Abstract
Indoor pesticide exposure is a growing concern, particularly from pyrethroids, a commonly used class of pesticides. Pyrethroid concentrations may be especially high in homes of immigrant farm worker families who often live in close proximity to agricultural fields, and are faced with poor housing conditions, causing higher pest infestation and more pesticide use. We investigate exposure of farm worker families to pyrethroids in a study of mothers and children living in Mendota, CA within the population-based Mexican Immigration to California: Agricultural Safety and Acculturation (MICASA) Study. We present pyrethroid exposure based on an ELISA analysis of urinary metabolite 3-phenoxybenzoic acid (3PBA) levels among 105 women and 103 children. The median urinary 3PBA levels (children = 2.56 ug/g creatinine, mothers = 1.46 ug/g creatinine) were higher than those reported in population based studies for the United States general population, but similar to or lower than studies with known high levels of pyrethroid exposure. A positive association was evident between poor housing conditions and the urinary metabolite levels, showing that poor housing conditions are a contributing factor to the higher levels of 3PBA seen in the urine of these farm worker families. Further research is warranted to fully investigate sources of exposure.
Keywords: Pyrethroid, 3PBA, ELISA, farm worker, children
1. Introduction
Since they were first introduced in the 1970s more than 30 synthetic pyrethroids have been commercialized (Khambay and Jewess, 2005). Their rapid control of insects, relatively low toxicity to mammals and rapid degradation in the environment has made pyrethroids a very important class of insecticides, and they are commonly used worldwide. The federally mandated phase-out of residential uses of the organophosphate pesticides chlorpyrifos and diazinon in 2001 has caused further increases in the usage of pyrethroids indoors (Horton et al., 2011; USEPA, 2001; USEPA, 2012; Williams et al., 2008).
This class of insecticides has a remarkably good safety profile, particularly compared to earlier materials that they replaced. There are cautions with regard to human exposure however. Many studies have shown that high levels of exposure to pyrethroids may cause significant toxicity and health effects. Pyrethroids are acute neurotoxins (Costa et al., 2008; Ray and Fry, 2006). They have shown immunotoxic effects (Blaylock et al., 1995; Emara and Draz, 2007) and negative effects on mammalian reproduction (Ji et al., 2011; Zhang et al., 2008), and they are reported to likely be carcinogenic to humans (USEPA, 2006).
Exposure in the general population results from ingestion of foods such as fruits and vegetables onto which the insecticide has been applied; drinking water; and inhalation, dermal contact and non-dietary ingestion resulting from residential indoor application. In agricultural communities poor housing conditions can make homes more difficult to clean, potentially leading to a larger pest problem and in turn an increased use of pesticides in the indoor environment (Arcury et al., 2007; Bradman et al., 2005; Early et al., 2006; Quandt et al., 2004). Factors related to farm proximity, including drift from agricultural application and take-home contamination from occupational use, may also have a large influence on exposure to pesticides (Curl et al., 2002; Harnly et al., 2005; Lu et al., 2000; You et al., 2004). Because children have an increased risk of exposure to environmental contaminants as compared to adults (Moya et al., 2004) children living in agricultural communities are especially susceptible to pesticide exposure (Arcury et al., 2007; Bradman et al., 2007).
A number of major pyrethroids such as permethrin, cypermethrin, deltamethrin, and fenvalerate are metabolized to 3-phenoxybenzoic acid (3PBA). This urinary metabolite has been commonly used as a generic biomarker for evaluating human exposure to multiple pyrethroid pesticides (Barr et al., 2010; Chuang et al., 2011; Kimata et al., 2009). Traditionally, instrumental analytical methods (Leng et al., 1997; Schettgen et al., 2002) are used to determine 3PBA in urine samples. More recently however, a lower-cost bioanalytical approach using an enzyme-linked immunosorbent assay (ELISA) has been developed and shown to be a suitable alternative method for the analysis of 3PBA in urine samples for exposure monitoring (Ahn et al., 2011; Shan et al., 2004).
The city of Mendota is located in agriculturally intensive Fresno County, in California’s Central Valley. According to the USDA’s 2007 Census of Agriculture, Fresno County had 6,081 farms, comprising over 1.6 million acres of land. It was ranked one of the top three counties in the U.S. for either total value or total acreage of agricultural products for the following commodities: cotton and cottonseed; vegetables, melons, potatoes, and sweet potatoes; fruits, tree nuts, and berries; grapes; tomatoes; vegetables harvested for sale; and almonds (USDA, 2009). With almost 29 million pounds of pesticide active ingredients applied in Fresno County, the highest of any county in California, in 2009 (CDPR, 2010) it can be assumed that there is a high risk of pesticide exposure to the farm workers, their families and people living in the surrounding areas. This study was conducted to examine the sources of pyrethroid pesticide exposure in the homes of farm worker families living in Mendota, California. We report housing conditions and exposure data collected in 2009 on pyrethroid pesticides measured by questionnaires and urinary concentrations of the metabolite 3PBA among 105 women, 23–51 years of age and 103 children, 2–8 years of age.
2. Materials and Methods
2.1 Study Population
The Mexican Immigration to California: Agricultural Safety and Acculturation (MICASA) Study is a prospective cohort sample of 467 hired farm worker family households from Mendota, CA designed to evaluate occupational and environmental exposures of significance for a farm working population. Households were sampled from randomly selected census blocks and, following door-to-door enumeration, those households containing at least one hired farm worker were contacted for recruitment. Eligible participants in the MICASA study included men and women, residing in Mendota, CA, ages 18–55 years, self-identified as Mexican or Central American, with at least one household member who worked in agriculture 45 days or more in the previous year (Stoecklin-Marois et al., 2011).
MICASA recruitment and baseline interviews were conducted between January 2006 and May 2007. A follow-up interview was conducted between February 2009 and June 2010. Recruitment for the home pyrethroid exposure study began in February of 2009 and sample collection took place between June and December of 2009.
In total, 843 participants, representing 467 households, completed the MICASA baseline interview. The analysis highlighted in this paper was designed to look at sources of pyrethroid exposure in the homes of the MICASA study population. As children typically have higher levels of exposure to pesticides (Moya et al., 2004), we restricted eligibility to those MICASA families with at least one child aged 7 or under at the time of recruitment in order to better understand the sources in this potentially highly exposed population. Among the MICASA households completing baseline interviews, 175 (37.5%) were eligible for participation in the home pyrethroid exposure study. Eligible households were placed in random order for contact. One hundred twenty seven households were contacted for recruitment before reaching our goal of 105 (82.7%) households who agreed to participate and were enrolled in the study. The remaining 22 households either could not be contacted or declined to participate. If a family had multiple eligible children, one child was randomly selected and enrolled. At the time of sample collection, children ranged from 2 to 8 years of age.
Written informed consent was obtained from each participant. Each study component was described verbally and in writing to the participant prior to obtaining written informed consent. Spanish was the primary language of the participants, thus the study description and written informed consent were provided in Spanish. All study procedures were approved by the University of California, Davis Institutional Review Board.
2.2 Sample Collection
Data collected between June and December of 2009 consisted of urine samples, questionnaires and food recall.
As collecting 24-hour urine samples can be difficult for participants, a more convenient end-of-day single spot void urine sample was collected from each mother and enrolled child. The end-of-day was chosen to allow for a consistent collection time from all participants. Plastic bonnets, to be placed under the toilet seat (Barr et al., 2010; Wilson et al., 2004), and pre-labeled standard plastic urine cups were provided for urine collection. Total volume of void, time of void collection and time of previous void were recorded. Participants were instructed to store their urine sample in the provided coolers with ice packs overnight until study staff retrieved the samples the following day. Urine samples were then refrigerated at the MICASA field office for generally less than one day, delivered on ice to UC Davis where they were stored in a −80°C freezer until sample extraction and analysis.
At the time of urine collection, a questionnaire was administered to the mothers. We obtained the frequency of pesticide use in both the hot and cold season of the previous year. Pesticide use encompassed any type of pest control including sprays, foggers, sticky traps, bait traps, gels, and any application by professional exterminators.
To assess the presence of insect or rodent problems in the home, we asked if anyone living in the home had seen rodents, rodent feces, live or dead roaches, roach feces or ants inside the home at any time in the last year.
The overall condition of the home was assessed by a series of questions looking at the presence of various common household disrepair items with the aim of creating a summed Home Disrepair Score. These individual disrepair items included water damage, mold, plumbing leaks, rotten wood, holes/worn spots in flooring, walls, ceiling and/or counters, peeling paint and home security. On the day of urine collection, a staff member inventoried all pesticide products in the home, including their EPA registration number and active ingredients.
Additional sources of data included pesticide use questions assessed at both MICASA baseline and follow-up interviews and asked of husband and wife in each household. Furthermore, at follow-up interview, an evaluation each participant’s home was conducted by trained staff members. Three assessments--Inside Housing Condition, Outside Housing Condition, and Home Cleanliness--were scored on a 4-point scale based on level of disrepair. The Inside Housing Condition included peeling paint, holes/cracks in walls or floors, stains on floors, and presence of rodents and/or insect feces inside the house. The Outside Housing Condition included peeling paint and landscape maintenance. Home Cleanliness assessed the difficulty level of keeping the home clean. The Inside Housing Condition was based only on the portion of the home visible during the interview, and the research staff did not look for the presence of water damage.
Participants were asked to complete a 24-hour food diary for the day prior to urine collection. The diary was split into four sections: breakfast, lunch, dinner, and snacks. The participants were instructed to write down everything they ate or drank during the entire 24-hour period, including the number of servings of each item consumed. Due to the low education level of participants, we did not consider it feasible to collect more detailed serving size information. No information was collected on participants’ time activity.
2.3 Laboratory Methods
Urine samples were prepared and analyzed for 3PBA using a competitive inhibition ELISA adapted from previously reported methods (Leng et al., 1997; Shan et al., 2004). Briefly, 0.5 mL urine aliquots were treated by acid hydrolysis, followed by solid phase extraction with a mixed-mode (C8 + Strong Anion Exchange, 200 mg Screen-A Tube, Phenomenex, Torrance, CA) column, and finally solvent exchanged into methanol (MeOH). The ELISA was performed using 96-well high binding micro titer plates (Nunc, Roskilde, Denmark) coated with coating antigen cAg06 (Shan et al., 2004), washed with 10mM phosphate buffered saline (PBS) + 0.05% Tween 20 (PBST), blocked with 0.5% bovine serum albumin in PBS. All standards, quality control (QC) samples and urine samples were prepared in 10% MeOH in PBS prior to the assay procedure. A 10-point (0.005 – 10,000 ng/mL) calibration curve was prepared for each assay using a serial 1:5 dilution from the highest standard solution. A blank 0 ng/mL standard in the same 10% MeOH/PBS solution was also prepared. Wells containing instrument blanks received a 50 uL aliquot of PBST, while all remaining wells received a 50 uL aliquot of anti-rabbit 3PBA antibody #294 (Shan et al., 2004) diluted 1:7000 in PBST. The goat anti-rabbit IgG-horseradish peroxidase conjugate (GAR-HRP; Sigma) was diluted 1:3000 in PBST. The substrate solution (3.3 uL of 30% H2O2, 400 uL of 0.6% tetramethylbenzidine (TMB) in dimethylsulfoxide (DMSO) per 25 mL of acetate buffer, pH 5.5) was added and color development was stopped after 15 min with 2 M H2SO4. The absorbance was measured using a Vmax micro plate reader (Molecular Devices, Menlo Park, CA) in dual wavelength mode at 450 nm – 650 nm. Urinary creatinine concentrations were determined using the methods described in Ahn et al. 2011 (Ahn et al., 2011).
2.4 QA/QC
All urine samples, blank samples and QC samples were analyzed in triplicate. The final concentrations were calculated from the means of the triplicate values. If one of the triplicate values fell below the LOD it was not considered in the calculation of the mean concentration. Mean values that fell between the LOD and the LOQ were retained in all further statistical analyses. For each set of extractions, one urine sample was randomly chosen for QC analysis and was spiked to a level of 10 ng/mL 3PBA before extraction in order to verify that there were no matrix effects from the sample extract and to check method accuracy. Five different urine samples were also extracted and analyzed in duplicate at different time points in the analysis to verify the method precision.
For further validation of the ELISA method, and in order to try to quantify any cross-reactivity that may have occurred, a set of six urine samples was sent to Emory University in Atlanta, GA where they were analyzed by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) using a well established method (Olsson et al., 2004).
2.5 Statistical Analysis
Summary statistics for both the volume based and creatinine adjusted 3PBA data were calculated. For concentrations below the limit of detection (LOD), an imputed value was assigned equal to the LOD divided by the square root of 2 (Barr et al., 2010; Hornung and Reed, 1990).
To determine predictive variables from the questionnaire data to include in a multivariate analysis, linear regression with both the log-transformed creatinine concentration and the variable of interest, referred to as the bivariate comparisons, were performed for each variable with 3PBA concentration. For each type of pesticide application, we created a continuous variable that represented the number of applications per year as well as a categorical variable indicating if that type of pesticide had been applied. Additional variables were also created summing pesticide applications across different application types.
Food diaries were translated to English, and individual food items were grouped into ten different food categories: Fruit, Vegetables, Legumes, Meats, Snack/Processed Foods, Dairy, Beverages, Grains, Mixed Foods (i.e. soup), and Other. Each category also had sub-categories for specific, popular food items. For example, in the Dairy category, sub-categories included Milk, Cheese and Other Dairy. Two variables were created for each food category and sub-category. First a categorical variable that indicated if the participant had consumed an item from a given category or sub-category. Second, a continuous variable was created with the number of servings in each category or sub-category.
Volume based 3PBA concentrations were log-transformed to better approximate a normal distribution and regressed against the log-transformed creatinine concentrations and the individual questionnaire variable (Barr et al., 2005). Two regression analyses were conducted for each variable, one including only data from the mothers and one including only data from the children (Barr et al., 2005).
There were a number of variables related to individual measures of home disrepair (e.g. mold, water damage, holes in the carpet, etc.) that were significant in the bivariate analysis. Because many of these measures of disrepair were correlated, item analysis was performed to select and evaluate the internal consistency of a set of items for a summative scale score. The resulting Home Disrepair Score is computed by summing the water damage, leaks, carpet damage, worn spots or holes in the counters and rotten wood indicators and has good internal consistency in our sample (Cronbach Coefficient Alpha = 0.81).
Multivariate regression was then performed to evaluate which questionnaire variables were most predictive of the urinary 3PBA concentrations. Three models were fit, one with the data from both the mothers and the children, one with data only from the children, and one with data only from the mothers. As an alternative, we also ran the models using the metabolite concentrations directly adjusted by the creatinine concentration. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
3. Results
3.1 Population Demographics & Questionnaire
Women in this study ranged in age from 23 to 51 years old; they had very low educational levels, with 46% having only a 6th grade education or lower; they were almost all married and lived in homes with 4 or more residents (Table 1). Pest problems were common with 59% reporting insect problems, 43% using pesticides indoors and 35% applying pesticides outside (Table 2). A Spearman rank correlation analysis was performed to see if there were associations between the two measures of home disrepair (Home Disrepair Score and Inside Housing Conditions) with pesticide application. Multiple significant correlations were observed (see Table S1 in Supporting Information), suggesting that poor housing conditions do lead to higher rates of pesticide application.
Table 1.
Socio-demographic characteristics of mothers in Home Pesticide Study assessed on MICASA baseline questionnaire, 2006–2007.
| Mother Characteristics (n=105) | % | |
| Age Range = 23.2 to 51.8 years | ||
| < 30 | 31 | |
| 30–34 | 25 | |
| 35–39 | 22 | |
| 40–44 | 18 | |
| >45 | 4 | |
| Education | ||
| No school | 4 | |
| ≤ 6th Grade education | 42 | |
| > 6th Grade education | 54 | |
| Marital Status | ||
| Married | 92 | |
| Single | 8 | |
| # of People in Household | ||
| 1–3 | 6 | |
| 4–6 | 73 | |
| > 6 | 21 | |
| Country of Birth | ||
| United States | 3 | |
| Mexico | 65 | |
| El Salvador | 29 | |
| Other | 3 | |
| Child Characteristics (n=103) | % | |
| Age Range = 1.6 to 8.4 years | ||
| < 3 | 4 | |
| 3–4 | 53 | |
| 5–6 | 31 | |
| >6 | 12 | |
| Sex | ||
| Male | 53 | |
| Female | 47 | |
Table 2.
Self-reported pesticide use and home disrepair indicators from the MICASA Home Pesticide Study questionnaire, 2009.
| Questionnaire Items (n=105) | % |
|---|---|
| Insect problem in home | 59 |
| Indoor pesticide use by household member | 43 |
| Had pesticides stored in the home at time of interviewa | 38 |
| Outdoor pesticide use by household member | 35 |
| Rodent problem in home | 18 |
| Peeling paint in home | 17 |
| Pesticide use by professional applicator | 13 |
| Mold in home | 13 |
| Holes in carpets or flooring in home | 11 |
| Holes or worn spots on counters in home | 7 |
| Holes or cracks in walls or ceiling in home | 7 |
| Water damage in home | 6 |
| Rotting wood in home | 6 |
Based upon staff evaluation
3.2 QA/QC
For each set of three triplicates, the sample standard deviation of the urinary 3PBA concentration was computed. The average 3PBA concentration was 2.51 with an average standard deviation of 0.42 ng/mL. Less than 10% of the samples had a triplicate that fell below the LOD resulting in that value being dropped from the calculation of the mean. Recoveries of the fortified urine samples ranged from 67 to 111% with an average (± standard deviation) of 82 ± 12%. The LOD of this analysis was estimated to be 0.1 ng of 3PBA in 1 mL urine. The limit of quantitation (LOQ) was determined to be 2 ng/mL. The percent difference (%D) between concentrations in duplicate aliquots of selective urine samples ranged from 3.6 to 28% with an average of 14%.
Six urine samples were also analyzed by HPLC-MS/MS to further validate the ELISA method. The square of the correlation coefficient between the 3PBA concentrations from the two laboratory methods for the six samples tested was R2 = 0.934, and the %D ranged from 7.9 to 30.6% with an average of 25%, with the ELISA resulting in higher concentrations in four of the samples, and lower concentrations in two of the samples.
3.3 Urinary 3PBA Concentrations
Urinary 3PBA concentrations in our study were detected in 80% of all samples with a range of 0.3–13 ng/mL (Table 3). There was no significant difference in the detection frequencies between mothers and children. However, adjustment for urinary creatinine resulted in a significantly higher concentration of urinary metabolites in children than in mothers (p=0.005). We calculated the correlation of urinary 3PBA concentrations between mothers and children. Urinary 3PBA concentrations from mothers and children in the same household were positively correlated for both volume based (R2=0.39, p < 0.0001) and creatinine adjusted concentrations (R2=0.37, p < 0.0001).
Table 3.
Summary statistics of volume based (ng/mL) and creatinine adjusted (ug/g) 3PBA concentrations of participant urine samples in MICASA Home Pesticide Study, 2009a.
| Percentilesc | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Category (n) | % NDb | Mean | GMc | 50th | 75th | 90th | 95th | Max | |
| Total (208) | 20.2 | ||||||||
| volume based (208) | 2.51 | 1.14 | 1.65 | 3.65 | 5.58 | 8.14 | 13.29 | ||
| creatinine adjusted (205) | 3.49 | 1.47 | 1.86 | 3.67 | 8.74 | 13.68 | 36.58 | ||
| Children (103) | 22.3 | ||||||||
| volume based (103) | 2.40 | 1.11 | 1.93 | 3.59 | 5.61 | 7.36 | 11.85 | ||
| creatinine adjusted (103)d | 3.83 | 1.97 | 2.56 | 5.96 | 10.11 | 15.47 | 36.58 | ||
| Mothers (105) | 18.1 | ||||||||
| volume based (105) | 2.62 | 1.17 | 1.63 | 3.65 | 5.60 | 8.24 | 13.29 | ||
| creatinine adjusted (102) | 3.15 | 1.10 | 1.46 | 2.58 | 5.27 | 10.37 | 21.95 | ||
Samples below LOD = LOD/√2, LOD = 0.1 ng/mL
%ND – percent non-detect
GM – geometric mean
Significantly higher than Mothers (p = 0.005)
3.4 Multivariate Analysis
Variables included in the multivariate analysis were based upon the results of the bivariate analysis. The Home Disrepair Score, derived from the combination of multiple questionnaire items, was significant in the bivariate analysis only for mothers, while the Inside Housing Conditions score, derived from the staff evaluation during the MICASA follow-up interview, was significant only for the children. Because these two scores were designed to measure similar housing characteristics, both scores along with the Outdoor Spray pesticide use variable from the MICASA baseline questionnaire and the log-transformed creatinine concentrations were included in all three multivariate models described below.
Multivariate models assessed factors associated with 3BPA concentrations in the combined sample (both mothers and children), children only and mothers only (Table 4). Both the Home Disrepair Score (p = 0.049) and Outdoor Spray (p = 0.03) were positive significant predictors of urinary 3PBA levels in the total study population model, which included log-transformed creatinine, the Home Disrepair Score, Outdoor Spray, Inside Housing Conditions and a Mother/Child variable (Table 4). The model restricted to children included food diary variables significant in the bivariate model: Apple (categorical), Milk (continuous), All Meat (continuous) and Cereal (continuous) as well as the log-transformed creatinine, the Home Disrepair Score, Outdoor Spray and Inside Housing Conditions. In this model Outdoor Spray (p = 0.07) and Inside Housing Conditions (p = 0.08) were marginally significant positive estimators of urinary 3PBA concentration. Cereal Total, while marginally significant, was negatively associated with urinary 3PBA in the children only data. In the mother only model we included the food diary variables Eggs (categorical), Beans (categorical), Grapes (categorical), Chicken (categorical), and Cereal (continuous) as well as log-transformed creatinine, the Home Disrepair Score, Outdoor Spray and Inside Housing Conditions. The Home Disrepair Score (p = 0.03), Outdoor Spray (p = 0.03), and Cereal Total (p = 0.04) were all significant positive estimators of urinary 3PBA levels in the mothers. The models with the metabolite concentrations directly adjusted for creatinine resulted in similar associations (see Table S2 in supporting information).
Table 4.
Multivariate analysis results showing the relationship between the log-transformed volume based urinary 3PBA concentrations and various pesticide use, home disrepair and food diary items.
| Total Study Population (n=208) | Percent Users |
Estimate | Standard Error |
t value | Pr > t |
|---|---|---|---|---|---|
| Creatininea | 0.61 | 0.11 | 5.4 | <.0001 | |
| Mother or Child | −0.31 | 0.16 | −1.9 | 0.06 | |
| Home Disrepair Scoreb | 20 | 0.17 | 0.09 | 2.0 | 0.049 |
| Outdoor Sprayc | 15 | 0.77 | 0.35 | 2.2 | 0.03 |
| Inside Housing Conditionsd | 40 | 0.26 | 0.19 | 1.4 | 0.17 |
| Children (n=103) | |||||
| Creatininea | 0.59 | 0.15 | 3.8 | 0.0003 | |
| Home Disrepair Scoreb | 19 | 0.16 | 0.10 | 1.7 | 0.10 |
| Outdoor Sprayc | 15 | 0.71 | 0.39 | 1.8 | 0.07 |
| Inside Housing Conditionsd | 40 | 0.38 | 0.21 | 1.8 | 0.08 |
| Apple Yes/No | 28 | −0.48 | 0.32 | −1.5 | 0.13 |
| Milk Total | 86 | −0.15 | 0.13 | −1.2 | 0.22 |
| All Meat Total | 76 | 0.25 | 0.15 | 1.7 | 0.10 |
| Cereal Total | 50 | −0.42 | 0.24 | −1.7 | 0.08 |
| Mothers (n=105) | |||||
| Creatininea | 0.53 | 0.18 | 3.0 | 0.0038 | |
| Home Disrepair Scoreb | 21 | 0.22 | 0.10 | 2.2 | 0.03 |
| Outdoor Sprayc | 14 | 0.88 | 0.39 | 2.3 | 0.03 |
| Inside Housing Conditionsd | 41 | −0.10 | 0.21 | −0.5 | 0.64 |
| Eggs Yes/No | 39 | −0.38 | 0.29 | −1.3 | 0.19 |
| Beans Yes/No | 53 | −0.37 | 0.29 | −1.3 | 0.21 |
| Grapes Yes/No | 12 | 0.54 | 0.43 | 1.3 | 0.21 |
| Chicken Yes/No | 24 | 0.58 | 0.35 | 1.7 | 0.10 |
| Cereal Total | 37 | 0.58 | 0.28 | 2.1 | 0.04 |
Log transformed values
Includes water damage, water leaks, carpet damage, counter damage and rotten wood assessed at time of urine collection
Data from baseline interview (2006–2007)
Staff evaluated data from follow-up interview (2009–2010)
4. Discussion
We assessed the exposure to pyrethroid pesticides in 105 women and 103 children in a farm worker population by laboratory measurements of the metabolite 3PBA in urine samples and by questionnaire data. This population had a relatively low educational level, with only about half of the adult women participating in our study reporting a 6th grade education or higher, in contrast to the 85% of U.S. adults who have a high school diploma (Stoops, 2004). The results from the ELISA method used to analyze urinary 3PBA concentrations in this study were validated by a more traditional instrumental method at the Emory University in Atlanta, GA. The suitability of this newer analytical technique for use in biological monitoring of 3PBA is further corroborated by Chuang et al. who analyzed over 100 urine samples and showed high correlation between ELISA and GC/MS data, with the square of the linear correlation coefficient R2 = 0.906 and no significant between the two methods of analysis for any given sample (Chuang et al., 2011).
Children had higher concentrations of this metabolite than their mothers. This result is consistent with multiple pesticide exposure studies and most likely has to do with differences in behavior that leads to higher non-dietary ingestion in children (Cohen Hubal et al., 2000). Once pyrethroid pesticides have entered the home, the carpets and cushioned furniture can act as repositories for pesticides (Colt et al., 2004; Starr et al., 2008). High levels of pesticides in carpet dust is a particular concern for young children who, due to their continual exploration of their environments, spend a large amount of time on the floor and have increased hand to mouth activity, resulting in increased exposure to the pollutants through dermal and non-dietary ingestion routes (Fenske et al., 1990; Gurunathan et al., 1998; Zartarian et al., 1997).
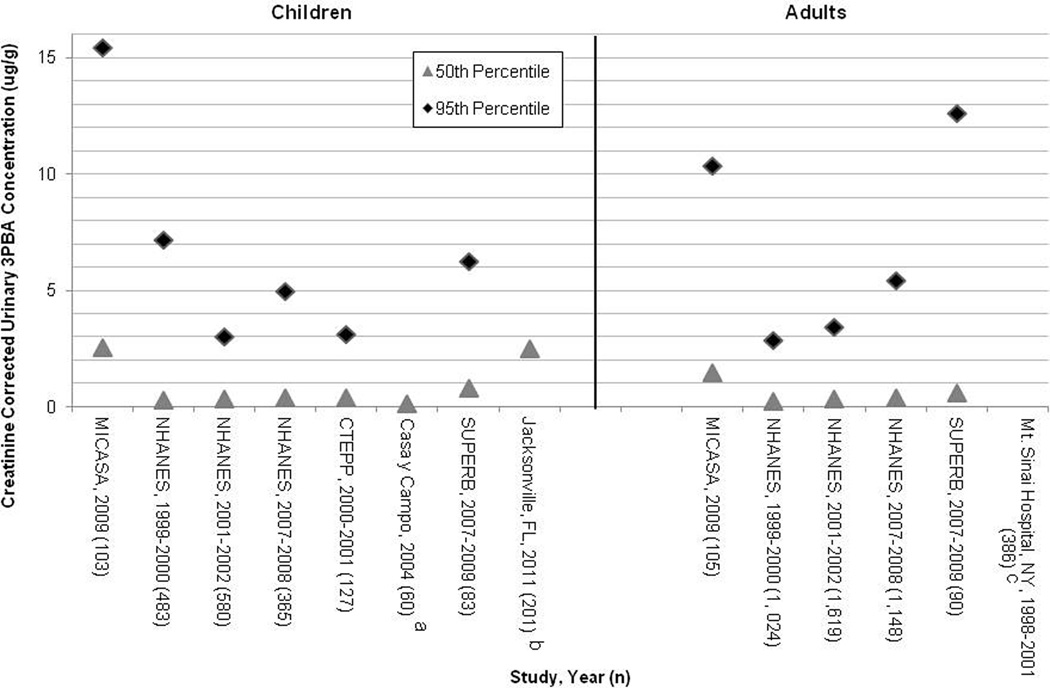
The median concentrations in the National Health and Nutrition Examination Survey (NHANES), a population based sample, collected from 1999 to 2000 were 0.30 and 0.26 ug/g creatinine for children (aged 6–11 years) and adults (aged 20–59 years), respectively, and only increased slightly to 0.33 and 0.30 ug/g creatine for children and adults, respectively, in samples collected from 2001 to 2002 (Barr et al., 2010). Median urinary 3PBA concentrations of NHANES samples collected from 2007 to 2008 increased a bit more to 0.42 and 0.38 ug/g creatinine for children and adults, respectively (CDC, 2013). In the Children’s Total Exposure to Persistent Pesticides (CTEPP) 2000 to 2001 study, a population based sample of preschool children living in Ohio, the median level was 0.32 ug/g creatinine for children aged 1–5 years (Morgan et al., 2007). The 2004 Casa y Campo study, a community-based project aiming to reduce pesticide exposure among farm workers and their families in eastern North Carolina, reported median concentrations of 3PBA in children aged 1–6 years of 0.15 ug/g creatinine (Arcury et al., 2007). In the 1999 to 2001 Center for the Health and Assessment of Mothers and Children of Salinas (CHAMACOS), a longitudinal birth cohort study of families in a largely agricultural area, the median level of the pregnant adult women in an agricultural community was below the LOD of 0.1 ug/g creatinine (Castorina et al., 2010). The 2007 to 2009 Study of Use of Products and Exposure Related Behavior (SUPERB), a study investigating behaviors of Northern California families that could influence exposure to environmental pollutants, reports median urinary 3PBA concentrations of 0.80 and 0.61 ug/g creatinine for children and adults respectively (Trunnelle et al., 2014). The 3PBA levels in the MICASA population, 2.56 ug/g in children and 1.46 ug/g in mothers, were approximately ten times higher than those in the NHANES, CTEPP and CHAMACOS studies and approximately 3 times higher that those in SUPERB. A 2011 biomonitoring study in which participants were recruited when they visited a health center in Jacksonville, FL, a city previously determined to have elevated rates of pesticide use, showed median urinary 3PBA levels of 2.5 ug/g in children aged 4–5 years (Naeher et al., 2010), a value similar to the median urinary 3PBA levels found in our population. Finally, the Children’s Environmental Health Study, a prospective study following a multiethnic urban cohort of mothers and infants delivered at Mount Sinai Hospital in New York City, found median urinary 3PBA levels to be 19.3 ug/g in pregnant adult women living in the area during the 1998 to 2001 sampling (Berkowitz et al., 2003) (Figure 1). While the Children’s Environmental Health Study in New York City reported much higher levels, it was suspected that the sumithrin sprayed to combat West Nile Virus in the area during the sampling campaign may have contributed to these findings (Berkowitz et al., 2003).
Figure 1.
Comparison of 50th and 95th percentile creatinine adjusted 3PBA concentrations (ug/g) of children (left) and women (right) reported in multiple studies, with number of subjects in parenthesis.
a95th percentile not reported
b95th percentile is 24.2 ug/g
c50th percentile is 18.3 ug/g, and 90th percentile is 126.9 ug/g
With urinary 3PBA levels higher than the NHANES, CTEPP, Casa y Campo, SUPERB and CHAMACOS studies, and comparable to the Jacksonville, Fl study, it is clear that the MICASA population has high concentrations of 3PBA in their urine, which may be indicative of pyrethroid pesticide exposure. One possible explanation for these results is the increasing use of pyrethroids for residential spraying since the 2001 federally mandated phase-out of residential uses of the organophosphate pesticides chlorpyrifos and diazinon (Horton et al., 2011; USEPA, 2001; USEPA, 2012; Williams et al., 2008). However, since the NHANES 2007–2008 results show only a minimal increase in 3PBA levels from the 1999–2002 results, it is more likely that higher urinary 3PBA levels in the MICASA population are due to residing in an agricultural community with higher pesticide use and therefore exposure. We also note that some of the 3PBA in the urine may have resulted from direct exposure to 3PBA in the environment, as it has been measured in dust (Starr et al., 2008). It is also important to note that, although our results were validated by a GC/MS method, it is possible for cross-reactivity to occur with another common pyrethroid metabolite, 4-fluoro-3-phenoxybenzoic acid (4F3PBA), in ELISA. This could potentially result in an over estimation of 3PBA concentrations in our study as compared to those using instrumental methods. However, the median 4F3PBA levels reported in the 2007–2008 NHANES were only 0.082 and 0.061 ug/g creatinine for children and adults, respectively (CDC, 2013). If cross-reactivity were to occur, these levels would still not account for the difference between NHANES and MICASA urinary 3PBA levels.
There were few associations between the metabolite and pesticide use data from the questionnaire administered at the time of urine collection. Although 59% of the population reported having an insect problem in the home, within the previous year, only 43% reported using pesticides inside their homes and 35% reported using pesticides outside their homes. When both adult household members were asked the pesticide use questions at baseline, 21% husband/wife pairs gave discordant answers to using indoor pesticides, and 27% gave discordant answers to using outdoor pesticides within the last year. These results suggest that actual pesticide use was higher than reported in our study and that both adult household members should be interviewed in the future in order to obtain a more accurate measure of pesticide use.
There were limited associations with urinary metabolite levels and food products, with some associations, such as grapes, corresponding to crops to which pyrethroids are applied. Associations with food items where pyrethroids are not used, or used very minimally, may represent other dietary trends, or spurious correlations, as have been found in previous studies assessing food categories and pesticide metabolites in urine (Riederer et al., 2008).
There were several limitations to this study, mostly related to the use of self-reported data. One such limitation was that we were unable to obtain food serving size information due to the low educational level of this population. Additionally, as mentioned above, there was a lack of consistent reporting of pesticide applications between husband and wife. There were also limited and inconsistent relationships between housing disrepair indicators and the metabolite data. The Home Disrepair Score, a sum of participants self-reported housing disrepair indices, was significantly associated with the mother’s urinary metabolite data but only marginally associated with metabolite levels in the children. In contrast, the Inside Housing Conditions, an interviewer assessed measure of housing disrepair indices, was marginally associated with children’s urinary metabolite data with a p-value of 0.08 and was not associated with the mother’s urinary metabolite data. A possible explanation for this inconsistency is that it was difficult for staff to assess home conditions for the Inside Housing Conditions while remaining sensitive to the participants in this community-based study. Additionally, all participants may not have consistently reported on their own housing conditions for the Home Disrepair Score. The difficulty in obtaining an accurate and reliable rating of housing condition is a limitation of this study. Also, there is often both within-day and between-day variability in the urinary concentrations of metabolites of non-persistent compounds like pyrethroids when only single spot urine samples are collected (Preau et al., 2010). Although inconsistent, the data does however support the idea that poor housing conditions lead to higher pesticide exposure, thus looking at both housing condition measurements (self-reported and interviewer assessed) can be an alternative mechanism in predicting pesticide exposure of people living in poorer housing conditions.
As a urinary metabolite of multiple pyrethroids, 3PBA can reflect exposure from various sources, not only those in the home environment. Unfortunately there were few correlations between the urinary 3PBA concentrations and the questionnaire data. Limitations of this study were difficulties in obtaining accurate pesticide use information, food serving size information, and reliable measures for housing conditions.
4.1 Conclusion
Despite its limitations, this study contributes to existing research by providing further evidence that farm working families may face higher exposures to pyrethroid pesticides. Additionally, this study provides evidence that poor housing conditions are a contributing factor to the higher exposure. Further research is warranted to fully investigate the impact of housing conditions.
Supplementary Material
Acknowledgements
We acknowledge Tamara Hennessy-Burt for assistance with statistical programming; Lisa Tang for assistance in both the field and the lab; the local field staff, particularly Gloria Andrade, Ana Cervantes, Alex Cervantes and Giselle Garcia, for all of their efforts; and all of the participants. We thank Dana Barr for analysis of validation samples. Funding from The California Endowment and the National Institute of Occupational Safety and Health, 2U50OH007550 and 1R01OH009293, and the NIEHS Superfund Research Program, P42ES04699 supported this research.
Relevant Abbreviations and Definitions
- MICASA
Mexican Immigration to California: Agricultural Safety and Acculturation
- 3PBA
3-phenoxybenzoic acid
- MeOH
methanol
- PBS
phosphate buffered saline
- PBST
phosphate buffered saline Tween 20
- QA
quality assurance
- QC
quality control
- GAR-HRP
goat anti-rabbit IgG-horseradish peroxidase conjugate
- TMB
tetramethylbenzidine
- DMSO
dimethylsulfoxide
- HPLC-MS/MS
high performance liquid chromatography-tandem mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantitation
- %D
percent detection
- NHANES
National Health and Nutrition Examination Survey
- CTEPP
Children’s Total Exposure to Persistent Pesticides
- CHAMACOS
Center for the Health and Assessment of Mothers and Children of Salinas
- SUPERB
Study of Use of Products and Exposure Related Behavior
- 4F3PBA
4-fluoro-3-phenoxybenzoic acid
Footnotes
Funding & Institutional Review:
All study procedures were approved by the University of California, Davis Institutional Review Board under protocol #200513483-2, Mexican Immigration to California: Agricultural Safety and Acculturation (MICASA).
Supplementary Data Available
There is supplementary data available for this manuscript. The results of the multivariate analysis looking at the relationship between the log-transformed creatinine adjusted urinary 3PBA concentrations and various pesticide use, home disrepair and food diary items can be seen in Table S1. The results from the Spearman rank correlation analysis showing associations between home disrepair and pesticide use can be seen in Table S2.
References
- Ahn KC, et al. Immunochemical analysis of 3-phenoxybenzoic acid, a biomarker of forestry worker exposure to pyrethroid insecticides. Anal. Bioanal. Chem. 2011;401:1285–1293. doi: 10.1007/s00216-011-5184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcury TA, Grzywacz JG, Barr DB, Tapia J, Chen H, Quandt SA. Pesticide urinary metabolite levels of children in eastern North Carolina farmworker households. Environ Health Perspect. 2007;115:1254–1260. doi: 10.1289/ehp.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, et al. Urinary Concentrations of Metabolites of Pyrethroid Insecticides in the General U.S. Population: National Health and Nutrition Examination Survey 1999–2002. Environ. Health Perspect. 2010;118:742–748. doi: 10.1289/ehp.0901275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G, et al. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ. Health Perspect. 2003;111:79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock BL, et al. Suppression of cellular immune responses in BALB/c mice following oral exposure to permethrin. Bull. Environ. Contam. Toxicol. 1995;54:768–774. doi: 10.1007/BF00206111. [DOI] [PubMed] [Google Scholar]
- Bradman A, et al. Association of housing disrepair indicators with cockroach and rodent infestations in a cohort of pregnant Latina women and their children. Environ. Health Perspect. 2005;113:1795–1801. doi: 10.1289/ehp.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, et al. Pesticides and their metabolites in the homes and urine of farmworker children living in the Salinas Valley, CA. J. Expo. Sci. Environ. Epidemiol. 2007;17:331–349. doi: 10.1038/sj.jes.7500507. [DOI] [PubMed] [Google Scholar]
- Castorina R, et al. Comparison of current-use pesticide and other toxicant urinary metabolite levels among pregnant women in the CHAMACOS cohort and NHANES. Environ. Health Perspect. 2010;118:856–863. doi: 10.1289/ehp.0901568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. National Health and Nutrition Examination Survey 2007–2008 Laboratory Data. [January 24, 2014];2013 http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBeginYear=2007.
- CDPR. Summary of Pesticide Use Report Data - 2009. [July 11, 2012];2010 http://www.cdpr.ca.gov/docs/pur/pur09rep/09sum.htm.
- Chuang JC, Van Emon JM, Trejo RM, Durnford J. Biological monitoring of 3-phenoxybenzoic acid in urine by an enzyme-linked immunosorbent assay. Talanta. 2011;83:1317–1323. doi: 10.1016/j.talanta.2010.07.077. [DOI] [PubMed] [Google Scholar]
- Cohen Hubal EA, et al. Children's Exposure Assessment: A Review of Factors Influencing Children's Exposure, and the Data Available to Characterize and Assess That Exposure. Environ. Health Perspect. 2000;108:475–486. doi: 10.1289/ehp.108-1638158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colt JS, et al. Comparison of pesticide levels in carpet dust and self-reported pest treatment practices in four US sites. J. Expo. Anal. Environ. Epidemiol. 2004;14:74–83. doi: 10.1038/sj.jea.7500307. [DOI] [PubMed] [Google Scholar]
- Costa L, Giordano G, Guizzetti M, Vitalone A. Neurotoxicity of pesticides: a brief review. Front. Biosci. 2008;13:1240–1249. doi: 10.2741/2758. [DOI] [PubMed] [Google Scholar]
- Curl CL, et al. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ. Health Perspect. 2002;110:787–792. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early J, Davis SW, Quandt SA, Rao P, Snively BM, Arcury TA. Housing characteristics of farmworker families in North Carolina. J. Immigr. Minor. Health. 2006;8:173–184. doi: 10.1007/s10903-006-8525-1. [DOI] [PubMed] [Google Scholar]
- Emara AM, Draz EI. Immunotoxicological study of one of the most common over-the-counter pyrethroid insecticide products in Egypt. Inhal. Toxicol. 2007;19:997–1009. doi: 10.1080/08958370701533483. [DOI] [PubMed] [Google Scholar]
- Fenske RA, Black KG, Elkner KP, Lee CL, Methner MM, Soto R. Potential exposure and health risks of infants following indoor residential pesticide applications. Am. J. Public. Health. 1990;80:689–693. doi: 10.2105/ajph.80.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S, et al. Accumulation of chlorpyrifos on residential surfaces and toys accessible to children. Environ. Health Perspect. 1998;106:9–16. doi: 10.1289/ehp.981069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnly M, McLaughlin R, Bradman A, Anderson M, Gunier R. Correlating agricultural use of organophosphates with outdoor air concentrations: a particular concern for children. Environ. Health Perspect. 2005;113:1184–1189. doi: 10.1289/ehp.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung R, Reed L. Estimation of average concentrations in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990;5:46–51. [Google Scholar]
- Horton MK, et al. Characterization of residential pest control products used in inner city communities in New York City. J. Expo. Sci. Environ. Epidemiol. 2011;21:291–301. doi: 10.1038/jes.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, et al. Effects of non-occupational environmental exposure to pyrethroids on semen quality and sperm DNA integrity in Chinese men. Reprod. Toxicol. 2011;31:171–176. doi: 10.1016/j.reprotox.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Khambay BPS, Jewess PJ. Pyrethroids. In: Gilbert LI, Gill SS, editors. Insect Control. London, UK: Elsevier B.V., Academic Press; 2005. pp. 1–27. [Google Scholar]
- Kimata A, Kondo T, Ueyama J, Yamamoto K, et al. Relationship between dietary habits and urinary concentrations of 3-phenoxybenzoic acid in middle-aged and elderly general population in Japan. Environ. Health Prev. Med. 2009;14:173–179. doi: 10.1007/s12199-009-0077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Kühn KH, Idel H. Biological monitoring of pyrethroids in blood and pyrethroid metabolites in urine: applications and limitations. Sci. Total Environ. 1997;199:173–181. doi: 10.1016/s0048-9697(97)05493-4. [DOI] [PubMed] [Google Scholar]
- Lu C, Fenske RA, Simcox NJ, Kalman D. Pesticide exposure of children in an agricultural community: evidence of household proximity to farmland and take home exposure pathways. Environ. Res. 2000;84:290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- Morgan MK, Sheldon LS, Croghan CW, Jones PA, Chuang JC, Wilson NK. An observational study of 127 preschool children at their homes and daycare centers in Ohio: environmental pathways to cis- and trans-permethrin exposure. Environ. Res. 2007;104:266–274. doi: 10.1016/j.envres.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Moya J, Bearer CF, Etzel RA. Children's behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics. 2004;113:996–1006. [PubMed] [Google Scholar]
- Naeher LP, et al. Organophosphorus and pyrethroid insecticide urinary metabolite concentrations in young children living in a southeastern United States city. Sci. Total Environ. 2010;408:1145–1153. doi: 10.1016/j.scitotenv.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Olsson AO, et al. A liquid chromatography--tandem mass spectrometry multiresidue method for quantification of specific metabolites of organophosphorus pesticides, synthetic pyrethroids, selected herbicides, and deet in human urine. Anal. Chem. 2004;76:2453–2461. doi: 10.1021/ac0355404. [DOI] [PubMed] [Google Scholar]
- Preau JL, Jr, Wong LY, Silva MJ, Needham LL, Calafat AM. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ. Health Perspect. 2010;118:1748–1754. doi: 10.1289/ehp.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt SA, et al. Agricultural and residential pesticides in wipe samples from farmworker family residences in North Carolina and Virginia. Environ. Health Perspect. 2004;112:382–387. doi: 10.1289/ehp.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray DE, Fry JR. A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacol. Ther. 2006;111:174–193. doi: 10.1016/j.pharmthera.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Riederer AM, Bartell SM, Barr DB, Ryan PB. Diet and nondiet predictors of urinary 3-phenoxybenzoic acid in NHANES 1999–2002. Environ. Health Perspect. 2008;116:1015–1022. doi: 10.1289/ehp.11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettgen T, Koch HM, Drexler H, Angerer J. New gas chromatographic-mass spectrometric method for the determination of urinary pyrethroid metabolites in environmental medicine. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2002;778:121–130. doi: 10.1016/s0378-4347(01)00452-2. [DOI] [PubMed] [Google Scholar]
- Shan G, Huang H, Stoutamire DW, Gee SJ, Leng G, Hammock BD. A sensitive class specific immunoassay for the detection of pyrethroid metabolites in human urine. Chem. Res. Toxicol. 2004;17:218–225. doi: 10.1021/tx034220c. [DOI] [PubMed] [Google Scholar]
- Starr J, Graham S, Stout D, Andrews K, Nishioka M. Pyrethroid pesticides and their metabolites in vacuum cleaner dust collected from homes and day-care centers. Environ. Res. 2008;108:271–279. doi: 10.1016/j.envres.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Stoecklin-Marois MT, Hennessy-Burt TE, Schenker MB. Engaging a Hard-to-Reach Population in Research: Sampling and Recruitment of Hired Farm Workers in the MICASA Study. J. Agric. Saf. Health. 2011;17:291–302. doi: 10.13031/2013.39803. [DOI] [PubMed] [Google Scholar]
- Stoops N. Educational Attainment in the United States: 2003. [December 16, 2011];2004 http://www.census.gov/prod/2004pubs/p20-550.pdf.
- Trunnelle KJ, et al. Urinary pyrethroid and chlorpyrifos metabolite concentrations in northern California families and their relationship to indoor residential insecticide levels, part of SUPERB. Environ. Sci. Technol. 2014 doi: 10.1021/es403661a. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- USDA. Census of Agriculture, County Profile, Fresno County, CA 2007. [July 6, 2012];2009 http://www.agcensus.usda.gov/Publications/2007/Online_Highlights/County_Profiles/California/cp06019.pdf.
- USEPA. Interim Reregistration Eligibility Decision for Chlorpyrifos. [June 12, 2012];2001 http://www.epa.gov/pesticides/reregistration/REDs/chlorpyrifos_ired.pdf.
- USEPA. Permethrin Facts (Reregistration Eligibility Decision (RED) Fact Sheet) [October 8, 2011];2006 http://www.epa.gov/oppsrrd1/REDs/factsheets/permethrin_fs.htm#health.
- USEPA. Pesticides: Regulating Pesticides: Pyrethroids & Pyrethrins. [July 10, 2012];2012 http://www.epa.gov/oppsrrd1/reevaluation/pyrethroids-pyrethrins.html.
- Williams MK, et al. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ. Health Perspect. 2008;116:1681–1688. doi: 10.1289/ehp.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NK, et al. Design and sampling methodology for a large study of preschool children's aggregate exposures to persistent organic pollutants in their everyday environments. J. Expo. Anal. Environ. Epidemiol. 2004;14:260–274. doi: 10.1038/sj.jea.7500326. [DOI] [PubMed] [Google Scholar]
- You J, Weston DP, Lydy MJ. A sonication extraction method for the analysis of pyrethroid, organophosphate, and organochlorine pesticides from sediment by gas chromatography with electron-capture detection. Arch. Environ. Contam. Toxicol. 2004;47:141–147. doi: 10.1007/s00244-003-3165-8. [DOI] [PubMed] [Google Scholar]
- Zartarian VG, Ferguson AC, Leckie JO. Quantified dermal activity data from a four-child pilot field study. J. Expo. Anal. Environ. Epidemiol. 1997;7:543–552. [PubMed] [Google Scholar]
- Zhang SY, et al. Permethrin may induce adult male mouse reproductive toxicity due to cis isomer not trans isomer. Toxicology. 2008;248:136–141. doi: 10.1016/j.tox.2008.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.