Abstract
As part of an ongoing mosquito surveillance program, 27 sites in the greater metropolitan Albuquerque area (Bernalillo County, New Mexico) were trapped from May through September 2004. Each site was sampled for 1 night weekly, using a standard CO2-baited Centers for Disease Control and Prevention light trap and a gravid trap. Captured mosquitoes were catalogued by location, species, and date, and selected pools were tested for West Nile virus (WNV) by reverse transcription–polymerase chain reaction. Based on previous surveillance, WNV was already established in the state of New Mexico. Surveillance during 2003, the 1st year of WNV detection in New Mexico mosquitoes, was focused on the bosque forest of the Rio Grande river valley. Surveillance during summer of 2004 was extended to additional areas around the city of Albuquerque, the state's largest population center. In addition to the standard surveillance objectives, a secondary goal was to determine whether foci of WNV activity were detectable in other habitats besides the riparian ecosystem of the Rio Grande, and in other species not previously identified as vectors. There was no demonstrable advantage to extending the traditional trapping area outside of the Rio Grande valley. Sites in the valley area had WNV-positive mosquitoes earlier in the season, and for a longer period than the added sites. In addition, riparian sites had the highest diversity of species, the largest numbers of Culex spp. captured, and the largest proportion of the WNV-positive mosquito pools from the study. Species found in other areas of the metropolitan area were also represented in the valley. Although WNV activity was detected in other areas of the city, its activity began later and ended earlier than in the river valley. We surmise that the greatest benefit to mosquito surveillance could be achieved by focusing on the river valley area.
Keywords: Albuquerque, New Mexico, mosquitoes, West Nile virus, surveillance
Introduction
In the summer of 2003, the 1st human cases of West Nile virus (WNV) were confirmed in New Mexico (Ettestad et al. 2004). It appears that WNV was introduced into New Mexico at about this time, because surveillance through the autumn of 2002 found no WNV in humans or mosquitoes (Ettestad et al. 2004). The emergence and subsequent spread of WNV in New Mexico was documented and studied by a collaborative research effort representing the city of Albuquerque Environmental Health Bio-Disease Management program, the New Mexico Department of Health, the University of New Mexico Biology Department, and the Johns Hopkins University Bloomberg School of Public Health. The primary objective of our efforts was to identify the mosquito vectors transmitting and maintaining WNV along the Rio Grande valley (DiMenna et al. 2006b).
The riparian ecosystem of the Rio Grande bosque represents a concentration of flora and fauna relative to the surrounding area of semiarid high desert typical of New Mexico (Finch and Tainter 1995). As such, it provided a likely focus to target the detection of mosquitoes carrying WNV and also represented a probable corridor for the rapid dispersion of the virus: the Rio Grande traverses the entire state of New Mexico in a north–south direction. Results of that study were published separately, but briefly, WNV-positive mosquitoes were captured from sites all along the valley and the species found to be infected were Culex tarsalis Coq., Culex salinarius Coq., and Culex quinquefasciatus Say. (DiMenna et al. 2006a, 2006b).
Having determined that WNV was widely established in the Rio Grande, we anticipated that it would now be an endemic pathogen in the area. Whereas the initial mosquito survey program had extended across the rural, sparsely inhabited desert regions crossed by the Rio Grande, we subsequently focused our efforts during 2004 near the city of Albuquerque. With a population of just over 448,000, Albuquerque is the largest population center in the state, representing approximately a quarter of the population of New Mexico, and occupying both banks of the Rio Grande in central New Mexico.
The existing mosquito surveillance program in Albuquerque has historically focused on the bosque region of the river valley because of the favorable conditions for mosquitoes in this environment. The bosque is dominated by cottonwood (Populus deltoides ssp. wizlizenii S. Wats.) forest, interspersed with nonnative Russian olive (Elaeagnus angustifolia L.) trees and salt cedar (Tamarix spp.). In 2003, 48 WNV-positive mosquito pools were obtained from the greater metropolitan Albuquerque Rio Grande valley, where over 18,000 mosquitoes were captured (DiMenna et al. 2006b). The bosque area is largely protected by conservation law, and remains sparsely populated. Numerous ranches and small farms surround the cottonwood forest; however, the majority of the Albuquerque population lives farther away from the river. For the 2004 season, we directed our efforts toward determining whether it might be valuable to extend the coverage of the WNV surveillance into areas outside of the bosque where the human population is denser but ecological conditions appeared to be less favorable for mosquitoes.
Within the city, numerous artificial water sources, such as golf courses, runoff ditches, and fountains provide mosquito breeding sites not found in the desert areas beyond the river valley in rural New Mexico. If mosquitoes were also found in densely populated parts of the city in the same abundance as in the bosque, a higher potential for a major WNV outbreak could exist than was expected. This study describes the results of these surveys, comparing the mosquito communities sampled in 4 regions within the metropolitan area. Distinct differences in abundances and species composition were observed. WNV activity in mosquitoes from all of these areas was documented, but certain regions within the city showed an earlier and more extensive level of WNV activity.
Materials and Methods
We identified several areas of Albuquerque outside of the bosque portion of the Rio Grande valley. These areas were characterized by denser human population than in the valley/bosque area, and they had water sources besides the river that could sustain mosquito communities. Each of these areas had characteristics that might merit attention from an expanded mosquito surveillance program:
West Mesa
This area is a few miles west of the Rio Grande. It is a plateau of volcanic rock that rises above the river basin, following along the river's course (Fig. 1). Between this mesa and the river is an area of Albuquerque's west side that has developed rapidly over the last decade. Many new subdivisions and several schools, golf courses, and parks have provided additional mosquito breeding grounds only a short distance from the bosque. Landscaping is relatively recent, however, and vegetation is less abundant than in the bosque.
Fig. 1.
The 4 subdivisions of the study area are shown in relation to their respective geography across the Albuquerque greater metropolitan area. Individual sites are not shown in order to maintain the privacy of local residents.
Northeast Heights
This area is in the northeast quadrant of the city and lies at the fringe of the western foothills of the Sandia Mountains (Fig. 1). The numerous subdivisions of the Heights have multiplied and grown denser during the last several decades, creating the most populous part of the city. All types of artificial water sources can be found here, among crowded neighborhoods. Older, denser vegetation forms the landscaping around homes and parks.
East Mountains
In this area, the human population is considerably sparser than in the remainder of the metropolitan area (Fig. 1). This area is located on the eastern slopes of the Sandia Mountains. There is, nonetheless, significant growth of smaller communities in this area. These homes are surrounded by wild growing Piñon-juniper vegetation that is quite dense where it is undisturbed. The east side of the mountain range receives considerably more precipitation than the west side, and large ranches have numerous artificial water containers. In addition, several springs at higher elevations wind their way down the slopes, and various arroyos frequently contain water throughout the year.
A total of 11 sites were selected to represent each of these 3 areas of the city. Sites were selected based on the presence of permanent water sources nearby, as well as proximity to residences, or other areas where exposure of humans to mosquitoes was considered to be frequent, such as golf courses, parks, or camping areas. In addition, 16 sites were maintained within the bosque. In total, 27 sites were monitored.
All sites were trapped for 1 night each week from the end of May until the end of September. Although some mosquitoes were still present at the end of the trapping season, their numbers were greatly reduced. Each site was trapped using a Centers for Disease Control and Prevention light trap (John Hock Company, Gainesville, FL) suspended 1.5 m from the ground and baited with a thermos canister containing approximately 1.5 kg of dry ice. A gravid trap was also placed at each site, at least 10 m from the light trap to avoid possible trap interaction. The gravid traps were baited with a standard 2-wk fermentation mixture of nonchlorinated water with horse manure, grass clippings, and bacterial culture (Pro-Pump® Liquid Live Bacteria High Count, Ecological Laboratories, Freeport, NY). Traps were set in the late afternoon, left overnight, and collected the following morning. Trap nets were placed in a cooler filled with dry ice to kill and preserve the mosquitoes.
All mosquitoes captured were stored at –82°C, separated by date, site, and trap type. Mosquitoes were later removed and sorted by species under a dissecting microscope according to published dichotomous keys (Carpenter and LaCasse 1955, Pratt and Barnes 1959). Mosquitoes were divided into pools of up to 50 individuals for testing. A database was kept of the numbers and species of all mosquitoes captured, organized by date, site, and trap type. Because each of the 4 regions in the study area consisted of different numbers of sites, the number of captures was standardized by averaging the total number of mosquitoes from each area by the number of sites. Periodically, pools were selected for WNV testing. Pools were chosen for testing based primarily on large numbers of individuals in a pool (typically ≥10), as well as on the likelihood of detecting WNV (highest priority was given to Culex species). Selected pools were sent to the University of Illinois Medical Entomology lab (Illinois Natural History Survey [INHS]) for WNV testing by reverse transcription–polymerase chain reaction (RT-PCR). The INHS lab used a QIAamp RNA kit (Qiagen, Valencia, CA) for viral lysis and RNA extraction. A TaqMan® PE 7700 sequence detection system was used for amplification and signal detection, according to the manufacturer's specifications, for 40 cycles (PE Applied Biosystems, Foster City, CA). Forward and reverse WNV primers were used (based on NY99 WNV strain). The primer sequences were selected according to published results (Lanciotti et al. 2000).
Results
During the summer of 2004, 25,973 mosquitoes were caught at the 27 sites in the Albuquerque study area (Table 1). Of these, 15,370 (59.2%) were various Culex species. The valley had the highest numbers of captures of all species except for Culiseta incidens Thomson, which was most frequently caught in the East Mountains. Although Culex mosquitoes represented the largest fraction of the mosquitoes caught, large numbers of Aedes vexans Meigen were also captured, primarily in the valley. Large numbers of other Aedes species, Aedes dorsalis Meigen and Aedes nigromaculis Ludlow in particular, were found in the valley and tended to appear in high densities only sporadically. Other Aedes species caught included Aedes sollicitans Walker and Aedes trivitattus Coq., but only in very small numbers. Numerous Anopheles spp. were caught, but their condition prevented them from being identified to species. Like Ae. vexans, Ae. dorsalis, Ae. nigromaculis, and Anopheles spp. were restricted almost entirely to the valley sites (Table 1). Two Psorophora columbiae (Dyar and Knab) were also recovered from the valley. This species is typically found much farther south and is rarely reported in the Albuquerque area (Darsie and Ward 1981, Schultz 1987). A significantly higher diversity of mosquito species was found in the valley than in any other area (χ2 = 15,778.3; df = 21; P < 0.001).
Table 1.
Total numbers of mosquitoes captured are shown by species for each of the subdivisions in the study area. Numbers have not been adjusted for the number of sites in each area.
| Valley | West Mesa | NE Heights | East Mountains | |
|---|---|---|---|---|
| Culex tarsalis | 1,891 | 53 | 139 | 599 |
| Culex salinarius | 6,922 | 367 | 1,641 | 163 |
| Culex quinquefasciatus | 2,563 | 151 | 835 | 46 |
| Aedes vexans | 6,235 | 3 | 13 | 16 |
| Aedes dorsalis | 390 | 0 | 3 | 0 |
| Aedes nigromaculis | 461 | 0 | 0 | 0 |
| Aedes spp. (other) | 26 | 0 | 1 | 9 |
| Culiseta inornata | 526 | 1 | 13 | 211 |
| Culiseta incidens | 19 | 0 | 10 | 1,167 |
| Psorophora columbiae | 2 | 0 | 0 | 0 |
| Anopheles spp. | 1,493 | 1 | 2 | 1 |
Sites in the valley area yielded the highest number of Culex captured (41.4% of all Culex), despite the fact that the valley had a more diverse mosquito community structure with many more non-Culex species, and only 55.8% of the mosquitoes captured in this area were Culex (Table 2). The valley area had a significantly higher number of mosquitoes captured than the other 3 areas (1-way analysis of variance [AN-OVA] F= 5.11; df = 3; P =0.007). This pattern was evident in the numbers of mosquitoes captured on a weekly basis as well (Fig. 2). Examination of the mosquitoes caught on a weekly basis also shows that mosquitoes in the valley tend to reach higher abundances earlier in the season than in other areas and to remain at peak numbers later in the summer (Fig. 2).
Table 2.
Total numbers of mosquitoes, and number of Culex, are shown, adjusted for the number of sites in each of the 4 areas. Also shown is the percentage of specimens captured in each area that are Culex spp.
| Area | No. sites | No. mosquitoes/site | No. Culex/site | % Culex in area |
|---|---|---|---|---|
| Valley | 16 | 1,281 (48.3%) | 715 (41.4%) | 55.8 |
| West Mesa | 2 | 288 (10.9%) | 286 (16.6%) | 99.1 |
| Northeast Heights | 5 | 531 (20.0%) | 523 (30.3%) | 98.4 |
| East Mountains | 4 | 553 (20.8%) | 202 (11.7%) | 36.5 |
Fig. 2.
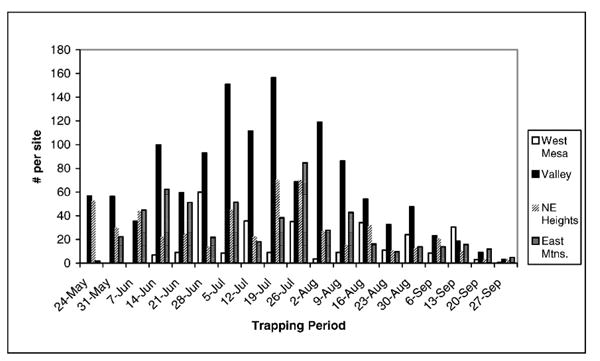
Average numbers of mosquitoes are shown for each area by trapping period, beginning the week of May 24, and ending the week of September 27.
A total of 785 pools were tested for WNV by RT-PCR (Table 3). Pools from the valley sites represented the largest fraction of the samples tested (69.4%). This was a slight overrepresentation from this area, which contained 59.3% of all of the sites, in part due to the larger average number of Culex caught in the valley compared with other areas. In addition, a number of other, non-Culex species were caught in the valley in sufficient numbers to merit WNV testing. Of the pools tested from the valley, 10.9% of all pools (Table 4), or 13.4 % of the Culex pools, were positive for WNV. The West Mesa area had a higher proportion of positives (20%); however, the mosquitoes captured at the West Mesa sites were almost entirely (99.1%) Culex, and all of the pools tested from this area were Culex. Among the pools tested from the valley area, 19.6% were non-Culex species. The pools tested from the valley sites represented 83.1% of the total positive pools (Table 4). All pools sent for testing from the Northeast Heights were Culex spp., which represented the majority (98.4%) of mosquitoes captured in this area, of which very few (3.4%) were WNV positive. In the East Mountains, Culex spp. were not as common (36.5% of all individuals). In this area, where altitudes are higher, Cs. incidens was caught most often (52.8%). Of all pools tested from this area, including 40 pools (46.5%) of Culiseta spp. (Cs. incidens and Culiseta inornata Williston), only 1 positive pool (1.16%) of Cx. tarsalis was found.
Table 3.
Pools tested for West Nile virus (WNV) by RT-PCR are shown by species. Pools containing Culex spp. were preferentially selected because of the known association between these mosquitoes and WNV. Other species were also tested when found in significant numbers.
| Species | Valley | West Mesa | NE Heights | East Mountains |
|---|---|---|---|---|
| Culex tarsalis | 79 | 4 | 9 | 35 |
| Cx. salinarius | 246 | 21 | 65 | 9 |
| Cx. quinquefasciatus | 115 | 10 | 43 | 2 |
| Aedes vexans | 76 | 0 | 0 | 0 |
| Ae. dorsalis | 11 | 0 | 0 | 0 |
| Ae. nigromaculis | 10 | 0 | 0 | 0 |
| Culiseta inornata | 10 | 0 | 0 | 4 |
| Cs. incidens | 0 | 0 | 0 | 36 |
| Total Pools | 547 | 35 | 117 | 86 |
| Total Culex Pools | 440 | 35 | 117 | 46 |
| % Culex | 80.4 | 100 | 100 | 53.5 |
Table 4.
Total numbers of pools tested for West Nile virus by RT-PCR are shown, as well as the average number of pools per site in each area. Positive pool results are shown, as are the percentages of positives among pools tested. The final column shows the percentage contribution of each area to the overall positive results.
| Area | No. pools | No. pools/site | No. sites with +pools/No. sites | +pools | % positive | % of total positives |
|---|---|---|---|---|---|---|
| Valley | 547 | 34 | 9/16 | 59 | 10.9 | 83.1 |
| West Mesa | 35 | 18 | 2/2 | 7 | 20 | 9.9 |
| NE Heights | 117 | 23 | 1/5 | 4 | 3.4 | 5.6 |
| East Mtns. | 86 | 22 | 1/4 | 1 | 1.16 | 1.4 |
The 1st positive mosquitoes of 2004 appeared in mid-June. During the week of June 14, WNV-positive Cx. salinarius were recovered from a single site in the valley. This site continued to have positive Cx. salinarius and Cx. quinquefasciatus pools tested during 6 out of 10 sampling periods until the 3rd week of August. This site was next to a ranch and stable facility located in the northern part of the Rio Grande valley that had dense vegetation with numerous cottonwood groves and heavy underbrush, as well as artificial landscaping. Water sources in the vicinity included the river area, as well as many water troughs and other artificial containers and irrigation ditches. In addition, an experimental constructed wetland facility was in close proximity to this site. No other sites had positive pools until the week of July 19, when 5 additional sites in the valley area yielded positive results. Among the sites that had positive pools in this period, 4 were located within approximately 1.5–2 km of the original positive valley site, nearly directly across the Rio Grande. The remaining site was farther to the south in the valley. Several of these sites had additional positive pools over the next several weeks, and all sites in the valley sub-sequently yielded WNV infected mosquitoes during 1 or more trapping periods.
A single site in the Northeast Heights had 2 positive pools during the week of July 19. This site had 4 positive pools but did not have any positive pools after the week of August 2. This site was a park-like setting, with extensive irrigation and runoff channels situated around a variety of artificial landscaping features. Positive mosquitoes were caught at both of the West Mesa sites nearly a month later, during the week of August 16. These sites yielded positive pools several times in the following month. Only 1 positive pool was recovered from the East Mountains area, and this occurred even later in the sampling season, during the week of August 23. Although trapping was continued until the last week of September, the last positive mosquito pools were caught the week of September 13, from the valley and the West Mesa.
Among the Culex, over two-thirds (70.1%) of the positive results were Cx. salinarius, with 47 positive pools. There were 17 positive Cx. quinquefasciatus pools, as well as 3 pools of Cx. tarsalis. Pools of Cx. salinarius were significantly more likely to be positive than pools of Cx. tarsalis, while pools of Cx. quinquefasciatus did not appear to be significantly more or less likely to be positive than expected (x2 =12.9; df = 2; P = 5 0.002). There were 5 positive pools of Ae. vexans collected from the valley sites, representing the only non-Culex species that was positive for WNV. Infection rates were comparable in Cx. quinquefasciatus and Cx. salinarius, and higher in both of these species than in Cx. tarsalis or Ae. vexans (Table 5).
Table 5.
Infection rates are shown for all species in which West Nile virus was detected in 2004. The Microsoft Excel® add-in developed by Biggerstaff (v 3.0, 2006) was used to calculate bias-corrected maximum likelihood estimates (MLEs) and 95% confidence intervals (CIs).
| Species | MLE infection rate | Lower limit (95% CI) | Upper limit (95% CI) |
|---|---|---|---|
| Culex quinquefasciatus | 9.40 | 5.92 | 14.30 |
| Cx. salinarius | 9.38 | 7.11 | 12.18 |
| Cx. tarsalis | 2.19 | 0.58 | 5.89 |
| Aedes vexans | 2.06 | 0.77 | 4.56 |
Discussion
Based on the results from the summer of 2004, we did not find sufficient evidence to merit expanding the coverage of our mosquito surveillance to other regions outside of the river valley in Albuquerque. Mosquitoes carrying WNV were found in all areas where trapping was conducted, confirming virus activity to be more widespread in the metropolitan area than previous surveillance had suggested. However, the traditional sites in the valley provided a much higher abundance and diversity of mosquitoes than other areas. The valley was also the area where WNV-positive mosquitoes were caught the earliest in the season and where they persisted the latest in the summer. Despite the fact that virus activity must be considered an important public health concern throughout the metropolitan area, little additional information was obtained by added surveillance efforts into other areas.
Larger quantities of most species of mosquitoes were trapped in the valley than any other area. This trend remained even when correcting for differences in the number of sampling sites. In addition, a greater variety of species was found in the valley than in any other area. No species was found exclusively in any other area; however, several species were found either exclusively or in higher numbers in the valley. Not only did the valley's greater diversity of species provide a better resource for studying the ecology of mosquitoes in the Rio Grande basin, but it also translated to a more complete picture of what species might be involved in arbovirus transmission. In time, WNV might be carried and transmitted by species not yet found to be involved in the spread of this virus in New Mexico. Several species found in the bosque in New Mexico have been shown to be competent carriers or bridge vectors for WNV in other parts of the country (Goddard et al. 2002, Turell et al. 2002). The valley could provide an ideal area to track and study newly involved vector species. Furthermore, 1 or more of the various mosquito species found here might be an important vector for other arboviruses that may be introduced to this area in the future.
The bosque had a higher rate of capture for Culex spp. than other areas. To optimize the efficiency of WNV surveillance, it is beneficial to maximize the number of known vectors captured. From a surveillance standpoint, sites in the valley provide an ideal combination of high numbers of all 3 Culex spp. found in Albuquerque, as well as a relatively high proportion of positive pools. Although Culex spp. were found throughout the metropolitan area, they were not as numerous in the other areas studied, and test results were not as likely to show positive pools. It is likely that the valley was the 1st area where WNV was established in Albuquerque. This may partially explain the higher probability of catching positive mosquitoes in the bosque than elsewhere in town. Over the next several years, other areas outside of the valley will become home to WNV-positive mosquitoes, although it appears that populations of these mosquitoes will be less numerous. In addition to being present in higher abundance, mosquitoes in the valley also appear to reach peak levels of activity earlier in the season and to remain in higher numbers for a longer period of time (Fig. 2). This suggests an earlier onset of mosquito–bird interactions, as well as a longer period of interaction. Increased or extended interactions between avian hosts and mosquito vectors would result in more cycles of viral amplification at an earlier time in the season. This increased opportunity for viral amplification, and dispersal, could be responsible for the greater likelihood of detecting WNV in mosquitoes from the valley area.
Sites in the valley yielded WNV-carrying mosquitoes earlier in the season than in other areas. One valley site had mosquitoes that tested positive for WNV over a month before any other sites. At the time that mosquitoes from other areas began to test positive for WNV, numerous sites in the valley had already yielded large numbers of positive pools. In addition, none of the other areas where trapping was conducted had positive mosquitoes any later than the middle of September, when the last of the positive pools from the valley were caught.
It is unclear why there was a difference in the WNV infection rates for Cx. salinarius and Cx. quinquefasciatus compared with Cx. tarsalis. This trend is worthy of further investigation. Statewide surveys for WNV in mosquitoes in 2003 found WNV infection rates to be much higher in Cx. quinquefasciatus caught in urban sites. That finding was based on results from across the state, including both urban and rural sites, and it appeared that Cx. tarsalis was the dominant vector sampled in rural areas, while Cx. quinque-fasciatus was the primary urban vector (DiMenna et al. 2006b). While Cx. tarsalis prefers to feed on avian hosts for blood meals early in the season (Tempelis et al. 1965), Cx. salinarius is a more opportunistic feeder (Carpenter and LaCasse 1955). Positive pools of Cx. quinquefasciatus were found in expected proportions, with an infection rate comparable to that of Cx. salinarius. This species is certainly also a significant factor in WNV dynamics, and its tendency to be found peridomestically suggests that it is an important vector of WNV to humans. If the latter 2 species are the predominant vectors of WNV in Albuquerque, the potential for transmission to humans and other animal species might be greater than it would have been if Cx. tarsalis was the primary vector in this region. The low infection rate in Cx. tarsalis is of interest, since it contradicts the accepted paradigm that this species is primarily responsible for amplification of the virus in avian populations. The low infection rate in 2004 does not appear to be an artifact of the sampling scheme; this trend has been noted again in 2005 (DiMenna, unpublished data). This unexpected result is in need of further explanation through additional research. The current data suggest that Cx. tarsalis may not play as significant a role in WNV transmission in New Mexico as was previously anticipated (Turell et al. 2002). Investigation of WNV in California has shown Cx. tarsalis to be an important vector species for WNV in that state (Goddard et al. 2002, Reisen et al. 2004).
The only non-Culex spp. found infected with WNV was Ae. vexans. This species has also been found to carry WNV in other states (Goddard et al. 2002). The occurrence of WNV-positive Ae. vexans in the valley is significant, since this species is found in high concentrations throughout the season. No positive pools of this species were found until mid-August, suggesting a possible scenario in which viral amplification in the environment had to reach a threshold after which Ae. vexans became involved in the cycle. In support of this argument, no positive pools of Ae. vexans had been detected in New Mexico through 2003, the 1st year in which WNV was detected in mosquitoes in this state. A low infection rate for this species suggests that it is unlikely to play a significant role in WNV transmission.
Given the evidence from the 2004 Albuquerque mosquito surveillance, we recommend maintaining a focus of trapping efforts on the Rio Grande bosque. Although human population is denser in other areas of the city, the valley appears to be the area where WNV is detected earliest and most often. In addition, it boasts the greatest diversity of mosquito species and the highest concentration of Culex spp. Future efforts will be directed toward developing an understanding of how the different species of Culex contribute to WNV transmission in this ecosystem. Studies of the many microhabitats within the bosque and environmental factors influencing the abundance and distribution of vector mosquito species will be beneficial in furthering our understanding of the mechanisms influencing the ecology of WNV in New Mexico.
Acknowledgments
Partial funding support for this research was provided by the National Science Foundation and National Institutes of Health (grant DEB-0328173, Ecology of Infectious Disease Program), and by the National Science Foundation's Un-dergraduate Mentorships in Environmental Biology (UMEB, grant DEB-0102773). Additional support was provided from The Johns Hopkins University Bloomberg School of Public Health W. Harry Feinstone Department of Molecular Microbiology and Immunology, The University of New Mexico Department of Biology, the Valles Caldera Trust, the New Mexico Depart-ment of Health, and the Albuquerque Environmental Health Department.
The authors would like to express their gratitude to all of the individuals and organizations whose kind permission allowed trapping to be conducted on their property. This includes several private landowners, the city of Albuquer-que, the Bureau of Reclamation, the U.S. Army Corps of Engineers, and the Bureau of Land Management.
Thanks also to our collaborators at the University of Illinois Medical Entomology Lab-oratory and the New Mexico Scientific Labora-tory Division for WNV testing.
Footnotes
All research was conducted through the Albuquerque Environmental Health Bio-Disease Management Laboratory at Montessa Park, 3600 Los Picaros Road SE, Albuquerque, NM 87105
References Cited
- Carpenter SJ, LaCasse WJ. Mosquitoes of North America (north of Mexico) Berkeley and Los Angeles: University of California Press; 1955. [Google Scholar]
- Darsie RF, Ward RA. Identification and geo-graphical distribution of the mosquitoes of North America, north of Mexico. Mosq Syst. 1981;1(Suppl):1–313. [Google Scholar]
- DiMenna MA, Bueno R, Parmenter RR, Norris DE, Sheyka J, Molina J, LaBeau EM, Hatton E, Glass GE. Comparison of mosquito trapping method efficacy for West Nile virus surveillance in New Mexico. J Am Mosq Control Assoc. 2006a;22(2):246–253. doi: 10.2987/8756-971x(2006)22[246:comtme]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMenna MA, Bueno R, Parmenter RR, Norris DE, Sheyka J, Molina J, LaBeau EM, Hatton E, Glass GE. The emergence of West Nile virus in mosquito (Diptera: Culicidae) communities of the New Mexico Rio Grande valley. J Med Entomol. 2006b;43(3):594–599. doi: 10.1603/0022-2585(2006)43[594:eownvi]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettestad P, Reynolds P, Powers N New Mexico Department of Health. West Nile virus in New Mexico, 2003. New Mexico Epidemiology Re-port. 2004;2004(1):1–4. http://www.health.state.nm.us/pdf/WNV-ER-010204.pdf. [Google Scholar]
- Finch DM, Tainter JA. Ecology, diversity, and sustainability of the Middle Rio Grande basin. Ft Collins, CO: U.S. Department of Agriculture, Forest Service; 1995. Gen. Tech. Rep. RM-GTR-268. [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8(12):1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38(11):4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt HD, Barnes RC. Identification keys for common mosquitoes of United States. Atlanta, GA: Public Health Service. CDC Training Guide, U.S. Department of Health, Education and Welfare; 1959. [Google Scholar]
- Reisen WK, Lothrop H, Chiles R, Madon M, Cossen C, Woods L, Husted S, Kramer V, Edman J. West Nile virus in California. Emerg Infect Dis. 2004;10(8):1369–1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JH. City of Albuquerque Environmental Health Department in-house report Occurrence and seasonal distribution of mosquitoes in Bernalillo County New Mexico 1986. 1987 [Google Scholar]
- Tempelis CH, Reeves WC, Bellamy RE, Lofy MF. A three-year study of the feeding habits of Culex tarsalis in Kern County, California. Am J Trop Med Hyg. 1965;14:170–177. doi: 10.4269/ajtmh.1965.14.170. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Sardelis MR, O'Guinn ML, Dohm DJ. Potential vectors of West Nile virus in North America. Curr Top Microbiol Immunol. 2002;267:241–252. doi: 10.1007/978-3-642-59403-8_12. [DOI] [PubMed] [Google Scholar]


