scholar]
- 22.Holmstrom N, Nilsson P, Carlsten J, Bowald S. Long-term in vivo experience of an electrochemical sensor using the potential step technique for measurement of mixed venous oxygen pressure. Biosens Bioelectron. 1998;13:1287–1295. doi: 10.1016/s0956-5663(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 23.Gough DA, Kumosa LS, Routh TL, Lin JT, Lucisano JY. Function of an implanted tissue glucose sensor for more than 1 year in animals. Sci. Transl. Med. 2010;2:42–53. doi: 10.1126/scitranslmed.3001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark LC, Jr, Wolf R, Granger D, Taylor Z. Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol. 1953;6:189–193. doi: 10.1152/jappl.1953.6.3.189. [DOI] [PubMed] [Google Scholar]
- 25.Dings J, Meixensberger J, Jager A, Roosen K. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery. 1998;43:1082–1095. doi: 10.1097/00006123-199811000-00045. [DOI] [PubMed] [Google Scholar]
- 26.Keddie S, Rohman L. Reviewing the reliability, effectiveness and applications of licox in traumatic brain injury. Nurs. Crit. Care. 2012;17:204–212. doi: 10.1111/j.1478-5153.2012.00499.x. [DOI] [PubMed] [Google Scholar]
- 27.van Santbrink H, Maas AI, Avezaat CJ. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery. 1996;38:21–31. doi: 10.1097/00006123-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Kwak J, Bard AJ. Scanning electrochemical microscopy. theory of the feedback mode. Anal. Chem. 1989;61:1221–1227. [Google Scholar]
- 29.Wangemann P, Schacht J. The Cochlea. Springer Handbook of Auditory Research. Vol. 8. New York, NY, USA: Springer-Verlag; 1996. Homeostatic mechanisms in the cochlea; pp. 130–185. [Google Scholar]
- 30.Sun P, Mirkin MV. Kinetics of electron-transfer reactions at nano-electrodes. Anal. Chem. 2006;78:6526–6534. doi: 10.1021/ac060924q. [DOI] [PubMed] [Google Scholar]
- 31.Clark JR, Warren FM, Abbott JJ. A scalable model for human scala-tympani phantoms. J. Med. Devices. 2011;5:014501. [Google Scholar]
- 32.Kontorinis G, Lenarz T, Stover T, Paasche G. Impact of the insertion speed of cochlear implant electrodes on the insertion forces. Otol. Neurotol. 2011;32:565–570. doi: 10.1097/MAO.0b013e318219f6ac. [DOI] [PubMed] [Google Scholar]
- 33.Todd CA, Naghdy F, Svehla MJ. Force application during cochlear implant insertion: An analysis for improvement of surgeon technique. IEEE Trans. Biomed. Eng. 2007 Jul.54(7):1247–1255. doi: 10.1109/TBME.2007.891937. [DOI] [PubMed] [Google Scholar]
- 34.Rajan GP, Kontorinis G, Kuthubutheen J. The effects of insertion speed on inner ear function during cochlear implantation: A comparison study. Audiol. Neurootol. 2013;18:17–22. doi: 10.1159/000342821. [DOI] [PubMed] [Google Scholar]
- 35.Hibbert DB, Weitzner K, Carter P. Voltammetry of platinum in artificial perilymph solution. J. Electrochem. Soc. 2001;148:E1–E7. [Google Scholar]
- 36.Antoine O, Durand R. RRDE study of oxygen reduction on Pt nanoparticles inside nafion®: H2 O2 production in PEMFC cathode conditions. J. Appl. Electrochem. 2000;30:839–844. [Google Scholar]
- 37.Magid E, Turbeck BO. The rates of the spontaneous hydration of CO2 and the reciprocal reaction in neutral aqueous solutions between 0° and 38°. Biochimica et Biophysica Acta (BBA)—General Subjects. 1968;165:515–524. doi: 10.1016/0304-4165(68)90232-8. [DOI] [PubMed] [Google Scholar]
- 38.Kuijpers W, Bonting SL. The cochlear potentials. II. the nature of the cochlear endolymphatic resting potential. Pflugers Arch. 1970;320:359–372. doi: 10.1007/BF00588214. [DOI] [PubMed] [Google Scholar]
- 39.Arakawa E, Marcus DC, Thalmann R. Dependence of endocochlear potential on vascular pH. Hear Res. 1987 Nov.31:1–7. doi: 10.1016/0378-5955(87)90209-7. [DOI] [PubMed] [Google Scholar]
- 40.Wakizono S, Komune S, Uemura T. Susceptibility of the endocochlear potential to pH and osmolarity changes in the perilymph of the cochlea in the guinea pig. Eur. Arch. Otorhinolaryngol. 1990;247:97–99. doi: 10.1007/BF00183176. [DOI] [PubMed] [Google Scholar]
- 41.Nimura Y, Mori Y, Inui T, Sohma Y, Takenaka H, Kubota T. Effects of CO2/HCO3− in perilymph on the endocochlear potential in guinea pigs. J. Physiol. Sci. 2007 Feb.57:15–22. doi: 10.2170/physiolsci.RP012006. [DOI] [PubMed] [Google Scholar]
- 42.Horrocks BR, Mirkin MV, Pierce DT, Bard AJ, Nagy G, Toth K. Scanning electrochemical microscopy. 19. ion-selective potentiometric microscopy. Anal. Chem. 1993;65:1213–1224. [Google Scholar]
- 43.Ballestrasse CL, Ruggeri RT, Beck TR. Calculations of the pH changes produced in body tissue by a spherical stimulation electrode. Ann. Biomed. Eng. 1985;13:405–424. doi: 10.1007/BF02407769. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Du Y, Fang C. Developing an iridium oxide film modified microelectrode for microscale measurement of pH. Electroanalysis. 2007;19:608–611. [Google Scholar]
- 45.Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997;74:1–20. doi: 10.1016/s0163-7258(96)00198-2. [DOI] [PubMed] [Google Scholar]
- 46.Okamura HO, Sugai N, Suzuki K, Ohtani I. Enzyme-histochemical localization of carbonic anhydrase in the inner ear of the guinea pig and several improvements of the technique. Histochem. Cell Biol. 1996 Oct.106:425–430. doi: 10.1007/BF02473302. [DOI] [PubMed] [Google Scholar]
- 47.Huang CQ, Shepherd RK, Carter PM, Seligman PM, Tabor B. Electrical stimulation of the auditory nerve: Direct current measurement in vivo. IEEE Trans. Biomed. Eng. 1999 Apr.46(4):461–470. doi: 10.1109/10.752943. [DOI] [PubMed] [Google Scholar]
- 48.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952 Aug.117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan CT, Svirsky M, Anwar A, Kumar S, Caessens B, Carter P, Treaba C, Roland JT., Jr Real-time measurement of electrode impedance during intracochlear electrode insertion. Laryngoscope. 2013 Apr.123:1028–1032. doi: 10.1002/lary.23714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lilly JC, Hughes JR, Alvord EC, Jr, Galkin TW. Brief, noninjurious electric waveform for stimulation of the brain. Science. 1955;121:468–479. doi: 10.1126/science.121.3144.468. [DOI] [PubMed] [Google Scholar]
- 51.Schmickler W, Santos E. Interfacial Electrochemistry. Berlin, Germany: Springer; 2010. The metal-solution interface; pp. 39–50. [Google Scholar]
- 52.Brummer SB, Turner MJ. Electrochemical considerations for safe electrical stimulation of the nervous system with platinum electrodes. IEEE Trans. Biomed. Eng. 1977 Jan.24(1):59–63. doi: 10.1109/TBME.1977.326218. [DOI] [PubMed] [Google Scholar]