Abstract
The first appearances of West Nile virus (family Flaviviridae, genus Flavivirus, WNV) in New Mexico were reported in late summer to early fall 2002. Several dead birds tested positive for WNV, and 78 equine cases were confirmed. All mosquito pools tested (n = 268) were negative. A statewide surveillance program was launched in May 2003 to study the emergence and spread of this new arbovirus in mosquitoes from the Rio Grande valley. Mosquitoes were trapped at 32 sites along a 750-km stretch of the Rio Grande valley. Sites were trapped for one night either weekly or biweekly, by using CO2-baited CDC light traps and gravid traps. Pools of captured mosquitoes were tested for WNV by reverse transcription-polymerase chain reaction. By mid-July 2003, WNV levels in the mosquito population had reached levels that were detectable by the surveillance program. Positive pools of mosquitoes were found in the Rio Grande valley from mid-July through late September. In total, 75 positive pools were found, from sites throughout the study area. The predominant species infected with WNV in this region were Culex tarsalis (Coquillett) in rural areas, and Culex salinarius (Coquillett) and Culex pipiens quinquefasciatus (Say) in urban areas. There were 202 human cases and 438 equine cases of WNV in New Mexico in 2003, which corresponded well in time with the positive mosquitoes. Our results seemed to be consistent with introduction of WNV in late summer 2002, followed by a period of transmission and amplification cycles between local avian hosts and mosquito vectors.
Keywords: West Nile virus, New Mexico Rio Grande, mosquitoes, emergence
The first cases of West Nile virus (family Flaviviridae, genus Flavivirus, WNV) were documented in the northeastern United States in 1999 (CDC 1999, Nash et al. 2001). In the following years, this arbovirus spread south and west across the country. By fall 2002, WNV had been detected in all states of the continental United States except for Oregon, Nevada, Utah, and Arizona (CDC 2002). In New Mexico, Idaho, and Washington, no human cases were reported, but animal infection was detected (Ettestad et al. 2004). In several counties in eastern and central New Mexico, 78 equine cases and several WNV-positive dead birds were reported, and it was fully expected that human cases would occur in 2003 (Ettestad et al. 2004). A statewide mosquito trapping program was designed to detect the presence of WNV in mosquito populations to provide insight into WNV ecology in the semiarid desert climate. Various studies had already established the relevant mosquito vectors in the eastern United States; however, there was still speculation as to which species would be involved in the southwestern United States (Turell et al. 2002). In New Mexico, Culex pipiens (L.) is not found, although other Culex are present (Carpenter and LaCasse 1955, Schultz 1987). Identifying which species would be responsible for WNV transmission was therefore a primary objective of this program.
Another goal was to characterize the pattern of introduction and subsequent spread of this pathogen. Not only would this serve as a general investigation of the introduction of WNV to the state but also it would represent a unique opportunity to study an emerging arbovirus as it entered a new environment. Because the New Mexico Rio Grande valley had not previously been exposed to WNV, this represented a chance to monitor a newly introduced virus as it spread through a naïve ecosystem. In addition to providing an understanding of the ecology of WNV in the state, this opportunity also might provide insight into how other pathogens might spread if introduced into this area, either naturally or intentionally.
We hypothesized two possible scenarios for the establishment of WNV. In the first scenario, the virus would be introduced from either the northern reaches of the river valley, near Alamosa, CO, or the southern end, at El Paso, TX, and gradually spread north or south along the river to the opposite end. Both Colorado and Texas had already reported human cases of WNV by 2002, indicating a potential for introduction from either direction. In the second scenario, the virus would become established throughout the valley nearly simultaneously. This would most likely occur if the virus was introduced by migratory birds in 2002 and then amplified at several latitudes at about the same time in 2003. If this was the case, the failure to find positive pools in 2002 would have been due to virus prevalence below a detectable threshold. In either scenario, we hoped to be able to draw some conclusions as to the nature of the establishment of WNV in New Mexico.
Materials and Methods
Collection sites were selected along the Rio Grande valley at intervals of 25–30 km (Fig. 1). Mosquito trapping of the area from Taos, NM (Arroyo Hondo), to San Marcial, NM (Ft. Craig), was conducted by Albuquerque Bio-Disease Management. Collaboration with local mosquito abatement programs extended the surveillance into Alamosa, CO, in the north and into El Paso, TX, in the south. In this way, the majority of the valley was covered throughout the state. The study area extended over ≈750 km of the river from north to south.
Fig. 1.
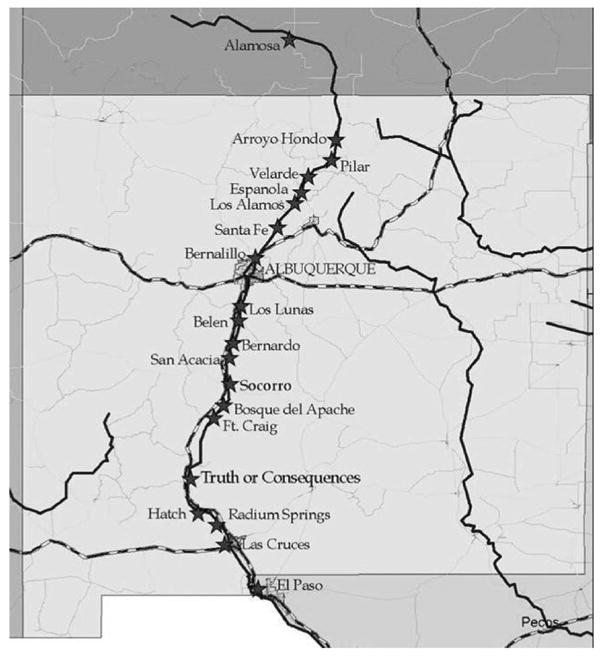
Map of New Mexico, southern Colorado, and western Texas, showing the location of all sites along the Rio Grande in the 2003 WNV study.
The state of New Mexico is largely composed of semiarid desert, with scrubby Chihuahuan Desert vegetation in the south, transitioning northward into piñon–juniper, and eventually Ponderosa pine at higher elevations. Winding through the state from north to south is the Rio Grande, bounded by a relatively fertile strip of riparian forest vegetation, known as bosque. The bosque is composed largely of cottonwood, Populus deltoides ssp. wizlizenii S. Wats., forest, interspersed with salt cedar, Tamarix spp., and Russian olive, Elaeagnus angustifolia L. The bosque provides an attractive refuge for wildlife seeking relief from the hot, dry conditions of the desert (Finch and Tainter 1995). As such, it serves as a corridor for the passage of migratory birds and concentrates diverse species, including insects, as well as human settlements (Yong and Finch 2002). Many years of trapping by the Albuquerque Bio-Disease Management program within the city had shown the valley to be an area heavily populated by mosquitoes.
Sites were trapped every other week from mid-May until late October or early November. Sites were no longer trapped once mosquito catches were nearly zero; north of Albuquerque, trapping ended in late October, and sites south of Albuquerque reached zero in mid-November. Each site was trapped using a gravid trap and two CDC light traps (John Hock Company, Gainesville, FL). The gravid trap was baited with a mixture of horse manure, grass clippings, and bacterial culture (Pro-Pump Liquid Live Bacteria High Count, Ecological Laboratories, Freeport, NY) and was set in the forest undergrowth. Gravid trap attractant was mixed in a large barrel and allowed to ferment for 5–7 d before use. All sites were supplied from the same mixture to ensure consistency of the samples collected. The two CDC light traps were baited with dry ice CO2, with one trap placed at 1.5 m from the ground, and the other trap suspended in the forest canopy, at ≈10–15 m. Results from the different trap types and microhabitats have been described in a separate study (DiMenna 2005). Traps were set in the afternoon or evening and collected the next morning. Trap nets were tied off and placed into a cooler filled with dry ice to kill and preserve the captured mosquitoes.
Mosquitoes were stored in a freezer at −82°C until being sorted and counted by species under a dissecting microscope, by using published keys (Pratt and Barnes 1959, Darsie and Ward 1981). Pools of up to 50 individuals of a species were saved for WNV testing.
Pools from each trapping period were selected for WNV testing. Pool selection was based primarily on the number of individuals of any given species in a sample. Typically, any pool with 15 or more individuals was tested, with Culex species or any gravid females being preferentially chosen, under the assumption that these might be the most likely to contain WNV-positive mosquitoes. Pools from the city of Albuquerque or certain collaborators with existing arrangements were sent to the New Mexico Department of Health Laboratory for testing. Specimens collected along the Rio Grande by our teams outside of the city of Albuquerque were sent to the University of Illinois Medical Entomology Laboratory (Illinois Natural History Survey, Champaign, IL) for WNV testing by reverse transcription-polymerase chain reaction (RT-PCR). Both laboratories used a QIAamp RNA kit (QIAGEN, Valencia, CA) for viral lysis and RNA extraction. A TaqMan PE 7700 sequence detection system was used for amplification and signal detection, according to the manufacturer's specifications, for 40 cycles (Applied Biosystems, Foster City, CA). The primer sequences were selected according to published results (Lanciotti et al. 2000).
Results
Infected mosquitoes were first captured concurrently at sites throughout the entire north–south range of the study during the last 2 wk of July 2003. The predominant species of mosquito found infected with WNV in the Rio Grande valley were Culex tarsalis (Coquillett), Culex pipiens quinquefasciatus (Say), and Culex salinarius (Coquillett) (Table 1).
Table 1. Total numbers of each species captured are shown, with the number of pools of each species that were tested for WNV by RT-PCR.
| Species | No. caught, total (%) | No. pools tested | No. positive pools | % positive |
|---|---|---|---|---|
| Cx. tarsalis | 7,791 (14.5) | 303 | 27 | 8.9 |
| Cx. salinarius | 12,047 (22.4) | 227 | 24 | 10.6 |
| Cx. p. quinquefasciatus | 4,526 (8.4) | 126 | 23 | 18.3 |
| Ae. vexans | 16,884 (31.5) | 151 | 0 | 0 |
| Ae. dorsalis | 9,130 (17.0) | 26 | 1 | 3.8 |
| Aedes spp. | 1,377 (2.6) | 34 | 0 | 0 |
| Culiseta spp. | 1,530 (2.9) | 47 | 0 | 0 |
| Anopheles spp. | 383 (0.7) | 22 | 0 | 0 |
| Total | 53,668 | 936 | 74 | 7.9 |
The numbers of positive pools of each species are shown, and the percentage of pools tested that were positive is indicated. Other Aedes species includes pooled results of Ae. nigromaculis and Ae. sollicitans.
The first positive pool was of Aedes dorsalis (Meigen), captured in Alamosa on 19 June. No positive pools were collected in the New Mexico Rio Grande valley until 3 wk later in mid-July 2003. The week of 12 July, four sites in the South Valley area of Albuquerque, and one in the North Valley, had multiple pools of Cx. tarsalis and Cx. salinarius that were WNV positive. At the same time, sites as far north as Arroyo Hondo, Velarde, and Española, and as far south as Ft. Craig, had WNV -positive mosquitoes from the same species as well as Cx. p. quinquefasciatus.
In the following trapping period, WNV was detected in mosquitoes captured throughout the state as far south as El Paso, TX, and Las Cruces, NM. In addition, several additional positive pools were captured in Albuquerque; in Alamosa, CO; and in nearly all of the northern New Mexico sites in between. The positive sites in the northern regions (Fig. 1) were Arroyo Hondo, Pilar, Velarde, Española, Santa Fe, and Bernalillo. The majority (76.9%) of the 13 sites in Albuquerque that had positive pools were located in the southern region of the city.
There was substantial temporal variation among infected pools of each Culex species (Fig. 2). In the first two trapping periods with WNV-infected pools, the majority (19 of 20) of positive pools were Cx. tarsalis (12 pools) and Cx. salinarius (seven pools). The number of infected pools of Cx. tarsalis was highest the week of 28 July and then quickly declined, whereas infected pools of Cx. p. quinquefasciatus were most numerous the week of 11 August. Overall infection rates for Culex mosquitoes are shown in Table 2.
Fig. 2. Positive pools of Culex spp. mosquitoes are shown for the trapping periods during which WNV was detected.
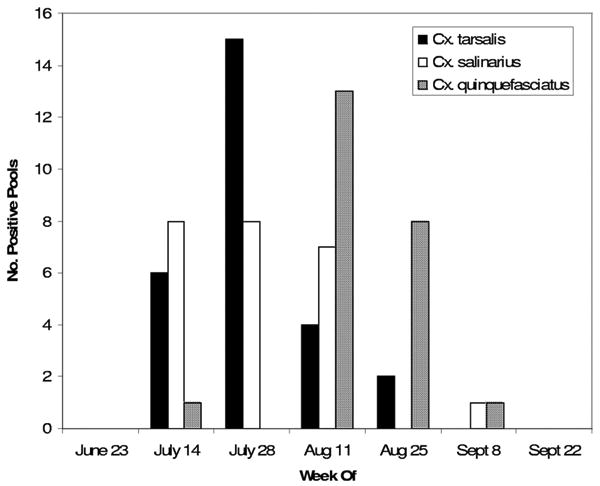
Table 2. Infection rates are shown for Culex spp. mosquitoes from 2003.
| Species | Infection rate (MLE)/1,000 | 95% CI |
|---|---|---|
| Cx. tarsalis | 4.98 | 3.36-7.14 |
| Cx. salinarius | 3.4 | 2.24-4.99 |
| Cx. p. quinquefasciatus | 18.23 | 11.68-27.65 |
The Microsoft Excel version 2.0 add-in developed by Biggerstaff (2004) was used to calculate bias-corrected maximum likelihood estimates and 95% CIs.
Overall infection rates were similar for all Culex spp. mosquitoes caught at rural sites (Fig. 3). Infection rates were slightly higher in Cx. tarsalis and Cx. salinarius caught at urban sites than for those of all three Culex spp. at rural sites. Much higher infection rates were found for Cx. p. quinquefasciatus in urban habitats.
Fig. 3.
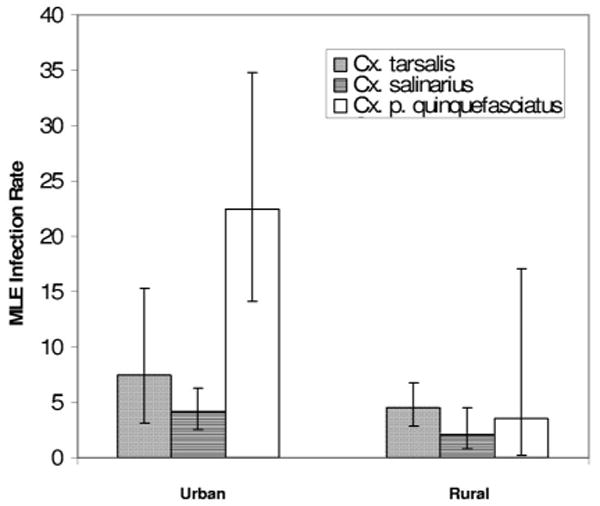
Maximum likelihood estimates (MLEs) of infection rate are shown with 95% CIs for Culex spp. mosquitoes captured in urban and rural sites.
The onset of symptoms of the first human case of WNV in New Mexico occurred on 9 July, within the city limits of Albuquerque (Fig. 4). Altogether, there were 209 confirmed cases of human WNV in New Mexico in 2003, four of which were fatal, and 438 equine cases (Ettestad et al. 2004). The majority of the human cases were reported before the week of 28 September; however, one human case had onset of symptoms the week of 19 October (Ettestad et al. 2004). Incidence of human disease was highest from late July until late August, with the peak number of cases reporting onset of symptoms the week of 11 August (Fig. 4). By mid-August, sites north of Albuquerque no longer yielded infected mosquitoes. By September only one site, Bosque del Apache National Wildlife Refuge, still had positive mosquitoes. Although nearly all of the sites within the Albuquerque area had at least one positive pool from mid-July to late August, only four sites in the far south valley area of the city had infected mosquitoes caught in several weeks throughout this peak period. Specific sites in Albuquerque are not named or mapped here to protect the privacy of the residential neighborhoods in which they were located.
Fig. 4.
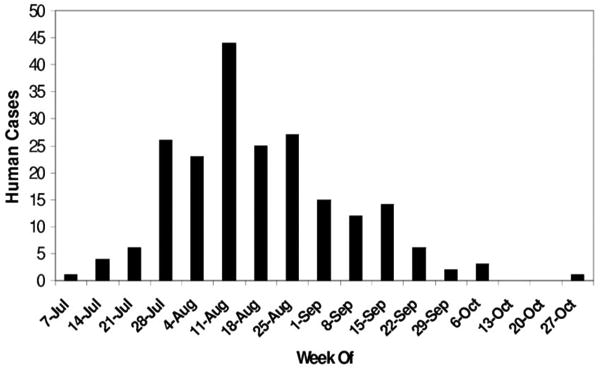
Human cases of WNV in New Mexico, 2003 are shown by the week during which onset of symptoms was reported. Human data are provided courtesy of the New Mexico Department of Health.
Discussion
West Nile virus was first detected among Culex mosquitoes in New Mexico at widely separate locations throughout the Rio Grande valley during the same 2-wk period in July 2003. This supports the hypothesis that the virus was introduced to avian hosts and mosquitoes throughout the valley in early 2003, but it was first detectable in mosquitoes only after a period of amplification. The virus may have been introduced by migratory birds passing through the bosque in spring 2003 or perhaps fall 2002. The occurrence of avian and equine cases in late autumn 2002 favors an earlier introduction, although these animal cases were focused in eastern New Mexico. The equine WNV cases of late 2002 suggest that WNV had at least gained a footing in eastern New Mexico, although the virus may not have become established or successfully overwintered. Low prevalence of mosquito infection before the outbreak of 2003 could explain the lack of detection of WNV in vector surveillance in 2002 as well as the absence of human cases in 2002. In addition, surveillance trapping was conducted over a much smaller area and with less frequency before 2003, and very few samples were tested, resulting in very low sensitivity for detecting infected mosquitoes. Once the virus became established in local populations of resident and perhaps migratory birds, whether in late 2002 or in early 2003, it would have required time to be amplified in the resident mosquito vectors in sufficient quantity to be detected by our surveillance efforts. It seems that this period of buildup occurred throughout the Rio Grande valley in the spring and early summer 2003. Temperature also may have influenced the amplification phase, with transmission beginning after a threshold value of average daily temperatures was achieved. By mid-July, WNV prevalence in mosquito populations had reached detectable levels.
The first instances of positive mosquitoes in mid-July 2003 occurred at approximately the same time as the first human and equine cases. The detection of several cases before detection of WNV in mosquitoes indicates that our surveillance system lacked sufficient sensitivity to serve as an early warning of risk. Concentration of mosquito surveillance at selected sites with increased intensity of sampling could refine the approach and allow it to be used as an effective indicator for risk of human or equine outbreaks, assuming appropriate criteria could be identified to select sentinel sites of value. Similar surveillance techniques have been used in other states throughout the country as a means of assessing risk and directing control measures and public health interventions (Scott et al. 2001, White et al. 2001, Barker et al. 2003, Takeda et al. 2003). The last trapping period to yield positive mosquito pools was approximately the first week of September. The majority of human and equine cases in the Rio Grande valley subsided by the third week of September. Although climate data are not analyzed here, heavy late-summer monsoon precipitation and unseasonably cold overnight temperatures are suspected to account for the unusual decrease in mosquito abundance in mid-September compared with previous years (R.B., personal observation). These weather conditions could have induced earlier diapause in mosquitoes, although it is more likely that lower temperatures suppressed fight activity. The occurrence of a human case with onset of symptoms in the last week of October indicates that infected vectors were still present even though our surveillance was not sensitive enough to detect them.
Repeated sampling of infected mosquitoes was only achieved at sites within Albuquerque. This could be explained by higher numbers of mosquitoes, especially Cx. p. quinquefasciatus that were typically captured in the city (DiMenna 2005), as well as a high rate of infection in this species, especially in urban habitats (Fig. 3). The tendency for infected Cx. p. quinquefasciatus to be caught most frequently at urban sites emphasizes the importance of habitat in understanding WNV transmission. Sampling sites in the city were in proximity to horse ranches, human development (including irrigated fields and runoff ditches), and an array of artificial containers that might serve as attractive larval habitats. Consequently, the urban sites might have had greater abundances of mosquitoes within the effective range of the traps. That most rural sites had only one or two positive pools suggests a low prevalence of WNV in the mosquito populations at rural sites in 2003. This is consistent with a period of buildup required for the virus to reach detectable levels. It is currently unclear as to what role adult mosquito overwintering in New Mexico may contribute to early season WNV amplification, and peak season infection rates, in the Rio Grande valley.
The three main species of Culex found in the New Mexico Rio Grande valley— Cx. tarsalis, Cx. salinarius, and Cx. p. quinquefasciatus—were infected with WNV. The infection rate in Cx. p. quinquefasciatus was much greater than in the other two species (Table 2). The significantly higher infection rate of Cx. p. quinquefasciatus could be explained by the tendency for this species to be caught in gravid traps rather than CDC light traps (DiMenna 2005). Mosquitoes caught in the gravid traps had presumably already taken a blood-meal, whereas mosquitoes caught in light traps may have consisted mainly of nulliparous females seeking their first bloodmeal. Although we found that all Culex spp. mosquitoes peaked in abundance in mid-July and were greatly reduced in number by mid- to late August, the temporal distribution of positive pools among these species differed. The transition in infected mosquitoes from Cx. tarsalis to Cx. p. quinquefasciatus suggests that there may be two separate cycles of WNV in mosquitoes. The first is an amplification cycle that took place between avian hosts and Cx. tarsalis mosquitoes early in the season. As the virus spread through multiple amplification cycles, it eventually infected Cx. salinarius and Cx. p. quinquefasciatus populations. Concentrations of Cx. p. quinquefasciatus in urban habitats would have increased the likelihood of this species becoming an important vector of human disease. In addition, late-season changes in host-seeking preferences in Cx. tarsalis are known to result in increased feeding of this species on mammalian hosts (Tempelis et al. 1965), thereby further increasing the risk of transmission to humans. A dual WNV life cycle has been postulated by others (Andreadis et al. 2001, Turell et al. 2002, Reisen et al. 2004). It is also possible that the temporal differences in infection could be a result of the necessity of a longer period of time required for WNV to become established in less competent vector species (Goddard et al. 2002). Although the high infection rate in Cx. p. quinquefasciatus could be an artifact of sampling procedures, it is of interest that infected pools of this species were most numerous the week of 11 August, the same week when the greatest number of human cases was reported. A more sensitive surveillance system could have detected the increase of infection rates in Cx. p. quinquefasciatus before the peak of human cases. It is unclear as to whether the increase in human cases was a result of a rise in infection rates of this species or a result of a change in host-preference in Cx. tarsalis.
Only one pool of mosquitoes from another species (Ae. dorsalis) was found to be WNV positive. Others have shown that Ae. dorsalis can transmit WNV (Goddard et al. 2002), but given that only a single pool was found, we are uncertain that this species has a significant role in WNV ecology in this region.
Further study may determine to what extent each Culex species contributes to actual transmission between hosts, whether it is between two avian hosts, or from avian to human. Such research might help in better understanding the nature of WNV ecology in this region. In addition, follow-up research is needed to determine how the presence of WNV will be manifested in subsequent years. Given the variety of geography and habitat types found throughout the Rio Grande valley, it seems possible that different areas might lend themselves to highly variable ecologies for the virus– host relationship as WNV becomes more established and integrated into the local communities.
Acknowledgments
We express our gratitude to all of the individuals and organizations whose kind permission allowed trapping to be conducted on properties, including pueblos and towns throughout the study area, numerous individuals, and the Sevilleta and Bosque del Apache National Wildlife Refuges, the Bureau of Reclamation, the U.S. Army Corps of Engineers, and the Bureau of Land Management. We also thank our collaborators at the Alamosa Mosquito Control District, New Mexico State University, and other municipal mosquito programs; Paul Ettestad, Pam Reynolds, and Nelson Powers at the New Mexico Department of Health for providing support and access to human case records; and the University of Illinois Medical Entomology Laboratory and the New Mexico Scientific Laboratory Division for WNV testing. Partial funding support for this research was provided by the National Science Foundation and National Institutes of Health (Grant DEB-0328173, Ecology of Infectious Disease Program), and by the National Science Foundation's Undergraduate Mentorships in Environmental Biology (Grant DEB-0102773). Additional support was provided from The Johns Hopkins University Bloomberg School of Public Health W. Harry Feinstone Department of Molecular Microbiology and Immunology, The University of New Mexico Department of Biology, the Valles Caldera Trust, and the Albuquerque Environmental Health Department.
References Cited
- Andreadis TG, Anderson JF, Vossbrinck CR. Mosquito surveillance for West Nile virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, Cx. salinarius, and Culiseta melanura. Emerg Infect Dis. 2001;7:670–674. doi: 10.3201/eid0704.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker CM, Reisen WK, Kramer VL. California state mosquito-borne virus surveillance and response plan: a retrospective evaluation using conditional simulations. Am J Trop Med Hyg. 2003;68:508–518. doi: 10.4269/ajtmh.2003.68.508. [DOI] [PubMed] [Google Scholar]
- Carpenter SJ, LaCasse WJ. Mosquitoes of North America (North of Mexico) University of California Press: Berkeley and Los Angeles, CA; 1955. [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. Outbreak of West Nile-like viral encephalitis-New York, 1999. MMWR Morb Mortal Wkly Rep. 1999;48:845–849. [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. West Nile virus activity – United States, November 21 – 26, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1072–1073. [Google Scholar]
- Darsie RF, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Mosq Syst. 1981;(Suppl 1):1–313. [Google Scholar]
- DiMenna M. PhD dissertation. The Johns Hopkins University, Bloomberg School of Public Health; Baltimore, MD: 2005. The emergence of West Nile virus in mosquito populations of the New Mexico Rio Grande. [Google Scholar]
- Ettestad P, Reynolds P, Powers N. New Mexico Epidemiology Report. New Mexico Department of Health; Santa Fe NM: 2004. West Nile virus in New Mexico, 2003. [Google Scholar]
- Finch DM, Tainter JA. Gen Tech Rep RM-GTR-268. U.S. Department of Agriculture, Forest Service; Ft. Collins, CO: 1995. Ecology, diversity, and sustainability of the middle Rio Grande basin. [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, et al. Rapid detection of West Nile virus from human clinical specimens, field–collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR Assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash D, Mostahari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, et al. The outbreak of West Nile virus in the New York City area in 1999. N Engl J Med. 2002;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- Pratt HD, Barnes RC. CDC Training Guide. U.S. Department of Health, Education and Welfare, Public Health Service; Atlanta, GA: 1959. Identification keys for common mosquitoes of United States. [Google Scholar]
- Reisen W, Lothrop H, Chiles R, Madon M, Cossen C, Woods L, Husted S, Kramer V, Edman J. West Nile virus in California. Emerg Infect Dis. 2004;10:1369–1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JH. Occurrence and seasonal distribution of mosquitoes in Bernalillo County, New Mexico, 1986. City of Albuquerque Environmental Health Department inhouse report; Albuquerque, NM: 1987. [Google Scholar]
- Scott TW, Wright SA, Eldridge BF, Brown DA. Cost effectiveness of three arbovirus surveillance methods in Northern California. J Am Mosq Control Assoc. 2001;17:118–123. [PubMed] [Google Scholar]
- Takeda T, Whitehouse CA, Brewer M, Gettman AD, Mather TN. Arbovirus surveillance in Rhode Island: assessing potential ecologic and climatic correlates. J Am Mosq Control Assoc. 2003;19:179–189. [PubMed] [Google Scholar]
- Tempelis CH, Reeves WC, Bellamy RE, Lofy MF. A three-year study of the feeding habits of Culex tarsalis in Kern County, California. Am J Trop Med Hyg. 1965;14:170–177. doi: 10.4269/ajtmh.1965.14.170. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Sardelis MR, O'Guinn ML, Dohm DJ. Potential vectors of West Nile virus in North America. Curr Top Microbiol Immunol. 2002;267:241–252. doi: 10.1007/978-3-642-59403-8_12. [DOI] [PubMed] [Google Scholar]
- White DJ, Kramer LD, Backenson PB, Lukacik G, Johnson G, Oliver J, Howard JJ, Means RG, Eidson M, Gotham I, et al. Mosquito surveillance and polymerase chain reaction detection of West Nile virus, New York state. Emerg Infect Dis. 2001;7:643–649. doi: 10.3201/eid0704.010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong W, Finch DM. Gen Tech Rep RMRS-GTR-99. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station; Ogden, UT: 2002. Stopover ecology of landbirds migrating along the middle Rio Grande in spring and fall. [Google Scholar]


