Abstract
Adolescence is associated with the onset of puberty, shifts in social and emotional behavior, and an increased vulnerability to social anxiety disorder. These transitions coincide with changes in amygdala response to social and affective stimuli. Utilizing an emotional face-matching task, we examined amygdala response to peer-aged neutral and fearful faces in relation to puberty and social anxiety in a sample of 60 adolescent females between the ages of 8 and 15. We observed amygdala activation in response to both neutral and fearful faces compared to the control condition, but did not observe differential amygdala activation between fearful and neutral faces. Right amygdala activity in response to neutral faces was negatively correlated with puberty and positively correlated with social anxiety, and these effects were statistically independent. Puberty and social anxiety did not relate to amygdala activation in response to fearful faces. These findings suggest that emotional differentiation between fearful and neutral faces may arise during later pubertal development and may result from decreasing sensitivity to neutral faces, rather than increasing sensitivity to threatening faces. Furthermore, these findings highlight the importance of considering individual differences in social anxiety when examining the neural response to social stimuli in adolescents.
Keywords: puberty, social anxiety, adolescence, faces, amygdala
The beginning of adolescence roughly corresponds to the onset of puberty, which initiates drastic changes in hormone levels and a cascade of physical changes in the body and the brain [1]. During adolescence, there are also dramatic shifts in motivation, social behavior and rates of psychopathology, particularly for girls [2-5]. One of the most notable social changes during adolescence is increasing independence from parental figures and corresponding reliance on close friendships and romantic relationships with peers; by the 7th grade, peer and parent relationships become equally important to adolescents, and by 10th grade, peer relationships become primary [3,6].
Both the value of social relationships and the facility for processing social stimuli increase during adolescence. For example, the ability to identify faces [7,8] and the ability to recognize and remember emotional expressions [9-12] continues to develop throughout adolescence. Adolescence may also be a period during which individuals are beginning to extract new information from faces, such as attractiveness, trustworthiness, social status and dominance, especially from peers [13].
Increasing sensitivity to emotional and social cues coincides with changes in neural structures, particularly the amygdala [14,15]. Indeed, the amygdala is one of the few regions of the brain that contains both estrogen and androgen receptors [16-18], indicating that its function may be directly influenced by hormonal changes during puberty. Recent studies have demonstrated that directly administering sex hormones can increase amygdala response to emotional faces [19]. Consistent with these data, pubertal development has been linked to increased amygdala reactivity to faces [20,21]; in fact, some studies have reported greater amygdala activation to faces in adolescents compared to adults [22-24].
In adults, amygdala activation is typically enhanced for emotional compared to neutral faces [25]. However, numerous studies across childhood and adolescence have failed to find differentiation between emotional and neutral faces [15,26-28] and some studies have even reported greater activation to neutral compared to fearful faces [29-31].
Although it seems clear that pubertal development is associated with changes in amygdala activation to faces, it is unclear whether pubertal development impacts the increased response of the amygdala to emotional compared to neutral facial expressions. Existing studies on this topic span a wide range of ages (i.e., 7 to 17)—and lack of emotional differentiation in amygdala response, or an increase in response to neutral faces, is more common in studies on younger [28,30] compared to older adolescents [22,23]. One possibility is that only adolescents advanced in pubertal development demonstrate adult-like amygdala differentiation between emotional and neutral faces, an effect that could be due to either increasing or decreasing response to threatening or neutral faces, respectively.
However, there are two additional factors that may impact the relationship between pubertal development and amygdala reactivity to emotional faces. First, existing studies have primarily probed amygdala activation using pictures of adult faces. Initial evidence suggests that children may process peer and adult faces differently; in a recent study with adolescents, greater amygdala activation was observed in response to neutral adult, compared to peer faces, and preferential amygdala activation was observed in response to happy peer faces and angry adult faces [32]. Thus, failures to find differential amygdala reactivity to emotional compared to neutral faces may reflect, to some degree, the stimuli used. If social focus shifts from parents to peers over the course of adolescence [3,33], emotional expressions of peers may become more relevant with pubertal development. The neural response to peer stimuli, particularly in regions like the amygdala that are influenced by sex hormones, may better index relevant changes in social and pubertal maturation.
It is also important to consider the potential impact of trait-like individual differences that covary with pubertal development—such traits might impact the relationship between puberty and amygdala activity to emotional faces [34]. Specifically, symptoms of social anxiety increase during adolescence for girls [2,35]—and anxiety in general has been shown to impact amygdala response to facial stimuli [29,34,36-40]. Social phobia, in particular, is associated with enhanced amygdala activation to emotional faces [34] and even among healthy adolescents, social anxiety symptoms postively correlate with amygala activation to emotional faces [40]. Thus, it is possible that pubertal effects on amygdala response to faces could reflect developmental increases in social anxiety. However, no studies have simultaneously assessed the impact of pubertal development and social anxiety symptoms on amygdala response to faces.
In summary, adolescence is a period characterized by increased amygdala reactivity to facial stimuli—an increase that may relate to pubertal development and the increased salience of social signals. Whereas adults consistently show differential amygdala activation to fearful compared to neutral faces, findings in adolescents are mixed. Variability across studies could be potentially due to small sample sizes, failure to account for pubertal stage, or confounding factors such as gender or anxious symptomatology; moreover, the majority of these studies have not utilized age-appropriate facial stimuli.
Accordingly, the goal of this study was to employ adolescent facial stimuli to examine the effect of puberty on amygdala activation to neutral and fearful faces in a relatively large sample (n = 60) of females between the ages of 8 and 15, when pubertal changes initially begin to arise. Age and puberty are often highly correlated; however, neurodevelopment, particularly in sex-hormone rich areas of the brain like the amygdala, may be more closely tied to measures of puberty than to age, and some studies have even found age and puberty to have dissociable effects [41]. In addition, we examined relationships between amygdala activation and social anxiety; because the effects of social anxiety may interact with puberty and confound amygdala response to faces, we examined the independence of these effects.
Method
Participants
A total of 75 girls between the ages of 8 and 15 participated in this study. Participants were part of the larger Impact of Puberty on Affect and Neural Development across Adolescence (IPANDA) study at Stony Brook University. Participants were recruited using a commercial mailing list of families in the Stony Brook area with daughters in the targeted age range, through posted flyers in locations likely to be frequented by families with children including grocery stores, libraries, and medical offices, through an online advertisement on Craigslist, and finally through references from other participating families. Brief phone screens were conducted with families that expressed interest, and eligible participants were invited to participate in the study.
Participants who did not complete all puberty measures (n = 5) or participants who had excessive motion during the fMRI portion of the study (n = 10) were excluded from analysis. The final sample for this study included 60 female participants (mean age = 12.49 years, SD = 1.89; see Table 1 for demographic details).
Table 1.
Demographic characteristics of the sample.
| Mean | SD | |
|---|---|---|
| Age | 12.49 | 1.89 |
| MASC social anxiety | 8.78 | 5.79 |
| PDS:P | 2.55 | .85 |
| PDS:SR | 2.52 | .86 |
| PBIP:P | 3.05 | 1.37 |
| PBIP:SR | 3.34 | 1.22 |
PDS = Pubertal Development Scale (P = parent, SR = self-report), PBIP = Picture-Based Interview about Puberty (P = parent, SR = self-report), MASC = Multidimensional Anxiety Scale for Children.
Pubertal Assessments
Pubertal Development Scale
The Pubertal Development Scale (PDS; [42] is a questionnaire version of a scale originally designed to be administered to children and adolescents in interview format [43]. In the version administered to girls, development is assessed across five physical domains: growth spurt, body hair, changes in skin, breast development, and menstruation. All items except for menstruation are rated on a scale from 1 (“not yet started”) to 4 (“seems complete”); the menstruation item is rated either 1 (“no”) or 4 (“yes”). An overall puberty score is calculated as the mean of the five items.
Participants and their parents completed computer-based versions of the self-rated and parent versions of the PDS (PDS:SR and PDS:P, respectively). Child-parent agreement on mean PDS is relatively high in 5th- and 6th-grade girls (Spearman rs of .71 and .80, respectively), as is agreement on PDS pubertal stage score (Spearman rs of .70 and .82, respectively; [42]. Internal consistency of the PDS:SR (Cronbach's α of .67 to .70) and PDS:P (Cronbach's α of .68 to .78) is moderate to high [42]. In the current study, Cronbach's α was .84 for the PDS:SR, and .88 for the PDS:P. Correlations between parent and child ratings of the PDS in the current study are reported in Table 2.
Table 2.
Correlations among puberty measures.
Correlations represent Pearson's r values.
p < .001. PDS = Pubertal Development Scale (P = parent, SR = self-report), PBIP = Picture-Based Interview about Puberty (P = parent, SR = self-report).
Picture-Based Interview about Puberty
The Picture-Based Interview about Puberty (PBIP; [44] is a two-item measure assessing pubertal development on a scale from 1to 5; ratings are anchored by pictures and accompanying verbal descriptions of each stage. The PBIP correlates highly with other measures of puberty, including the PDS (rs of .72 to .81) and a physical examination (rs of .75 to .88; [45]. For correlations between the PBIP and PDS in the current study see Table 2. The PBIP was designed to be administered by an interviewer. However, in order to increase consistency of administration and to decrease participants' potential discomfort, participants in the current study were given a fully automated computer-based interview in which a recorded voice provided a verbal description of the developmental stages; this recording was timed to correspond to pictures of each stage that appeared onscreen. No interviewer was present in the room. As participants viewed the automated interview, they filled out a paper response-rating sheet on which they indicated their level of development on each of the items. The slideshow-based PBIP was administered in a private room to participants (PBIP:SR) and their parents (PBIP:P) separately.
Latent Puberty Factor Score
Participant and parent ratings on both puberty measures were highly correlated, with correlation coefficients ranging from .81 to .91; see Table 2 for all correlations. In order to estimate common variance across measures of puberty, the pubertal measures (PDS:SR, PDS:P, PBIP:SR, and PBIP:P) were modeled as observed indicators of a dimensional puberty latent variable in a confirmatory factor analytic framework. The latent puberty factor scores for participants were estimated and used in subsequent analyses. This latent variable modeling was conducted using Mplus software with a robust maximum likelihood estimator (MLR) to account for observed variables with non-normal distributions [46]. A one-factor model was estimated, and examination of model-implied correlations suggested the presence of a reporter-based measurement effect—that is, the self-report measures correlate in part due to being self-report. As such, the residuals of the two self-report measures (i.e., PDS:SR and PBIP:SR) were allowed to correlate to model this source of covariance. Model identification precluded including a second correlated residual for the two parent-report measures.
This one-factor model of the four puberty indicators produced excellent fit on the comparative fit index (CFI = .98), surpassing the common threshold of >.95 suggestive of good fit (Hu & Bentler, 1999). Similarly, the Tucker Lewis Index (TLI = .91) surpassed the common threshold of .90, suggesting an acceptable fit. The root mean square error of approximation (RMSEA = .23) did not reach the common threshold of < .06, however, suggesting some areas of misfit appearing to result from residual correlations between parent-report measures. In further support of our model, all four indicators loaded significantly (p < .001), and with relatively high magnitude, on the latent factor. Additionally, resultant factor scores were very highly determined (98.5%), indicating that factor score indeterminacy was not an analytic concern. The R-squared values for the four indicators were as follows: .846 for PDS:SR, .947 for PDS:P, .747 PBIP:SR, and .881 for PBIP:P, indicating that our single latent factor accounted for between 75% and 95% of the variance of each of our four indicators.
Assessment of Anxious Symptomatology
The Multidimensional Anxiety Scale for Children
Participants completed a computer-based version of the Multidimensional Anxiety Scale for Children (MASC; [47]. The MASC is a 39-item self-report questionnaire assessing symptoms over the course of the week prior to assessment. Items cover a wide range of anxious symptoms, which are divided four subscales: physical symptoms (e.g., “I get dizzy or faint feelings”), harm avoidance (e.g., “I check to make sure things are safe”), social anxiety (e.g., “I worry about other people laughing at me”), and separation anxiety (e.g., “I try to stay near my mom or dad”). Responses are rated on a scale from 0 (“never true about me”) to 3 (“often true about me”) and are summed to create an overall anxiety score. The current analyses focus on the social anxiety subscale of the MASC.
The MASC has generally strong psychometric properties. Internal reliability in both clinical and non-clinical samples of children is high, with Cronbach's αs from .87 to .93 [48-52]. In the current sample, Cronbach's α was .86. Three-week test-retest reliability in unselected children and adolescents is strong with a single-case ICC of .78 [47]; one-year test-retest reliability is moderate (r = .52; [48]. In 8- to 16-year-olds diagnosed with anxiety disorders or attention deficit hyperactivity disorder (ADHD), three-week test-retest reliability is satisfactory (single-case ICC of .65), and three-month test-retest reliability is excellent with a single-case ICC of .87 [52]. The MASC shows both convergent and divergent validity in clinical samples [48,49,51,52]. The social anxiety subscale of the MASC is also psychometrically robust, with Cronbach's αs of .82 to .90 [48,51,53] and moderate to strong test-retest reliability in clinical and non-clinical samples over a range of time delays [47,48,53]. In the current study, Cronbach's α was .85 for the social anxiety subscale.
Paradigm
Participants completed an emotional face-matching task adapted from Hariri and colleagues [54] using happy, fearful, sad and neutral faces selected from the NIMH Child Emotional Faces Picture Set [55]. Selected facial stimuli ranged in age from 10-16 years old with a mean age of 13.42. All faces had a direct gaze, and an equal number of male and female faces were presented. Only neutral and fearful faces were analyzed for this study. Based on the ratings provided by Egger and colleagues [55] from 20 adult volunteers, there were no significant differences between fearful and neutral faces on inter-rater agreement concerning the expression of the face (t = .338, p = .737) or overall goodness of the stimuli (t = .989, p = .328), but fearful faces were rated as significantly more intense (t = -2.055, p = .046) and more representative of the expression (t = 4.504, p = .001) than neutral faces. Shape matching was used as a baseline control condition.
During each trial, a single target face or shape was presented at the top of the screen and two additional faces or shapes were presented at the bottom of the screen. Participants were instructed to select the face or shape on the bottom of the screen that matched either the emotional expression of the target face, or the target shape, that was displayed on the top. During emotional face-matching trials, the non-matching facial expression was always neutral. During neutral face-matching trials, the non-matching facial expression was fearful on 50% of trials and happy on 50% of trials. Each trial type was presented a total of 16 times in four 20-second blocks. Each block consisted of 4 trials displayed for 5 seconds each. Blocks were counterbalanced and alternated between face and shape matching conditions. A schematic of the design is presented in Figure 1.
Figure 1.
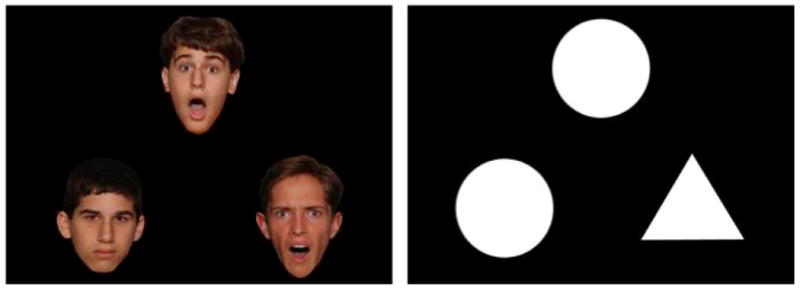
A trial from the fearful face matching condition (left) and the shape matching condition (right). Each block consisted of 4 trials displayed for 5 seconds each; participants were instructed to select the face or shapes on the bottom that matched either the emotional expression or the shape that was displayed on the top.
fMRI data acquisition and analysis
Images were acquired on a whole body 3-Tesla Siemens TrioTrim scanner (Siemens Medical, Erlangen, Germany) with a 12-channel head coil. An EPI sequence was used to acquire 324 T2 star-weighted whole brain volumes for analysis of BOLD signal. Scanning parameters were as follows: TR = 2.1s, TE = 23ms, Flip angle = 83 degrees, slices = 37 3.5mm interleaved slices parallel to the AC-PC.
Data analysis was performed using SPM8. Standard preprocessing procedures were applied including slice time correction, realignment for motion correction, co-registration, normalization to standard Montreal Neurological Institute space and spatial smoothing using an 8mm FWHM Gaussian kernel. Additional motion correction was applied using ArtRepair [56] for 16 participants with more than 2mm, but less than 5mm, of movement. For these participants, spikes in motion were corrected by interpolating volumes in excess of the motion cutoff; no more than 10% of scans were interpolated for any given participant. Data for those participants were then reprocessed using corrected volumes. Participants were excluded from analysis if they had greater than 5mm of motion or if they required interpolation on more than 10% of scans. Participant SPMs were created at the first level from a model that specified the onset of each face and shape matching condition. For participants whose data was motion corrected with ArtRepair, first level analyses were deweighted to account for interpolated volumes. Random effects analyses were then conducted at the second level to test for statistical differences between fearful faces and shapes, neutral faces and shapes, and fearful and neutral faces using contrasts created at the first level for each individual. A height threshold was set to .05 FWE to correct for multiple comparisons.
The Automated Anatomical Labeling (AAL) atlas [57] in WFU Pickatlas was used to create a mask for the right and left amygdala. Eigenvariates representing right and left amygdala activation were then extracted and imported in SPSS for analysis. In SPSS, Pearson's correlations and partial correlations were used to assess relationships between variables.
Procedures
Upon arrival to the lab, participants and their parents were introduced to a member of the research team who was trained in consenting procedures. In a private room, the study was explained to both the participant and the parent; written informed consent was then obtained from the parent, and written informed assent was obtained from the participant. Pubertal assessments and fMRI scans were conducted in the context of other questionnaires and tasks, which were randomized over the course of the visit.
Before participating in the fMRI scans, all participants underwent a 20- to 30-minute session in a mock fMRI scanner to acclimate to the environment of the scanner and to become familiar with task procedures. The mock scanner is similar in size and appearance to the actual scanner, and is equipped with speakers and a computer screen to simulate the noises and computer tasks used in the actual machine. In the mock scanner, participants were trained to reduce head motion using MoTrak software, which allowed them to view their head movement against a bull's-eye with a crosshair superimposed. Participants viewed a short cartoon, and playback paused whenever the tracking cursor moved out of the bull's-eye. Participants were then introduced to the emotional face-matching task and the other fMRI tasks, and completed short practice versions of each.
Families were paid $20 per hour for their participation in the study, and participants were given an additional $20 to $29 in earnings from other tasks with monetary incentives; thus, in total, participants and their families were paid approximately $100 to $130. Participants were also offered a choice of small prizes such as candy and stickers for completing each study task. This study was formally approved by the Institutional Review Board of Stony Brook University.
Results
Correlations between age, puberty, and social anxiety
There were no significant correlations between social anxiety scores on the MASC and age (r = -.014, p = .914) or the latent puberty measure (r = .061, p = .664). Puberty and age were strongly correlated (r = .830, p < .001).
Neutral face compared to shape matching
Whole brain analysis for the effect of neutral face matching compared to shape matching revealed peak activation in bilateral occipital gyrus (BA 17), right fusiform gyrus (BA 37), right amygdala, bilateral middle prefrontal gyrus (BA 8/9/46), left medial prefrontal gyrus (BA 6), bilateral precentral gyrus (BA 6), right superior parietal lobe (BA 7), and right precuneus (BA 7). Table 3 and Figure 2 (top) display peak activations associated with this contrast.
Table 3.
Peak activations for neutral face compared to shape matching.
| Cluster Size | T | Z | Talairach Coordinates (X Y Z) | Region | BA | |||
|---|---|---|---|---|---|---|---|---|
| 1596 | 22.83 | >8 | 19.13 | -91.13 | -5.83 | R | Inferior Occipital Gyrus | 17 |
| 21.67 | >8 | -17.84 | -90.59 | -10.01 | L | Inferior Occipital Gyrus | 17 | |
| 17.9 | >8 | 37.94 | -45.12 | -15.57 | R | Fusiform Gyrus | 37 | |
| 383 | 12.91 | >8 | -21.38 | -27.22 | -4.07 | L | Lateral Geniculum Body | |
| 12.32 | >8 | 23.35 | 0.37 | -18.71 | R | Amygdala | ||
| 11.81 | >8 | 19.54 | -4.03 | -11.99 | R | Amygdala | ||
| 334 | 10.02 | 7.62 | 41.22 | 6.58 | 32.62 | R | Precentral Gyrus | 9 |
| 7.99 | 6.55 | 52.24 | 9.55 | 40.3 | R | Middle Frontal Gyrus | 8 | |
| 7.9 | 6.5 | 33.36 | -11.42 | 63.22 | R | Precentral Gyrus | 6 | |
| 182 | 8.91 | 7.07 | -36.52 | 3.26 | 30.99 | L | Precentral Gyrus | 6 |
| 7.89 | 6.5 | -40.09 | 18.88 | 25.21 | L | Middle Frontal Gyrus | 46 | |
| 6.74 | 5.78 | -51.29 | 18.24 | 32.16 | L | Middle Frontal Gyrus | 9 | |
| 46 | 7.86 | 6.47 | -40.63 | -10.69 | 58.43 | L | Precentral Gyrus | 6 |
| 6.46 | 5.59 | -33.34 | -18.88 | 64.98 | L | Precentral Gyrus | 6 | |
| 6.3 | 5.49 | -29.62 | -11.45 | 65.75 | L | Precentral Gyrus | 6 | |
| 20 | 7.14 | 6.04 | 26.06 | -58.09 | 40.65 | R | Superior Parietal Lobule | 7 |
| 14 | 5.87 | 5.19 | 3.99 | -60.65 | 29.23 | R | Precuneus | 7 |
| 15 | 5.65 | 5.03 | -3.54 | 0.64 | 56.53 | L | Medial Frontal Gyrus | 6 |
All cluster and peak activations are significant at less than p = .05, FWE corrected. BA = Brodmann's area, R = right, L = left.
Figure 2.
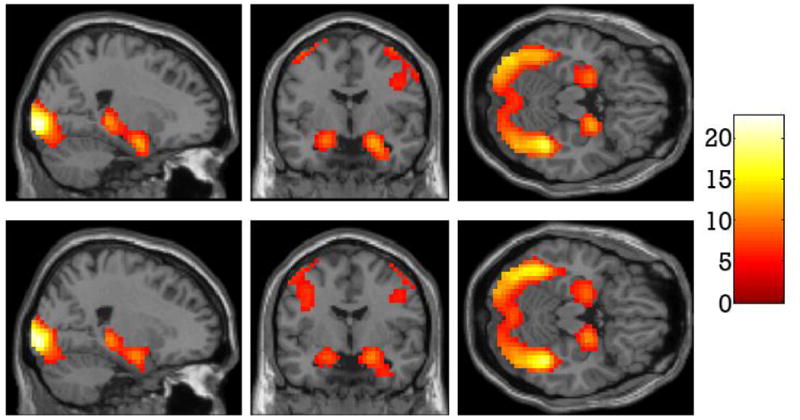
Regions of the brain more active for neutral face matching compared to shape matching (top). Regions of the brain more active for fearful face matching compared to shape matching (bottom).
Fearful face compared to shape matching
Whole brain analysis for the effect of fearful face matching compared to shape matching revealed enhanced BOLD response to fearful faces in bilateral occipital gyrus (BA 18), the right fusiform gyrus (BA 37), bilateral precentral gyrus (BA 6/9), bilateral middle frontal gyrus (BA 9/46), left inferior frontal gyrus (BA 13), right thalamus, right amygdala, right superior parietal lobe (BA 7) and right precuneus (BA 7). Results from this contrast are presented in Table 4 and Figure 2 (bottom).
Table 4.
Peak activations for fearful face compared to shape matching.
| Cluster Size | T | Z | Talairach Coordinates (X Y Z) | Region | BA | |||
|---|---|---|---|---|---|---|---|---|
| 1782 | 23.52 | >8 | -21.54 | -90.57 | -10.07 | L | Fusiform Gyrus | 18 |
| 22.93 | >8 | 22.88 | -90.8 | -9.34 | R | Inferior Occipital Gyrus | 18 | |
| 19.6 | >8 | 37.94 | -45.12 | -15.57 | R | Fusiform Gyrus | 37 | |
| 410 | 12.09 | >8 | 41.22 | 6.58 | 32.62 | R | Precentral Gyrus | 9 |
| 9.23 | 7.23 | 45.11 | 26.24 | 23.74 | R | Middle Frontal Gyrus | 46 | |
| 8.94 | 7.08 | 48.43 | 1.42 | 46.67 | R | Precentral Gyrus | 6 | |
| 431 | 10.81 | >8 | -51.35 | 14.16 | 35.38 | L | Middle Frontal Gyrus | 9 |
| 9.64 | 7.44 | -36.53 | -0.47 | 30.64 | L | Precentral Gyrus | 6 | |
| 7.26 | 6.11 | -43.58 | 27.74 | 11.57 | L | Inferior Frontal Gyrus | 13 | |
| 409 | 10.72 | >8 | -21.38 | -27.22 | -4.07 | L | Lateral Geniculum Body | |
| 10.6 | >8 | 19.29 | -27.78 | 0.17 | R | Thalamus | ||
| 10.59 | >8 | 19.53 | -7.76 | -12.34 | R | Amygdala | ||
| 23 | 7.39 | 6.19 | 26.02 | -58.44 | 44.22 | R | Superior Parietal Lobule | 7 |
| 10 | 6.1 | 5.35 | 3.99 | -60.65 | 29.23 | R | Precuneus | 7 |
All cluster and peak activations are significant at less than p = .05, FWE corrected. BA = Brodmann's area, R = right, L = left.
Fearful face matching compared to neutral face matching
Whole brain analyses contrasting fearful face matching with neutral face matching showed increased BOLD responses to fearful faces in bilateral occipital cortex (BA 17/18), right precuneus (BA 7) and the thalamus. Peak and cluster activations are listed in Table 5 and displayed in Figure 3.
Table 5.
Peak activations for fearful face compared to neutral face matching conditions.
| Cluster Size | T | Z | Talairach Coordinates (X Y Z) | Region | BA | |||
|---|---|---|---|---|---|---|---|---|
| 1788 | 10.84 | >8 | 15.38 | -91.46 | -2.33 | R | Lingual Gyrus | 17 |
| 10.12 | 7.67 | -10.54 | -95.05 | -3.1 | L | Lingual Gyrus | 17 | |
| 9.7 | 7.47 | 26.62 | -75.92 | -7.87 | R | Lingual Gyrus | 18 | |
| 53 | 7.09 | 6 | 22.35 | -61.79 | 40.24 | R | Precuneus | 7 |
| 6.09 | 5.34 | 26.18 | -68.22 | 28.89 | R | Precuneus | 7 | |
| 13 | 6.24 | 5.45 | 19.29 | -27.78 | 0.17 | R | Thalamus | |
All cluster and peak activations are significant at less than p = 05, FWE corrected. BA = Brodmann's area, R = right, L = left.
Figure 3.
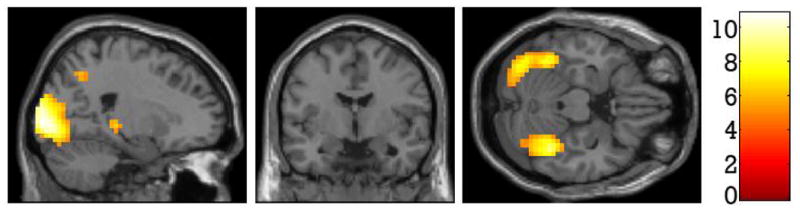
Regions of the brain more active for fearful face matching compared to neutral face matching.
Amygdala correlations
Table 6 presents correlations between average amygdala activity and age, puberty, and social anxiety scores on the MASC. Right amygdala activity during neutral face matching compared to shape matching was negatively correlated with age and puberty, and positively correlated with social anxiety. Left amygdala activity during neutral face matching compared to shape matching was not significantly related to age, puberty, or social anxiety. Neither right nor left amygdala activation during fearful face matching compared to shape matching related to age, puberty, or social anxiety scores.
Table 6.
Correlations between average amygdala activation (during neutral face matching compared to shape matching and fearful face matching compared to shape matching) and age, puberty, and social anxiety scores.
| Neutral > Shape: | Neutral > Shape: | Fear > Shape: | Fear > Shape: | |
|---|---|---|---|---|
| Left Amygdala | Right Amygdala | Left Amygdala | Right Amygdala | |
| Age | -.109 | -.280* | -.088 | -.217 |
| Puberty | -.027 | -.301** | .063 | -.048 |
| MASC | .135 | .342*** | -.115 | -.046 |
| Social anxiety |
MASC = Multidimensional Anxiety Scale for Children. Correlations represent Pearson's r values.
p < .05,
p < .025,
p < .01.
Correlations with p values < .025 remained significant after correcting for multiple comparisons.
Partial correlations were then conducted to examine the relationship between right amygdala activation, age and puberty while controlling for social anxiety. The negative correlations between right amygdala activation during neutral face matching compared to shape matching and age (r = -.304, p = .019) and puberty (r = -.343, p = .008) remained significant after controlling for social anxiety. The positive relationship between the social anxiety and right amygdala activation during neutral face matching compared to shape matching also remained significant when controlling for puberty (r = .378, p = .003) or age (r = .360, p = .005).
Discussion
This study used a recently developed set of adolescent facial stimuli to examine changes in the neural response to neutral and fearful faces in relation to puberty and social anxiety symptoms across adolescence. Neutral and fearful faces both elicited increased amygdala activation compared to shapes. Right amygdala activation to neutral, but not fearful, faces was negatively related to both age and puberty, and positively related to social anxiety. Thus, greater amygdala activation to neutral faces was characteristic of less pubertally developed, and more socially anxious, adolescent girls. The negative relationship between puberty and amygdala activation to neutral faces compared to shapes remained significant even when controlling for social anxiety—increasing slightly in magnitude. When controlling for puberty, the positive relationship between amygdala activation to neutral faces compared to shapes and social anxiety also remained significant. Thus, puberty and social anxiety had independent and opposing effects on amygdala response to neutral faces.
We did not, however, observe differential amygdala activity when comparing fearful to neutral faces. This replicates findings from studies using adult face sets with participants in a similar pubertal range [15,28] and extends these findings by demonstrating that a lack of differentiation is not specific to adult faces. The current results suggest that amygdala differentiation between fearful and neutral faces may only emerge during the latest stages of pubertal development. Further, in the current study, puberty-related decline in amygdala activation was specific to neutral faces, suggesting that later-developing emotional differentiation may result from decreasing sensitivity to neutral faces, rather than increasing sensitivity to threatening faces.
This puberty-related decline in amygdala response to neutral faces is consistent with a recent study demonstrating that participants in mid to late puberty have reduced amygdala activation to neutral faces compared to those in early puberty [15]. Amygdala activation is thought to reflect salience [58], indicating that reduced amygdala activation to neutral faces across puberty may reflect changes in the relative meaning of neutral social cues. A decline in amygdala reactivity to neutral stimuli, therefore, might reflect the increasing ability of adolescents to extract salient emotional cues and discriminate emotionality—and neutral peer faces may become less ambiguous over the course of pubertal development. As children mature, and their ability to discriminate between emotional and neutral social stimuli increases, they may be better able to categorize neutral faces as non-threatening or irrelevant stimuli. This ability may stem from an increased focus on interactions with peers, which provide opportunities to practice the evaluation and interpretation of ambiguous facial expressions. This reduced reactivity to neutral facial expressions, then, may facilitate social exploration in unfamiliar or ambiguous social situations, and allow for the formation of new peer relationships.
Though we only focused on female participants, and therefore cannot generalize these finding to males, studies with mixed-gender samples in both childhood and mid to late adolescence have also observed reductions in amygdala response to neutral faces as a function of age and/or puberty, or a lack of amygdala differentiation between emotional and neutral faces [15,28]. Thus, the current results are broadly consistent with studies that include both male and female participants.
Whereas amygdala response to neutral faces was negatively associated with puberty, it was positively related to social anxiety symptoms. A limited number of studies that have specifically examined social phobia in adolescence using adult face sets have found increased activation in response to emotional faces [34,40]. The current findings are more consistent with studies in adult social anxiety, which report increased amygdala activation to neutral faces [59]. Neutral peer faces may be particularly ambiguous or threatening for more socially anxious adolescents [60]. While social anxiety symptoms and puberty were not significantly correlated in this sample (cf. Deardorff et al., 2007; Reardon, Leen-Feldern & Hayward, 2009), controlling for social anxiety strengthened the negative relationship between puberty and amygdala activation to neutral faces, suggesting that relationships between the neural response to social stimuli and puberty may be masked to some degree by the opposing impact of social anxiety symptoms.
Although the current study focused on activation of the amygdala, changes in the affective processing of social stimuli are likely reflected in broader networks that involve the amygdala, particularly frontal and parietal networks implicated in emotion regulation and attentional control [61,62]. It will be important for future research to examine whether activation within and across these networks relate to changes in social and affective processing over the course of puberty. Furthermore, it will be important to examine other individual difference factors such as emerging symptoms of depression, relative reliance on parental figures and peers, and romantic relationships that may also impact neural activation to peer social stimuli. In addition, although the focus of the current study was on neutral and fearful faces, changes in the processing of other emotional faces, particularly happy faces, may also reflect important developmental changes. One advantage of the current study is the use of peer-aged facial stimuli; however, this stimulus set is relatively new and future studies might further validate the classification of these facial expressions as well as valence and arousal ratings in a adolescent sample; ratings were not obtained from participants in the current study. Finally, as we did not track eye movements during this task, we cannot rule out the possibility that the results of the current study are partially due to puberty- and anxiety-related changes in attention to the non-matching emotional faces during neutral face-matching trials, rather than the processing of neutral faces.
In conclusion, the current study suggests that reductions in amygdala reactivity to neutral peer faces may be a critical change that occurs over that course of pubertal development and may explain, in part, why amygdala differentiation between neutral and emotional faces is found more consistently in adult than adolescent and child populations. In addition, increased social anxiety symptoms predicted greater amygdala activation to neutral faces. Thus, pubertal development and social anxious symptoms exert opposing effects on amygdala activation to neutral social stimuli. These results highlight the importance of assessing both pubertal development and social anxiety when evaluating amygdala response to social stimuli during adolescence.
Figure 4.
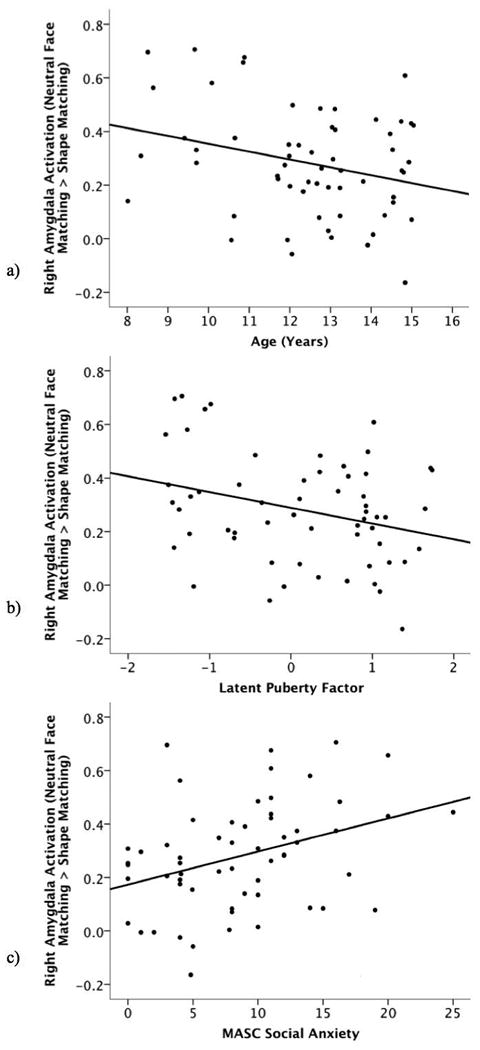
Scatter plots depicting correlations between right amygdala activation during the neutral face matching compared to shape matching condition and (a) age, (b) puberty, and (c) MASC social anxiety.
Acknowledgments
This work was supported by National Institute of Mental Health Grant RO1 MH097767 to Greg Hajcak Proudfit. We would like to give special thanks to Emily Hale-Rude and the rest of the iPANDA team.
References
- 1.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7:1040–1042. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 2.Deardorff J, Hayward C, Wilson KA, Bryson S, Hammer LD, Agras S. Puberty and gender interact to predict social anxiety symptoms in early adolescence. The Journal Of Adolescent Health: Official Publication Of The Society For Adolescent Medicine. 2007;41:102–104. doi: 10.1016/j.jadohealth.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown BB. Handbook of adolescent psychology. In: Lerner RM, Steinberg L, editors. Adolescents' relationships with peers. 2nd. Hoboken, NJ US: John Wiley & Sons Inc; 2004. pp. 363–394. [Google Scholar]
- 4.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohde P, Lewinsohn PM, Klein DN, Seeley JR, Gau JM. Key characteristics of major depressive disorder occurring in childhood, adolescence, emerging adulthood, adulthood. Clinical Psychological Science: A Journal Of The Association For Psychological Science. 2013;1:41–53. doi: 10.1177/2167702612457599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furman W, Buhrmester D. Age and sex differences in perceptions of networks of personal relationships. Child Development. 1992;63:103–115. doi: 10.1111/j.1467-8624.1992.tb03599.x. [DOI] [PubMed] [Google Scholar]
- 7.Germine LT, Duchaine B, Nakayama K. Where cognitive development and aging meet: Face learning ability peaks after age 30. Cognition. 2011;118:201–210. doi: 10.1016/j.cognition.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Carey S, Diamond R. From piecemeal to configurational representation of faces. Science. 1977;195:312–314. doi: 10.1126/science.831281. [DOI] [PubMed] [Google Scholar]
- 9.Durand K, Gallay M, Seigneuric A, Robichon F, Baudouin JY. The development of facial emotion recognition: The role of configural information. Journal of Experimental Child Psychology. 2007;97:14–27. doi: 10.1016/j.jecp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Herba CM, Phillips ML. Annotation: Development of facial expression recognition from childhood to adolescence: Behavioural and neurological perspectives. Journal of Child Psychology and Psychiatry. 2004;45:1185–1198. doi: 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- 11.Herba CM, Landau S, Russell T, Ecker C, Phillips ML. The development of emotion-processing in children: Effects of age, emotion, and intensity. Journal of Child Psychology and Psychiatry. 2006;47:1098–1106. doi: 10.1111/j.1469-7610.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- 12.Thomas LA, De Bellis MD, Graham R, LaBar KS. Development of emotional facial recognition in late childhood and adolescence. Developmental Science. 2007;10:547–558. doi: 10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 13.Scherf KS, Behrmann M, Dahl RE. Facing changes and changing faces in adolescence: A new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Developmental Cognitive Neuroscience. 2012;2:199–219. doi: 10.1016/j.dcn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biological Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: Role of pubertal maturation and relation to measures of negative affect. Developmental Neuropsychology. 2011;36:429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark AS, MacLusky NJ, Goldman-Rakic PS. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123:932–940. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- 17.Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biological Psychiatry. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mrna expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. The Journal Of Comparative Neurology. 2001;439:208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- 19.Bos PA, van Honk J, Ramsey NF, Stein DJ, Hermans EJ. Testosterone administration in women increases amygdala responses to fearful and happy faces. Psychoneuroendocrinology. 2013;38:808–817. doi: 10.1016/j.psyneuen.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Moore WE, 3rd, Pfeifer JH, Masten CL, Mazziotta JC, Iacoboni M, Dapretto M. Facing puberty: Associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience. 2012;7:35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeifer JH, Masten CL, Moore WE, 3rd, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: Resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 25.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 26.Deeley Q, Daly EM, Azuma R, Surguladze S, Giampietro V, Brammer MJ, Hallahan B, Dunbar RIM, Phillips ML, Murphy DGM. Changes in male brain responses to emotional faces from adolescence to middle age. Neuroimage. 2008;40:389–397. doi: 10.1016/j.neuroimage.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagliaccio D, Luby JL, Gaffrey MS, Belden AC, Botteron KN, Harms MP, Barch DM. Functional brain activation to emotional and nonemotional faces in healthy children: Evidence for developmentally undifferentiated amygdala function during the school-age period. Cognitive, Affective & Behavioral Neuroscience. 2013;13:771–789. doi: 10.3758/s13415-013-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 30.Lobaugh NJ, Gibson E, Taylor MJ. Children recruit distinct neural systems for implicit emotional face processing. NeuroReport: For Rapid Communication of Neuroscience Research. 2006;17:215–219. doi: 10.1097/01.wnr.0000198946.00445.2f. [DOI] [PubMed] [Google Scholar]
- 31.Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 32.Marusak HA, Carré JM, Thomason ME. The stimuli drive the response: An fmri study of youth processing adult or child emotional face stimuli. Neuroimage. 2013;83:679–689. doi: 10.1016/j.neuroimage.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- 34.Blair KS, Geraci M, Korelitz K, Otero M, Towbin K, Ernst M, Leibenluft E, Blair RJR, Pine DS. The pathology of social phobia is independent of developmental changes in face processing. The American Journal of Psychiatry. 2011;168:1202–1209. doi: 10.1176/appi.ajp.2011.10121740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 36.Beesdo K, Lau JYF, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Leibenluft E, Ernst M, Pine DS. Fmri predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology. 2007;191:97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- 38.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pine DS. Research review: A neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 40.Killgore WDS, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. NeuroReport: For Rapid Communication of Neuroscience Research. 2005;16:1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- 41.Goddings AL, Burnett Heyes S, Bird G, Viner RM, Blakemore SJ. The relationship between puberty and social emotion processing. Developmental Science. 2012;15:801–811. doi: 10.1111/j.1467-7687.2012.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. The Journal Of Adolescent Health: Official Publication Of The Society For Adolescent Medicine. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- 43.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 44.Dorn LD, Susman EJ. Puberty script: Assessment of physical development in boys and girls. Cincinati, OH: Cincinnati Children's Hospital Medical Center; 2002. [Google Scholar]
- 45.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Development. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muthén LK, Muthén BO. Mplus user's guide. 7th. Los Angeles, CA: Muthén & Muthén; 2013. [Google Scholar]
- 47.March JS, Sullivan K, Parker J. Testäìretest reliability of the multidimensional anxiety scale for children. Journal of Anxiety Disorders. 1999;13:349–358. doi: 10.1016/s0887-6185(99)00009-2. [DOI] [PubMed] [Google Scholar]
- 48.Baldwin JS, Dadds MR. Reliability and validity of parent and child versions of the multidimensional anxiety scale for children in community samples. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:252–260. doi: 10.1097/01.chi.0000246065.93200.a1. [DOI] [PubMed] [Google Scholar]
- 49.Rynn MA, Barber JP, Khalid-Khan S, Siqueland L, Dembiski M, McCarthy KS, Gallop R. The psychometric properties of the masc in a pediatric psychiatric sample. Journal of Anxiety Disorders. 2006;20:139–157. doi: 10.1016/j.janxdis.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Dierker LC, Albano AM, Clarke GN, Heimberg RG, Kendall PC, Merikangas KR, Lewinsohn PM, Offord DR, Kessler R, Kupfer DJ. Screening for anxiety and depression in early adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:929–936. doi: 10.1097/00004583-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Muris P, Merckelbach H, Ollendick T, King N, Bogie N. Three traditional and three new childhood anxiety questionnaires: Their reliability and validity in a normal adolescent sample. Behaviour Research And Therapy. 2002;40:753–772. doi: 10.1016/s0005-7967(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 52.March JS, Parker JDA, Sullivan K, Stallings P, Conners CK. The multidimensional anxiety scale for children (masc): Factor structure, reliability, and validity. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 53.March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The multidimensional anxiety scale for children (masc): Factor structure, reliability, and validity. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 54.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: A comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 55.Egger HL, Pine DS, Nelson E, Leibenluft E, Ernst M, Towbin KE, Angold A. The nimh child emotional faces picture set (nimh-chefs): A new set of children's facial emotion stimuli. International Journal of Methods in Psychiatric Research. 2011;20:145–156. doi: 10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazaika P, Whitfield-Gabrieli S, Reiss A. Artifact repair for fmri data from high motion clinical subjects. Human Brain Mapping; Chicago, IL: 2007. [Google Scholar]
- 57.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the mni mri single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 58.Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: A pet activation study of positive and negative affect. Neuropsychopharmacology. 2003;28:726–733. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- 59.Cooney RE, Atlas LY, Joormann J, Eugène F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral? Psychiatry Research: Neuroimaging. 2006;148:55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Winton EC, Clark DM, Edelmann RJ. Social anxiety, fear of negative evaluation and the detection of negative emotion in others. Behaviour Research And Therapy. 1995;33:193–196. doi: 10.1016/0005-7967(94)e0019-f. [DOI] [PubMed] [Google Scholar]
- 61.Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen X, Yao L, Liu Y, Ding M. Causal interactions in attention networks predict behavioral performance. The Journal of Neuroscience. 2012;32:1284–1292. doi: 10.1523/JNEUROSCI.2817-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


