Abstract
Transmission of Plasmodium falciparum is hyperendemic in southern Zambia. However, no data on the entomologic aspects of malaria transmission have been published from Zambia in more than 25 years. We evaluated seasonal malaria transmission by Anopheles arabiensis and An. funestus s.s. and characterized the blood feeding behavior of An. arabiensis in two village areas. Transmission during the 2004–2005 rainy season was nearly zero because of widespread drought. During 2005–2006, the estimated entomologic inoculation rate values were 1.6 and 18.3 infective bites per person per transmission season in each of the two village areas, respectively. Finally, with a human blood index of 0.923, An. arabiensis was substantially more anthropophilic in our study area than comparable samples of indoor-resting An. arabiensis throughout Africa and was the primary vector responsible for transmission of P. falciparum.
Introduction
Anopheles arabiensis Patton is one of the most recognized malaria vectors in Africa.1, 2 Even in the presence of other notorious vector species such as An. gambiae s.s. Giles and An. funestus s.s. Giles, An. arabiensis plays a major role, contributing a large proportion of the infectious mosquito bites that drive intense malaria transmission in communities throughout sub-Saharan Africa.3–7 However, this species differs dramatically from An. gambiae s.s. and An. funestus s.s. by its striking variation in anthropophily across continental Africa and Madagascar (Table 1), and more exophilic and exophagic behaviors.1, 7–9 Anopheles arabiensis frequently feeds on cattle, goats, chickens, dogs, and other available wild and domestic animals.10–13 Additionally, An. arabiensis are known to bite humans outdoors7 as well as indoors and have been collected in window exit traps, illustrating a behavior where some mosquitoes exit the house after feeding rather than remaining to rest on the interior walls where they are easily collected.9, 14, 15 These feeding and resting behaviors complicate the role of An. arabiensis in malaria transmission, the ease with which this role is effectively determined through traditional field sampling techniques, and ultimately malaria control. The behavioral characteristics and involvement of An. arabiensis in malaria transmission is vastly different from region to region in Africa and can be understood only in the local context of available hosts, blood feeding preferences, and additional vector species.
Table 1. Human blood index (HBI) of indoor-resting Anopheles arabiensis throughout Africa*.
| Human blood index of indoor-resting Anopheles arabiensis | ||
|---|---|---|
|
| ||
| HBI | Country | Reference |
| .859, .924 | Kenya | Joshi et al., 50 Service et al., 51 Fontaine et al., 52, 53 Garrett-Jones et al. 32 |
| 0.39 | Kenya | Highton et al. 54 |
| 0.631 | Kenya | Mnzava et al.12 |
| 0.23, 0.67 | Kenya | Githeko et al.11 |
| 0.2 | Eritrea | Waka et al.42 |
| 0.915 | Ethiopia | Hadis et al.40 |
| 0.552 | Ethiopia | Habtewold et al.41 |
| 0.88 | Ethiopia | Adugna and Petros39 |
| 0.82 | The Gambia | Bøgh et al.43 |
| 0.738 | Senegal | Fontenille et al.3 |
| 0.6, 0.68 | Senegal | Lemason et al.48 |
| 0.55, 1.0 | Nigeria | White and Rosen,55 Garrett-Jones et al.32 |
Data represent indoor collections of An. arabiensis in unsprayed houses. Multiple values are indicative of repeated studies.
The Southern Province of Zambia experiences hyperendemic transmission of Plasmodium falciparum.16 However, little is known about Anopheles mosquito ecology in Zambia, and how vector species composition and their relative roles in transmission vary throughout the country. Few studies on the mosquitoes in Zambia have been published, most of which were performed before differentiating members of the An. gambiae and An. funestus complexes became possible17–20 and was incorporated into standard practice for entomologic field investigations.10,21,22 The distribution of members of the An. gambiae and An. funestus species complexes in Zambia and their relative importance to malaria transmission is a basic question that remains to be answered. Data gathered to date suggest that although An. gambiae s.s. has been reported from the Northwestern Province23and from the Zambezi valley in Zimbabwe,2 malaria transmission in the Southern Province of Zambia is maintained primarily by An. arabiensis in the apparent absence of An. gambiae s.s., with the less-abundant An. funestus s.s. playing a secondary role (Thuma P, unpublished data).10,21
Our specific aims were to investigate the seasonal intensity of malaria transmission in the Macha region of the Southern Province of Zambia by An. arabiensis and An. funestus s.s. through estimation of the entomologic inoculation rates (EIRs). We also aimed to characterize the blood feeding behavior of indoor-resting An. arabiensis in this area by determining the human blood index and blood host preferences in the context of relative host availability. Herein, we provide necessary information on the seasonality and vectorial potential of An. arabiensis populations in southern Zambia, which can be compared with other behaviorally diverse populations of this species throughout Africa, and more importantly, data which may be directly applied towards evaluating the success of future malaria control programs. Furthermore, severe drought conditions in the Southern Province during 2004– 2005 have allowed the resilience of the mosquito population to be explored in terms of mosquito population rebound after extended dry conditions and the resumption of malaria transmission the following season.
Materials and Methods
Study area
The Johns Hopkins Malaria Research Institute's field station in Macha, Zambia is located in the Southern Province at approximately 16.39292°S, 26.79061°E at an elevation of approximately 1,000 meters above sea level. The habitat is characterized as Miombo woodland. Average annual rainfall varies greatly in Zambia, but averages 600–1,000 mm (24–40 inches) in the southern parts of the country.2 There is one rainy season each year that lasts from approximately November to April, followed by cool dry (April– August) and hot dry (August–November) seasons. Two village areas that were representative of the local demography and landscape, easily accessible, had a history of high malaria incidence, and were amenable to participating in the study were selected for monthly mosquito sampling. Chidakwa and Lupata areas are located approximately 5 km from each other in close proximity to the research facility and Macha Mission Hospital. One hundred forty and 244 people were enumerated in Chidakwa and Lupata, respectively, in the houses sprayed for mosquito collections. Bed nets were rarely encountered in these areas until March 2006 when 1,000 nets were distributed to the community independently of this project. No indoor residual spraying has occurred in this region.
Mosquito collection and handling
Engorged field specimens of An. arabiensis were collected by pyrethrum spray catch24 and human landing catch25 in Chidakwa and Lupata during November 2004–May 2005 and November 2005–May 2006. Each village area was sampled intensely for one week each month. During each collection week, human landing catches were performed on Monday and Wednesday nights, and pyrethrum spray catches were carried out on Thursday, Friday, and Saturday mornings. Collection methods were approved by the Johns Hopkins Bloomberg School of Public Health Committee on Human Research (CHR) and the University of Zambia Research Ethics Committee (UNZREC) (H26.03.08.01.A2). Collectors were informed of potential risks associated with the human landing catches and were screened every two weeks for malaria. For human landing catches, the same four houses in each village area were sampled throughout each of the two consecutive years. Teams of collectors were rotated among the houses on different collection nights to minimize sampling bias. Pyrethrum spray catches were executed with a household pyrethroid spray and were used to calculate the average number of An. arabiensis and An. funestus s.s. per house per night. Fifteen houses were sprayed each of the three mornings with collections ending no later than 10:00 AM. Additional pyrethrum spray catches were performed throughout the 2,000-km2 Macha catchment area and were included only in the determination of the human blood index for An. arabiensis and not in host preference calculations. Immediately after collection, specimens were killed by freezing, morphologically identified,1,26 packed individually in tubes containing silica gel desiccant and cotton, and stored at room temperature. Rainfall was measured manually from a rain gauge positioned on the research station grounds centrally located between the village areas.
Preparation of DNA and polymerase chain reaction (PCR)
DNA was extracted separately from mosquito abdomens and head/thoraces by a modified salt procedure as previously described.27The identity of all specimens morphologically identified as An. gambiae s.l. or An. funestus s.l. was confirmed by PCR.28,29 Head/thorax DNA extractions were screened for P. falciparum by nested PCR.30 DNA extracted from NF54 P. falciparum culture was used as a positive control for each set of reactions. Mosquito blood meals from all An. arabiensis collected by pyrethrum spray catch were identified by PCR, using 2 μL of the DNA extraction volume from engorged abdomens as template.27 Novel chicken/guinea fowl forward and reverse primers were manually designed to be used with the existing multiplexed primer set, using sequence data retrieved from GenBank: chicken (accession no. DQ236093, Guinea fowl (Numida meleagris, NC_006382, AP003322). Because unique forward primers for both chicken and guinea fowl could not be selected within the fragment of interest, a downstream chicken/guinea fowl reverse primer was designed to work specifically with the reverse complement of the existing universal primer (UNFOR1029: 5′-TAACCTGAATCGGAAGCCAACC-3′ and Chick1123R: 5′-GAAGAGGATAAGTAGGATGGTGAAG-3′). The expected chicken/guinea fowl PCR product size was 95 basepairs. Additionally, one nucleotide of the original universal reverse primer, UNREV1025, was altered to a mixed base to accommodate sequence differences in avian cytochrome b (UNREV1025B: 5′-GGTTG[T/G]CCTCCAATTCAT-GTTA-3′). Primer sequences were checked for Tm compatibility and self-complementarity using Primer3 software.31 These two novel avian primers were included in the original multiplexed set together with the novel universal reverse UNREV1025B. DNA amplifications were visualized after electrophoresis on an ethidium bromide-stained 2% agarose gels. All gels were run with GeneRuler 100-basepair DNA ladder (Fermentas Inc., Hanover, MD).
Human blood index and forage ratios
The human blood index (HBI) was determined for only indoor-resting An. arabiensis. Outdoor-resting collections were attempted to produce a more unbiased estimate of the HBI,32 but were not useful because of low mosquito numbers. The blood meals from all An. arabiensis collected by pyrethrum spray catch in Chidakwa, Lupata, and in supplemental regional collections were analyzed by PCR as described above27 and included in the HBI calculation. Mixed blood meals were each treated as two separate blood meals in calculations.
Numbers of people, cattle, goats, dogs, cats, pigs, and poultry (chickens plus guinea fowl) per household were obtained from each family in Chidakwa and Lupata in whose houses mosquito collections were performed to determine mosquito feeding preference relative to the local availability of different vertebrate species.33 The proportion of all potential hosts that each animal comprised was calculated to obtain the unbiased percentage of blood meals expected to be taken from each vertebrate species. The significance of differences between observed and expected blood meals from engorged An. arabiensis was analyzed by chi-square test. Only blood meals from Chidakwa and Lupata were included in the forage ratio analysis.
Entomologic inoculation rate
The human biting rate was calculated directly from human landing catches as the average number of bites per person per night.25 The EIR was calculated as the product of the human biting rate and the sporozoite rate.25 For comparative purposes, sporozoite rates were determined from An. arabiensis collected by both human landing catch and pyrethrum spray catch.34 The nightly EIR was multiplied by 30 days to obtain an approximation of the monthly transmission intensity in each village area.25 Monthly EIRs in each village area were then added together to derive an approximate seasonal EIR (November–May).
Results
Seasonality
A total of 394 An. arabiensis were collected by both spray catch and human landing catch in both Chidakwa and Lupata village areas over the entire two-year study period. During the 2004–2005 rainy season, Zambia experienced a severe drought, receiving only about 44% of the expected rainfall in the Southern Province.35 The Macha region received approximately 457 mm (18 inches) of rain between November 2004 and May 2005, and 914 mm (36 inches) of rain between November 2005 and May 2006. As a result, mosquito numbers were exceptionally low in both villages (Figure 1A and B). The EIR during the 2004–2005 rainy season was too low to be calculated by standard entomologic sampling techniques in both villages. Only one sporozoite-positive An. arabiensis and one sporozoite-positive An. funestus s.s. were collected between November 2004 and May 2005, both in Lupata. Doubling the amount of rainfall in the 2005– 2006 rainy season resulted in an approximately 10-fold increase in the numbers of An. arabiensis resting inside human sleeping houses each night (Figure 1A and B). Mosquito numbers peaked in February in Lupata in the 2005–2006 transmission season as compared with April during the 2004–2005 transmission season, and there was less of a lag period between the start of the rains and measurable mosquito activity in both village areas during 2005–2006 (Figure 1A and B). Transmission intensity by An. arabiensis increased gradually throughout the 2005–2006 rainy season, peaking in April 2006 (Figure 1C). The seasonal pattern of transmission intensity by An. arabiensis directly corresponded with malaria cases admitted to the Macha Mission Hospital during this period (Figure 1C). No An. funestus s.s. were collected during the 2005–2006 transmission season.
Figure 1.
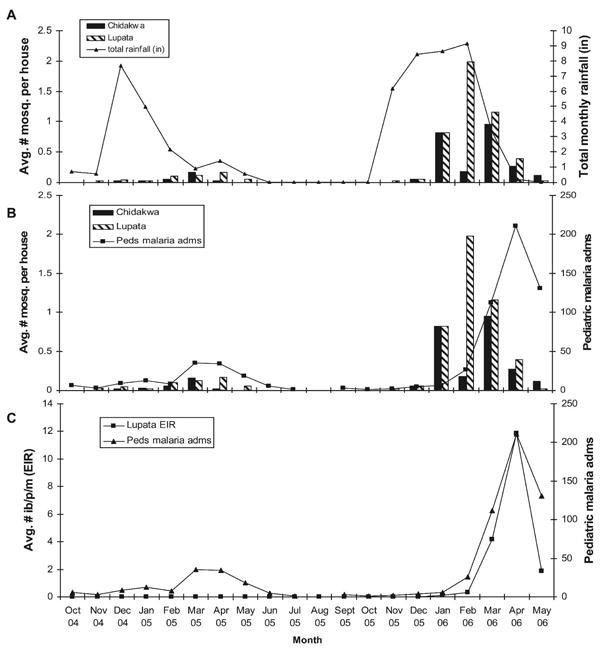
Total rainfall, indoor-resting density and entomologic inoculation rates (EIRs) of Anopheles arabiensis, and confirmed cases of Plasmodium falciparum malaria admitted to the pediatric ward of the Macha Mission Hospital, in Macha, Zambia, 2004–2006. Mosquito (mosq.) indoor-resting densities were determined by pyrethrum spray catch. Mosquito resting and EIR data were collected from Chidakwa and Lupata village areas only, whereas hospital admissions were from communities throughout the region. Peds malaria adms = pediatric malaria admission; ib/p/m = infective bites/person/month.
Blood feeding behavior
The multiplexed PCR diagnostic successfully identified 455 (80%) of 565 blood meals from engorged An. arabiensis. The 565 blood meals analyzed included 146 An. arabiensis collected from villages thoughout the 2,000-km2 Macha area; the remaining An. arabiensis blood meals were from the Chidakwa and Lupata samples. Of the 455 identified blood meals, 420 were human, resulting in a human biting index of 0.923 for indoor-resting An. arabiensis. The remaining blood meals were taken from dogs, goats, cattle, chickens, and a pig (Table 2). Thirteen An. arabiensis fed on multiple hosts. In relation to host availability in Chidakwa and Lupata, chi-square values were highly significant in both villages, indicating that the bias towards human feeding by An. arabiensis was indicative of a specific host preference for people and not a reflection of relative host availability (Table 3).
Table 2. Blood meals identified from indoor-resting Anopheles arabiensis in Macha, Zambia, 2004–2006.
| Blood host | No. |
|---|---|
| Human | 420 |
| Cattle | 11 |
| Dog | 5 |
| Goat | 3 |
| Pig | 1 |
| Chicken | 2 |
| Human plus cattle | 4 |
| Human plus dog | 5 |
| Human plus chicken | 3 |
| Human plus goat | 1 |
Table 3. Numbers of observed and expected blood meals from indoor-resting Anopheles arabiensis based on relative host availability in Chidakwa and Lupata villages, Macha, Zambia*.
| Host | Chidakwa | Lupata | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| % of surveyed hosts | No. of observed blood meals | No. of expected blood meals | χ2 | % of surveyed hosts | No. of observed blood meals | No. of expected blood meals | χ2 | |
| Human | 33.30 | 57 | 27.66 | 31.11 | 27.26 | 134 | 49.07 | 147.01 |
| Cattle | 17.62 | 4 | 14.62 | 7.72 | 10.50 | 1 | 18.90 | 16.95 |
| Goat | 17.62 | 2 | 14.71 | 10.98 | 18.44 | 0 | 33.19 | 33.19 |
| Dog | 5.48 | 2 | 4.55 | 1.43 | 6.26 | 3 | 11.27 | 6.07 |
| Pig | 5.71 | 0 | 4.74 | 4.74 | 4.69 | 1 | 8.44 | 6.56 |
| Poultry | 17.86 | 3 | 14.82 | 9.43 | 31.40 | 1 | 56.52 | 54.54 |
Poultry includes both chickens and guinea fowl. Cats were surveyed but no cat blood meals were identified. For Chidakwa village, n = 68, χ2 = 67.38, degrees of freedom (df) = 6, P < 0.001. For Lupata village, n = 140, χ2 = 212.39, df = 6, P < 0.001.
Entomologic inoculation rate
The estimated seasonal EIR for each village depended greatly on the sampling methodology used (human landing catches or pyrethrum spray catches). Despite providing good estimates of the human biting rate, too few mosquitoes were collected during human landing catches to detect low sporozoite infection rates. Therefore, the EIR estimated entirely from human landing catches underestimated the true transmission intensity in each village area. The EIR calculated entirely from human landing catches in Chidakwa during 2005–2006 was 0, and was 3.75 infective bites per person per transmission season (November–May) in Lupata. This figure from Lupata represents only one infected mosquito collected in April 2006. Alternatively, pyrethrum spray catches produced large enough mosquito collections to obtain reasonable estimates of the sporozoite rate. Therefore, the EIR was calculated using the human biting rates derived from the human landing catches, and the sporozoite rates determined from the pyrethrum spray catch collections for each village area each month (Table 4). These estimates were considerably higher, at 1.608 infective bites per person per season in Chidakwa, and 18.330 infective bites per person per season in Lupata.
Table 4. Seasonal entomologic inoculation rates (EIRs) of Plasmodium falciparum by Anopheles arabiensis in Chidakwa and Lupata communities during the 2005–2006 rainy season*.
| Month | Chidakwa | Lupata | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HBR | Sporozoite rate | Monthly EIR | HBR | Sporozoite rate | Monthly EIR | |
| Nov 2005 | 0.00 | 0.00% | 0.000 | 0.00 | 0.00% | 0.000 |
| Dec 2005 | 0.00 | 0.00% | 0.000 | 0.00 | 0.00% | 0.000 |
| Jan 2006 | 0.67 | 4.30% | 0.858 | 0.13 | 2.40% | 0.090 |
| Feb 2006 | 0.50 | 0.00% | 0.000 | 0.88 | 1.20% | 0.315 |
| Mar 2006 | 0.50 | 0.00% | 0.000 | 2.63 | 5.30% | 4.182 |
| Apr 2006 | 0.00 | 41.70% | 0.000 | 1.88 | 21.10% | 11.869 |
| May 2006 | 0.13 | 20.00% | 0.75 | 0.13 | 50.00% | 1.875 |
| Total | 1.608 | 18.330 | ||||
Human biting rates (HBRs) are expressed as the number of bites per person per night and were determined by human landing catch. Sporozoite rates were determined from pyrethrum spray catches performed the same week in each area. Monthly EIRs were estimated by multiplying each average nightly EIR by 30 days.
Discussion
In this study, we describe malaria transmission dynamics over a two-year period in southern Zambia. During 2004– 2005, mosquito activity was substantially reduced because of a severe, extended drought in the region. These dry conditions, coupled with high temperatures, resulted in a 40–90% loss of the maize crop and scarce water availability in the Southern Province.35 Confirmed malaria cases in the Macha Mission Hospital were also reduced during 2004–2005 (Figure 1B and C). Under these drought conditions, only about one An. arabiensis was collected in every 5–25 houses throughout the 2004–2005 transmission season, and 10 An. funestus s.s. were recorded over that entire collection period from both village areas. Despite rains during the 2005–An. funestus s.s. were collected. Thus, although An. funestus s.s. is known to be a malaria vector in this region, transmission during 2005–2006 was driven solely by An. arabiensis. Anopheles funestus has previously been reported to be sensitive to drought and changing environmental conditions. Mouchet and others36 observed the gradual disappearance of An. funestus after extended dry conditions and accompanying habitat modification.
Despite the extended drought conditions, An. arabiensis numbers rebounded strongly during the 2005–2006 season when the rains returned (Figure 1A and B). In semi-arid regions of Eritrea, An. arabiensis density and malaria transmission intensity display similar patterns in relation to rainfall, as well as spatial and seasonal heterogeneity among sites.37 Although Chidakwa and Lupata communities were separated by approximately 5 km, indoor-resting density, human biting rate, and malaria transmission by An. arabiensis were all much higher in Lupata than in Chidakwa. This finding suggests localized and spatially heterogeneous transmission intensity among villages in this region. Topographically, Lupata is spread out over a series of hilltops separated by a 1–2-km-long, low drainage area where water collects in large, stagnant, sunlit pools. Anopheles arabiensis and An. quadriannulatus (Theobald) larvae have been identified from these sites, which are near (< 100 meters) to concentrated human habitation on either side of the drainage area. In contrast, Chidakwa is located in a flatter area with fewer identifiable mosquito larval sites. Low, wet areas in Chidakwa where we have observed An. gambiae s.l. larvae typically dry up within a few days after a heavy rain. These differences in the extent and temporal availability of suitable larval habitat for An. arabiensis may account for some of the differences observed in transmission intensity between these two closely situated villages. These results are consistent with those reported by Shililu and others, who recorded 15–17-fold difference in transmission intensity by An. arabiensis between two villages located in the same ecological zone in Eritrea.37 Additionally, Mendis and others5 reported a negative correlation between the EIR and the distance from breeding sites.
Figure 1 illustrates the progression of the transmission season from the start of the rains in October 2005, to peak indoor-resting density of An. arabiensis in February–March 2006, followed by a peak in both confirmed pediatric malaria cases and An. arabiensis EIR in April 2006. Although few An. arabiensis were collected late in the rainy season during April and May, a higher proportion of these were infective and actively transmitting P. falciparum (Figure 1B and C and Table 4). These environmental, entomologic, and clinical data are biologically cohesive. As the 2005–2006 rainy season progressed, mosquito numbers increased as the ground became saturated and more breeding sites were available. Mid-season, infection levels built up in both the vector and reservoir populations as more mosquitoes were actively biting people indoors and picking up and transmitting parasites. Late in the rainy season, breeding sites began to dry up as the rains ceased, and mosquito numbers decreased. However, a large proportion of the few remaining An. arabiensis were infective, presumably because of mosquito longevity and increased exposure to gametocytemic persons throughout the season. Malaria cases peaked at the end of the rainy season because the highest proportion of mosquitoes were actively transmitting.
The 2005–2006 seasonal EIR for An. arabiensis was estimated to be approximately 1.6 in Chidakwa and 18.3 in Lupata, which had increased from undetectable levels during the 2004–2005 drought. Little is known about the entomologic aspects of malaria transmission in this region prior to the 2004–2005 drought for comparative purposes. Preliminary pyrethrum spray catches performed in Lupata village area in March 2002 confirmed that the primary vectors in this region were An. arabiensis and An. funestus s.s., and from those data a monthly EIR of approximately 7.8 infective bites per person was estimated (Thuma P, unpublished data), with An. arabiensis contributing all the infectious bites. This March 2002 EIR of 7.8 derived from spray catches probably significantly underestimates the actual EIR. However, even those limited data demonstrate that malaria transmission in Lupata in 2001–2002 was substantially higher than in either 2004–2005 or 2005–2006, and that malaria transmission in 2005–2006 had not resumed to pre-drought levels despite the increase in rainfall. These levels of transmission intensity by An. arabiensis are low compared with those reported in other parts of Africa. Mendis and others compared the EIR of An. arabiensis and An. funestus s.s. in a village in Mozambique and determined that the two species were equally important vectors: An. funestus s.s. delivered 14 infective bites per year and An. arabiensis delivered an additional 13 infective bites.5 In Tanzania, annual EIRs ranged from 122 to 320 for An. arabiensis and from 30 to 88 for An. funestus s.s.6 Shililu and others reported between 0 and 70 infective bites per person per year by An. arabiensis in different ecologic zones in Eritrea.37 Finally, in Senegal, EIRs over four consecutive years ranged from 7 to 63 infective bites per person per year, with An. arabiensis responsible for 66% of transmission, An. gambiae 31%, and An. funestus s.s. 3%.3The relative importance of each of these vector species also is known to fluctuate seasonally and by habitat composition.7,38 Therefore, in an area such as Macha, Zambia, with hyperendemic transmission maintained by An. arabiensis and An. funestus, it is important to determine how transmission intensity fluctuates spatially and seasonally, especially given the differential response of these two vector species to natural variability in environmental conditions. This information is crucial to direct future research and control efforts.
It is unclear why mosquito numbers in Chidakwa during February 2006 were so low (Figure 1A and B). High rainfall during the latter part of January could have flushed out mosquito breeding sites just prior to the collection period, or extremely wet or cooler weather conditions during that three-day collection period could have suppressed mosquito activity. Corresponding collections in Lupata during February 2006 occurred exactly one week later, perhaps when weather conditions were more amenable to mosquito activity or a new cohort had emerged. Sampling mosquitoes at least twice per month in each village would alleviate such stochastic influences on the data and decrease the variation in estimated indoor mosquito–resting density. 25
Ninety-two percent of the blood meals identified from An. arabiensis in the Macha region came from human hosts, indicating that this population of An. arabiensis in southern Zambia is highly anthropophilic compared with similar collections of indoor-resting An. arabiensis in other parts of Africa (Table 1). This finding is consistent with its status as maintaining hyperendemic levels of malaria transmission in the virtual absence of other vector species. Although there was inevitably some sampling bias because mosquitoes were only collected from inside human sleeping huts, the blood host choice of An. arabiensis was significantly biased towards humans despite the identification of additional blood meals from the variety of the other abundant domestic animals present in the study villages (Tables 2 and 3). There are many factors that influence the human feeding behavior of anophelines that are reflected in the HBI. For example, differences in HBI were noted according to whether the dwellings sampled were inhabited by humans, livestock, or mixed populations,39–41 if collections were from indoors, outdoors, or multiple biotopes,32,42 the relative proximity of houses to livestock enclosures,41,43 the relative availability of different potential hosts,44,45 and whether houses were sprayed with insecticide.15,32 In Macha, the houses sampled were not mixed, but usually < 20 meters away from livestock enclosures, and had never been sprayed with a residual insecticide.
Blood meals from two different vertebrate hosts were identified in 13 (2.8%) of 455 An. arabiensis (Table 2). The most common combination of mixed blood meals was human plus dog. Dogs were not the most common domestic animal in the villages. However, small puppies in particular were commonly observed inside houses whereas the larger livestock and poultry were penned outdoors at night. The presence of multiple blood meals from these combinations of hosts suggests that at least some individual An. arabiensis are feeding both indoors and outdoors during the same or consecutive nights. Multiple blood meals from the same host were not evaluated. Multiple blood meals have been widely reported in field-collected An. gambiae and An. funestus complex mosquitoes, and have been determined to be necessary for some mosquitoes to complete their first gonotrophic cycle.46,47 Mixed or double blood meals have been frequently reported for An. arabiensis, and represented between 2% and 26% of the total blood meals analyzed from engorged specimens collected inside human dwellings.3,11,40,41,43,48 Lemasson and others reported four specimens of An. gambiae s.l. that each contained blood from three different vertebrate species.48 Multiple feeding behavior can be epidemiologically important if infectious An. arabiensis feed more frequently on healthy people each gonotrophic cycle, and especially if the infection status of the mosquito increases its propensity to take multiple blood meals.49
The novel avian primers successfully identified chicken blood meals from engorged An. arabiensis (results confirmed by sequencing). However, inclusion of the avian primers in the multiplexed primer set decreased the amplification efficiency of the human-specific primer, indicating that dimerization or some other interference between these primers was occurring. This effect could not be alleviated by adjusting the primer concentrations or amplification settings. Therefore, for human malaria vectors, we recommend using the original multiplexed primer set27 and then if desired screening the failed reactions for the presence of avian DNA separately because avian blood meals were present at such a low rate. The quality of the DNA extractions where blood meal identification failed was verified. Therefore, blood meals that were not identified presumably contained vertebrate DNA that was too degraded by digestion for successful amplification, or the mosquitoes fed on a host not included in the diagnostic PCR.
In summary, we have described the first entomologic field investigations performed in the Southern Province of Zambia in more than 25 years. Transmission of P. falciparum is hyperendemic, and maintained primarily by An. arabiensis with An. funestus s.s. contributing secondarily, in the apparent absence of An. gambiae s.s. Transmission was reduced virtually to 0 during the 2004–2005 rainy season because of severe and widespread drought throughout the region. During 2005–2006 when rainfall was double that of the previous year, indoor-resting densities of An. arabiensis increased 10-fold, and transmission of P. falciparum rebounded to estimated annual EIR values of approximately 1.6 and 18 infective bites per person in each of the two study villages. Transmission was maintained solely by An. arabiensis in 2005–2006 because no An. funestus s.s. were detected. Transmission intensity was also spatially hetereogenous, with vastly different An. arabiensis larval sites, indoor-resting densities, human biting rates, and EIRs in communities just 5 km apart from each other. Finally, An. arabiensis was substantially more anthropophilic in our study area than comparable samples obtained for indoor-resting An. arabiensis from other parts of Africa. Collectively, these data will be directly used to evaluate the efficacy of future vector control interventions planned for this region. They are also representative of the magnitude at which mosquito populations and transmission of P. falciparum can resume in malaria-endemic areas after lengthy drought conditions.
Acknowledgments
We thank Harry Hamapumbu for organizing and managing the field team, and Petros Moono, Patricia Muleya, Pamela Sinywimaanzi, Fidelis Chanda, Lusyomo Chikobolo, Collence Munsanje, Rodwell Moono, Peter Simakwati, Guide Hansumo, Scene Mudenda, Betham Dubeka, Frederick Mwiinga, Buster Musanje, Maron Mulota, and Kalizya Sinyangwe for performing mosquito collections.
Financial support. This study was supported in part by funding to Douglas E. Norris from the Johns Hopkins Malaria Research Institute, a Johns Hopkins Malaria Research Institute pre-doctoral fellowship award to Rebekah J. Kent, a Johns Hopkins Bloomberg School of Public Health Global Field Experience Fund award to Rebekah J. Kent, and a National Institute of Environmental Health Sciences training award (T32ES07141) to Rebekah J. Kent.
References
- 1.Gillies MT, Coetzee M. A Supplement to The Anophelinae of Africa South of the Sahara. Johannesburg: South African Institute for Medical Research; 1987. Publication no. 55. [Google Scholar]
- 2.Coetzee M, Craig M, Le Sueur D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today. 2000;16:74–77. doi: 10.1016/s0169-4758(99)01563-x. [DOI] [PubMed] [Google Scholar]
- 3.Fontenille D, Lochouarn L, Diatta M, Sokhna C, Dia I, Diagne N, Lemasson JJ, Ba K, Tall A, Rogier C, Trape JF. Four years' entomological study of the transmission of seasonal malaria in Senegal and the bionomics of Anopheles gambiae and An. arabiensis. Trans R Soc Trop Med Hyg. 1997;91:647–652. doi: 10.1016/s0035-9203(97)90506-x. [DOI] [PubMed] [Google Scholar]
- 4.Shililu JI, Maier WA, Seitz HM, Orago AS. Seasonal density, sporozoite rates and entomological inoculation rates of Anopheles gambiae and Anopheles funestus in a high-altitude sugarcane growing zone in western Kenya. Trop Med Int Health. 1998;3:706–710. doi: 10.1046/j.1365-3156.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 5.Mendis C, Jacobsen JL, Gamage-Mendis A, Bule E, Dgedge M, Thompson R, Cuamba N, Barreto J, Begtrup K, Sinden RE, Høgh B. Anopheles arabiensis and An. funestus are equally important vectors of malaria in Matola coastal suburb of Maputo, southern Mozambique. Med Vet Entomol. 2000;14:171–180. doi: 10.1046/j.1365-2915.2000.00228.x. [DOI] [PubMed] [Google Scholar]
- 6.Ijumba JN, Mosha FW, Lindsay SW. Malaria transmission risk variations derived from different agricultural practices in an irrigated area of northern Tanzania. Med Vet Entomol. 2002;16:28–38. doi: 10.1046/j.0269-283x.2002.00337.x. [DOI] [PubMed] [Google Scholar]
- 7.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 8.Ralisoa Randrianasolo BO, Coluzzi M. Genetical investigations on zoophilic and exophilic Anopheles arabiensis from Antananarivo area (Madagascar) Parassitologia. 1987;29:93–97. [PubMed] [Google Scholar]
- 9.Ameneshewa B, Service MW. Resting habits of Anopheles arabiensis in the Awash river valley of Ethiopia. Ann Trop Med Parasitol. 1996;90:515–521. doi: 10.1080/00034983.1996.11813077. [DOI] [PubMed] [Google Scholar]
- 10.Bransby-Williams WR. House catches of adult Anopheles gambiae species B in two areas of Zambia. East Afr Med J. 1979;11:557–561. [PubMed] [Google Scholar]
- 11.Githeko AK, Service MW, Mbogo CM, Atieli FK, Juma FO. Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta Trop. 1994;58:307–316. doi: 10.1016/0001-706x(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 12.Mnzava AEP, Mutinga MJ, Staak C. Host blood meals and chromosomal inversion polymorphisms in Anopheles arabiensis in the Baringo district of Kenya. J Am Mosq Control Assoc. 1994;10:507–510. [PubMed] [Google Scholar]
- 13.Mwangangi JM, Mbogo CM, Nzovu JG, Githure JI, Yan G, Beier JC. Blood-meal analysis for anopheline mosquitoes sampled along the Kenyan coast. J Am Mosq Control Assoc. 2003;19:371–375. [PubMed] [Google Scholar]
- 14.Thompson MC, Connor SJ, Quinones ML, Jawara M, Todd J, Greenwood BM. Movement of Anopheles gambiae s.l. malaria vectors between villages in The Gambia. Med Vet Entomol. 1995;9:413–419. doi: 10.1111/j.1365-2915.1995.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 15.Githeko AK, Adungo NI, Karanja DM, Hawley WA, Vulule JM, Seroney IK, Ofulla AV, Atieli FK, Ondijo S, Genga IO, Odada PK, Situbi PA, Oloo JA. Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol. 1996;82:306–315. doi: 10.1006/expr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 16.Larkin GL, Thuma PE. Congenital malaria in a hyperendemic area. Am J Trop Med Hyg. 1991;45:587–592. doi: 10.4269/ajtmh.1991.45.587. [DOI] [PubMed] [Google Scholar]
- 17.DeMeillon B. A Note on Anopheles gambiae and Anopheles funestus in Northern Rhodesia, Entomological Studies. Johannesburg: South African Medical Research Institute; 1937. p. 306. Publication no. 7. [Google Scholar]
- 18.Adams PC. Some observations on the flight of stained anophelines at Nkana, Northern Rhodesia. Ann Trop Med Parasitol. 1940;34:35–43. [Google Scholar]
- 19.Pielou DP. Anopheline mosquitoes breeding in fish dams, pools, and streams in northern Rhodesia. Proc R Entomol Soc Lond. 1947;22:18–23. [Google Scholar]
- 20.Paterson HE. Direct evidence for the specific distinctness of forms A, B, and C of the An. gambiae complex. Riv Malariol. 1964;43:191–196. [PubMed] [Google Scholar]
- 21.Shelley AJ. Observations on the behavior of An. gambiae species B in Kambole village in the Zambezi valley, Zambia. Ann Trop Med Parasitol. 1973;67:237–248. doi: 10.1080/00034983.1973.11686884. [DOI] [PubMed] [Google Scholar]
- 22.Kent RJ, Coetzee M, Mharakurwa S, Norris DE. Feeding and resting behaviour of the mosquito Anopheles longipalpis in an area of hyperendemic malaria transmission in southern Zambia. Med Vet Entomol. 2006 doi: 10.1111/j.1365-2915.2006.00646.x. published online 10/17.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann T, Licht M, Elissa N, Maega BT, Chimumbwa JM, Watsenga ET, Wondji CS, Simard F, Hawley WA. Population structure of An. gambiae in Africa. J Hered. 2003;94:133–147. doi: 10.1093/jhered/esg024. [DOI] [PubMed] [Google Scholar]
- 24.Service MW. Mosquito Ecology: Field Sampling Methods. Second. London: Elsevier Applied Science; 1976. [Google Scholar]
- 25.Beier J. Vector incrimination and entomological inoculation rates. Methods Mol Med. 2002;72:3–10. doi: 10.1385/1-59259-271-6:01. [DOI] [PubMed] [Google Scholar]
- 26.Gillies MT, DeMeillon B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region) Second. Johannesburg: South African Institute for Medical Research; 1968. Publication no. 54. [Google Scholar]
- 27.Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome b. Am J Trop Med Hyg. 2005;73:336–342. [PMC free article] [PubMed] [Google Scholar]
- 28.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 29.Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;6:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- 30.Snounou G, Viriyakoso S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 31.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 32.Garrett-Jones C, Boreham PFL, Pant CP. Feeding habits of anophelines (Diptera: Culicidae) in 1971–1978, with reference to the human blood index: a review. Bull Entomol Res. 1980;70:165–185. [Google Scholar]
- 33.Hess AD, Hayes RO, Tempelis CH. The use of forage ratio technique in mosquito host preference studies. Mosq News. 1968;28:386–389. [Google Scholar]
- 34.Githeko AK, Service MW, Mbogo CM, Atieli FK, Juma FO. Plasmodium falciparum sporozoite and entomological inoculation rates at Ahero rice irrigation scheme and the Miwani sugar-belt in western Kenya. Ann Trop Med Parasitol. 1993;87:379–391. doi: 10.1080/00034983.1993.11812782. [DOI] [PubMed] [Google Scholar]
- 35.FEWS NET. Food Security Update. Washington DC: US Agency for International Development; 2005. Available from http://www.fews.net/centers/?f=zm. [Google Scholar]
- 36.Mouchet J, Faye O, Julvez J, Manguin S. Drought and malaria retreat in the Sahel, west Africa. Lancet. 1996;348:1735–1736. doi: 10.1016/s0140-6736(05)65860-6. [DOI] [PubMed] [Google Scholar]
- 37.Shililu J, Ghebremeskel T, Mengistu S, Fekadu H, Zerom M, Mbogo C, Githure J, Novak R, Brantly E, Beier JC. High seasonal variation in entomologic inoculation rates in Eritrea, a semi-arid region of unstable malaria in Africa. Am J Trop Med Hyg. 2003;69:607–613. [PubMed] [Google Scholar]
- 38.Fontenille D, Simard F. Unravelling complexities in human malaria transmission dynamics in Africa through a comprehensive knowledge of vector populations. Comp Immunol Microbiol Infect Dis. 2004;27:357–375. doi: 10.1016/j.cimid.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Adugna N, Petros B. Determination of the human blood index of some anopheline mosquitoes by using ELISA. Ethiop Med J. 1996;34:1–10. [PubMed] [Google Scholar]
- 40.Hadis M, Lulu M, Makonnen Y, Asfaw T. Host choice by indoor-resting Anopheles arabiensis in Ethiopia. Trans R Soc Trop Med Hyg. 1997;91:376–378. doi: 10.1016/s0035-9203(97)90245-5. [DOI] [PubMed] [Google Scholar]
- 41.Habtewold T, Walker AR, Curtis CF, Osir EO, Thapa N. The feeding behaviour and Plasmodium infection of Anopheles mosquitoes in southern Ethiopia in relation to use of insecticide-treated livestock for malaria control. Trans R Soc Trop Med Hyg. 2001;95:584–586. doi: 10.1016/s0035-9203(01)90086-0. [DOI] [PubMed] [Google Scholar]
- 42.Waka M, Hopkins RJ, Akinpelu O, Curtis C. Transmission of malaria in the Tesseney area of Eritrea: parasite prevalence in children, and vector density, host preferences, and sporozoite rate. J Vector Ecol. 2005;30:27–32. [PubMed] [Google Scholar]
- 43.Bøgh C, Clarke SE, Pinder M, Sanyang F, Lindsey SW. Effect of passive zooprophylaxis on malaria transmission in The Gambia. J Med Entomol. 2001;38:822–828. doi: 10.1603/0022-2585-38.6.822. [DOI] [PubMed] [Google Scholar]
- 44.Diatta M, Spiegel A, Lochouarn L, Fontenille D. Similar feeding preferences of Anopheles gambiae and A. arabiensis in Senegal. Trans R Soc Trop Med Hyg. 1998;92:270–272. doi: 10.1016/s0035-9203(98)91005-7. [DOI] [PubMed] [Google Scholar]
- 45.Killeen GF, McKenzie FE, Foy DB, Bøgh C, Beier JC. The availability of potential hosts as a determinant of feeding behaviours and malaria transmission by African mosquito populations. Trans R Soc Trop Med Hyg. 2001;95:469–476. doi: 10.1016/s0035-9203(01)90005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillies MT. Recognition of age-groups within populations of Anopheles gambiae by the pre-gravid rate and the sporozoite rate. Ann Trop Med Parasitol. 1954;48:58–74. doi: 10.1080/00034983.1954.11685599. [DOI] [PubMed] [Google Scholar]
- 47.Gillies MT. The pre-gravid phase of ovarian development in Anopheles funestus. Ann Trop Med Parasitol. 1955;49:320–325. doi: 10.1080/00034983.1955.11685681. [DOI] [PubMed] [Google Scholar]
- 48.Lemasson JJ, Fontenille D, Lochouarn L, Dia I, Simard F, Ba K, Diop A, Diatta M, Molez JF. Comparison of behavior and vector efficiency of Anopheles gambiae and An. arabiensis (Diptera: Culicidae) in Barkedji, a Sahelian area of Senegal. J Med Entomol. 1997;34:396–403. doi: 10.1093/jmedent/34.4.396. [DOI] [PubMed] [Google Scholar]
- 49.Koella JC, Sorensen FL, Anderson RA. The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc R Soc Lond B Biol Sci. 1998;1398:763–768. doi: 10.1098/rspb.1998.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi GP, Service MW, Pradhan GD. A survey of species A and B of the Anopheles gambiae Giles complex in the Kisumu area of Kenya prior to insecticidal spraying with OMS-43 (fenithrothion) Ann Trop Med Parasitol. 1975;69:91–104. doi: 10.1080/00034983.1975.11686988. [DOI] [PubMed] [Google Scholar]
- 51.Service MW, Joshi GP, Pradhan GD. A survey of Anopheles gambiae (species A) and An. arabiensis (species B) of the An. gambiae Giles complex in the Kisumu area of Kenya following insecticidal spraying with OMS-43 (fenithrothion) Ann Trop Med Parasitol. 1978;72:377–386. doi: 10.1080/00034983.1978.11719332. [DOI] [PubMed] [Google Scholar]
- 52.Fontaine RE, Pull J, Payne D, Pradhan GD, Joshi GP, Pearson JA. Evaluation of Fenithrothion (OMS 43) for Malaria Control in a Large-Scale Epidemiological Trial, Kisumu, Kenya. Geneva: World Health Organization; 1976. WHO/VBC/76.645. [Google Scholar]
- 53.Fontaine RE, Pull JH, Payne D, Pradhan GD, Joshi GP, Pearson JA, Thymakis MK, Ramos Camacho ME. Evaluation of fenithrothion for the control of malaria. Bull World Health Organ. 1978;56:445–452. [PMC free article] [PubMed] [Google Scholar]
- 54.Highton RB, Bryan JH, Boreham PFL, Chandler JA. Studies on the sibling species Anopheles gambiae Giles and A. arabiensis Patton (Diptera: Culicidae) in the Kisumu area, Kenya. Bull Entomol Res. 1979;69:43–53. [Google Scholar]
- 55.White GB, Rosen P. Comparative studies on sibling species of the Anopheles gambiae Giles complex (Diptera: Culicidae). II. Ecology of species A and B in savanna around Kaduna, Nigeria, during transition from wet to dry season. Bull Entomol Res. 1973;62:613–625. [Google Scholar]


