Abstract
During the summer of 2001, field studies were performed to evaluate the effects of carbon dioxide (CO2) and 1-octen-3-ol (octenol) on the ability to collect Aedes albopictus with Centers for Disease Control (CDC) Fay–Prince traps. Results from these studies indicated that Ae. albopictus is significantly more attracted to CO2- or CO2 + octenol–baited CDC Fay–Prince traps than unbaited or octenol-baited traps. However, the difference between the responses to CO2 and CO2 + octenol was not statistically different, indicating that CO2 is driving the response of Ae. albopictus to CDC Fay–Prince traps.
Keywords: Aedes albopictus, attractants, octenol, carbon dioxide, Fay–Prince trap
Aedes albopictus (Skuse), the Asian tiger mosquito, has become one of the most notorious human-biting mosquitoes in the southeastern and mid-Atlantic regions of the United States. This species is well established in most urban and suburban areas and is rapidly becoming the most important pest species in these regions. The species has been implicated in the transmission of several mosquito-borne diseases, such as dengue, yellow fever, La Crosse encephalitis, eastern equine encephalitis, and West Nile encephalitis (Mitchell 1991, Mitchell et al. 1992, Vazeille-Falcoz et al. 1999, Gerhardt et al. 2001, Holick et al. 2002). Recent laboratory studies have demonstrated that this species is a highly competent vector for West Nile virus (Turell et al. 2001a, 2001b; Sardelis et al. 2002). Its widespread distribution and vector potential make Ae. albopictus an urgent problem.
Previous studies have investigated the efficacy of 1-octen-3-ol (octenol) in conjunction with carbon dioxide (CO2) to attract various mosquito species, to enhance vector-borne disease surveillance, and as a potential control method (Kline 1994, 2002; Vaidyanathan and Edman 1997a, 1997b; Kline and Lemire 1998; Kline and Mann 1998; Rueda et al. 2001; van den Hurk et al. 2001). The results indicated that octenol has species-specific effects. However, few studies have ventured into urban and suburban habitats and none have focused on Ae. albopictus. Only 1 study has utilized the Centers for Disease Control (CDC) Fay–Prince trap (Canyon and Hii 1997), which originally was developed to target Aedes aegypti (L.) (Fay and Prince 1970) and also has been utilized in efforts to collect Ae. albopictus.
Popularity of commercial mosquito traps, which utilize CO2 and octenol as olfactory attractants, is increasing in areas where Ae. albopictus is the primary mosquito species. Because of the increase in use of these traps by homeowners and the potential for this species to transmit endemic viruses (e.g., West Nile virus), it has become important to understand the effect of octenol on the collection of Ae. albopictus. The purpose of this study was to evaluate the effectiveness of octenol and CO2 at increasing the efficacy of CDC Fay–Prince traps for collecting Ae. albopictus.
During the weeks beginning on July 23, August 13, and September 10, 2001, trials were conducted at 12 locations in Baltimore City, Howard County, and Anne Arundel County, Maryland. The 12 sites were chosen because they had active populations of adult Ae. albopictus and a significant number of artificial containers providing breeding grounds for the tiger mosquitoes. Containers found at each site included tire piles, flowerpots, tarps, pool covers, birdbaths, children's toys, corrugated pipes, and cemetery vaults.
A CDC Fay–Prince battery–powered trap (model 712, John W Hock Co., Gainesville, FL) was operated at each site. Each trap was suspended from a natural or man-made structure 0.9 m above the ground and equipped with an attractant combination (see below). The traps were set each day between 1100 and 1400 h, and operated for 24 h. Collected mosquitoes were kept cool until arrival in the laboratory, killed in the freezer, sorted on a chill table, and identified to species by morphological characteristics by using a series of dichotomous keys (Darsie and Ward 1981, Slaff and Apperson 1989). The number of male and female mosquitoes of each species was counted, recorded, and entered into a database. Each species was pooled (into groups of up to 40) and tested for West Nile virus by the Maryland Department of Health and Mental Hygiene (results not reported here).
The 4 attractant schemes evaluated in the field trials were no bait, octenol, CO2, and CO2 + octenol. Carbon dioxide was released from a 1.9-liter plastic Igloo® beverage cooler containing approximately 1.4 kg of dry ice suspended directly above the trap. The approximate release rate of CO2 was 53 g/h. Three milliliters of octenol (Aldrich, Milwaukee, WI) was supplied in a 5-ml microreaction vial (Supelco, Bellefonte, PA) fitted with a plastic cap and neoprene septa with a 1.5-mm hole bored through it. The octenol was released by inserting a pipe-cleaner wick (Dills 15-cm), which had been doubled over, into the hole such that 1.5 cm protruded above the septum. This provided a release rate of approximately 44 mg/h. The reaction vial was attached 5 cm below the entrance to the trap.
A gridlike Latin-square design, typically used to evaluate different baiting schemes, was not practical or possible in an urban and suburban environment. However, our method allowed us to test each of the 4 schemes simultaneously in triplicate at sites that were similar in their potential for breeding by Ae. albopictus. Sampling was conducted on 4 consecutive nights with the bait at each site chosen randomly such that each bait was only tested once at a given site in a given week. Three replicates of the trial were run. Attractant, date, and site effects were evaluated by using 3-way analysis of variance for total number of female Ae. albopictus. SigmaStat (SPSS Inc., Chicago, IL) was used for all statistical analyses.
Over the 144 trap nights, a total of 1,121 female Ae. albopictus was collected. No statistical difference was found between the numbers of female tiger mosquitoes collected for each trial. A small, overall difference among sites was seen; however, Tukey's post hoc comparison revealed that the individual sites did not differ significantly. The overall response for the average number of tiger mosquitoes caught each night for each bait type was CO2 + octenol > CO2 > no bait > octenol (Table 1). However, the response to CO2 + octenol did not differ significantly from the response to CO2. The only statistically significant difference found was between the response to CO2 + octenol or CO2 and no bait or octenol (P ≤ 0.05, Tukey's studentized range test) and this response was replicated during each trial (Fig. 1).
Table 1.
Mean number of female Aedes albopictus (SE) collected for each bait type (n = 36).
| Bait | Mean (SE) |
|---|---|
| No bait | 1.06 (0.21) |
| Octenol | 0.81 (0.20) |
| CO2 | 13.14 (1.60) |
| CO2 + octenol | 16.14 (2.52) |
Fig. 1.
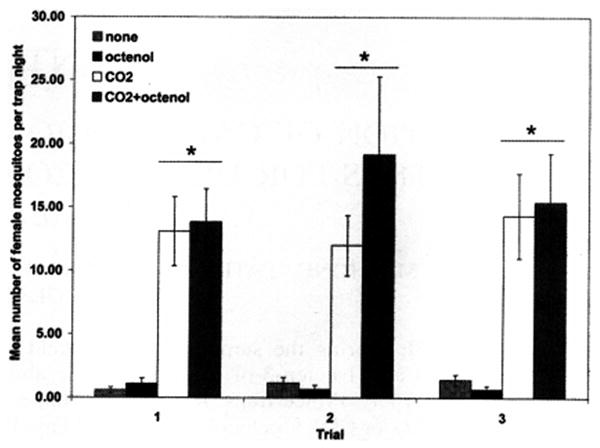
Mean (females/trap night) response of Aedes albopictus collected in Centers for Disease Control Fay–Prince traps baited with octenol, CO2, and CO2 + octenol for each trial (* P ≤ 0.05).
After the initial report that octenol is a potential attractant for mosquitoes (Takken and Kline 1989), several studies have investigated its use in a variety of habitats and for a range of mosquito species (van Essen et al. 1994, van den Hurk et al. 1997, Beavers et al. 1998, Kline and Mann 1998, Mboera et al. 2000). This is the 1st study to investigate the effectiveness of CO2 and octenol in collecting Ae. albopictus. Similar to these previous studies, octenol alone was not seen to be a potent attractant. Furthermore, although CDC Fay–Prince traps baited with CO2 + octenol collected the greatest number of female Ae. albopictus, the difference between the mean number of the species collected in these double-baited traps and the traps baited with CO2 alone was not statistically significant (P > 0.05, Tukey's studentized range test). The difference between the mean number of Ae. albopictus collected in traps baited with CO2 or CO2 + octenol and unbaited traps or traps baited with octenol alone was statistically significant (P ≤ 0.05, Tukey's studentized range test). The results of this study suggested that octenol did not decrease catch success for Ae. albopictus, which has been reported with Ae. aegypti (Canyon and Hii 1997), and that CO2 is driving the response of Ae. albopictus to a CDC Fay–Prince trap. Because this study evaluated the response of tiger mosquitoes to baited and unbaited Fay-Prince traps and did not differentiate the potential visual effect of the contrasting black and white panels of the Fay–Prince trap, future studies might include additional trap types with the same attractant combinations. Nonetheless, the addition of octenol for surveillance purposes or to augment the trapping capability of commercial mosquito traps appears to be unnecessary for Ae. albopictus.
Acknowledgments
We thank the employees of the Mosquito Control Section at the Maryland Department of Agriculture who assisted with this project.
References Cited
- Beavers GM, Hanafi HA, Tetreault GE. Response of mosquitoes (Diptera: Culicidae) to carbon dioxide and octenol in Egypt. J Egypt Soc Parasitol. 1998;28:303–312. [PubMed] [Google Scholar]
- Canyon DV, Hii JLK. Efficacy of carbon dioxide, 1-octen-3-ol, and lactic acid in modified Fay–Prince traps as compared to man-landing catch of Aedes aegypti. J Am Mosq Control Assoc. 1997;13:66–70. [PubMed] [Google Scholar]
- Darsie RF, Ward RA. Identification and geographical distribution of mosquitoes of North America, North of Mexico. Mosq Syst. 1981;1(Suppl):1–313. [Google Scholar]
- Fay RW, Prince WH. A modified visual trap for Aedes aegypti. Mosq News. 1970;30:20–23. [Google Scholar]
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, Panella NA, Powell EE, Nasci RS. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg Infect Dis. 2001;7:807–811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick J, Kyle A, Ferraro W, Delaney RR, Iwaseczk M. Discovery of Aedes albopictus infected with West Nile virus in southeastern Pennsylvania. J Am Mosq Control Assoc. 2002;18:131. [PubMed] [Google Scholar]
- Kline DL. Olfactory attractants for mosquito surveillance and control: 1-octen-3-ol. J Am Mosq Control Assoc. 1994;10:280–287. [PubMed] [Google Scholar]
- Kline DL. Evaluation of various models of propane-powered mosquito traps. J Vector Ecol. 2002;27:1–7. [PubMed] [Google Scholar]
- Kline DL, Lemire GF. Evaluation of attractant-baited traps/targets for mosquito management on Key Island, Florida, USA. J Vector Ecol. 1998;23:171–185. [PubMed] [Google Scholar]
- Kline DL, Mann MO. Evaluation of butanone, carbon dioxide, and 1-octen-3-ol as attractants for mosquitoes associated with north central Florida bay and cypress swamps. J Am Mosq Control Assoc. 1998;14:289–297. [PubMed] [Google Scholar]
- Mboera LE, Takken W, Sambu EZ. The response of Culex quinquefasciatus (Diptera: Culicidae) to traps baited with carbon dioxide, 1-octen-3-ol, acetone, butyric acid and human foot odour in Tanzania. Bull Entomol Res. 2000;90:155–159. doi: 10.1017/s0007485300000262. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ. Vector competence of North and South American strains of Aedes albopictus for certain arboviruses: a review. J Am Mosq Control Assoc. 1991;7:446–451. [PubMed] [Google Scholar]
- Mitchell CJ, Niebylski ML, Smith GC, Karabatsos N, Martin D, Mutebi JP, Craig GB, Jr, Mahler MJ. Isolation of eastern equine encephalitis virus from Aedes albopictus in Florida. Science. 1992;257:526–527. doi: 10.1126/science.1321985. [DOI] [PubMed] [Google Scholar]
- Rueda LM, Harrison BA, Brown JS, Whitt PB, Harrison RL, Gardner RC. Evaluation of 1-octen-3-ol, carbon dioxide, and light as attractants for mosquitoes associated with two distinct habitats in North Carolina. J Am Mosq Control Assoc. 2001;17:61–66. [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, O'Guinn ML, Andre RG, Roberts DR. Vector competence of three North American strains of Aedes albopictus for West Nile virus. J Am Mosq Control Assoc. 2002;18:284–289. [PubMed] [Google Scholar]
- Slaff M, Apperson CS. A key to the mosquitoes of North Carolina and the mid-Atlantic states. NC State Univ Agric Ext Serv Publ AG. 1989:1–38. [Google Scholar]
- Takken W, Kline DL. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J Am Mosq Control Assoc. 1989;5:311–316. [PubMed] [Google Scholar]
- Turell MJ, O'Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001a;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Sardelis MR, Dohm DJ, O'Guinn ML. Potential North American vectors of West Nile virus. Ann N Y Acad Sci. 2001b;951:317–324. doi: 10.1111/j.1749-6632.2001.tb02707.x. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R, Edman JD. Sampling methods for potential epidemic vectors of eastern equine encephalomyelitis virus in Massachusetts. J Am Mosq Control Assoc. 1997a;13:342–347. [PubMed] [Google Scholar]
- Vaidyanathan R, Edman JD. Sampling with light traps and human bait in epidemic foci for eastern equine encephalomyelitis virus in southeastern Massachusetts. J Am Mosq Control Assoc. 1997b;13:348–355. [PubMed] [Google Scholar]
- van den Hurk AF, Beebe NW, Ritchie SA. Responses of mosquitoes of the Anopheles farauti complex to 1-octen-3-ol and light in combination with carbon dioxide in northern Queensland, Australia. Med Vet Entomol. 1997;11:177–180. doi: 10.1111/j.1365-2915.1997.tb00310.x. [DOI] [PubMed] [Google Scholar]
- van den Hurk AF, Johansen CA, Zborowski P, Phillips DA, Pyke AT, Mackenzie JS, Ritchie SA. Flaviviruses isolated from mosquitoes collected during the first recorded outbreak of Japanese encephalitis virus on Cape York Peninsula, Australia. Am J Trop Med Hyg. 2001;64:125–130. doi: 10.4269/ajtmh.2001.64.125. [DOI] [PubMed] [Google Scholar]
- van Essen PH, Kemme JA, Ritchie SA, Kay BH. Differential responses of Aedes and Culex mosquitoes to octenol or light in combination with carbon dioxide in Queensland, Australia. Med Vet Entomol. 1994;8:63–67. doi: 10.1111/j.1365-2915.1994.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Vazeille-Falcoz M, Adhami J, Mousson L, Rodhain F. Aedes albopictus from Albania: a potential vector of dengue viruses. J Am Mosq Control Assoc. 1999;15:475–478. [PubMed] [Google Scholar]


