Abstract
As part of the West Nile virus surveillance program for the state of New Mexico, 13 sites along the Rio Grande River were sampled for mosquitoes during spring and summer 2003. We evaluated 3 different trapping procedures for their effectiveness at capturing selected species of mosquitoes. The 3 methods used were a dry ice-baited Centers for Disease Control and Prevention (CDC) light trap set 1.5 m above the ground (standard method), a CDC light trap suspended within the forest canopy, and a gravid trap set on the ground. Thirteen sites were sampled for 10 1-night periods biweekly from May through September. The relative numbers of captured Culex tarsalis, Cx. salinarius, Cx. quinquefasciatus, and Aedes vexans as well as the numbers of total recorded captures of all species were compared for each trapping method. Significant differences were observed for each species by location and by trapping method. Culex tarsalis was most commonly caught in canopy or standard CDC traps, especially in cottonwood bosque. Culex salinarius was found most frequently in association with marshy water, and was most often caught in gravid or standard light traps. Culex quinquefasciatus was captured almost exclusively in gravid traps within urban areas. Aedes vexans was primarily sampled in standard CDC light traps and found most frequently in wooded areas near floodplains. With the exception of Cx. quinquefasciatus, no species was collected significantly more frequently in gravid or canopy traps than in the standard CDC light trap. Our findings do not support altering the methods currently used in New Mexico, namely, the use of 1.5-m CDC light traps and gravid traps. An increased use of gravid traps seems to be warranted in monitoring urban vector populations (specifically Cx. quinquefasciatus and Cx. salinarius) that may be involved in human transmission.
Keywords: Mosquitoes, trapping methods, New Mexico, Rio Grande, West Nile virus
Introduction
The emergence of West Nile virus (WNV) in the northeastern United States in 1999 marked the beginning of the steady and rapid spread of this arbovirus across the country (Peterson and Roehrig 2001, Roehrig et al. 2002). In addition to raising concerns as to the public health significance of this new threat, the WNV phenomenon has rekindled interest in mosquito control and ecology at the local and regional level. The city of Albuquerque, NM, has maintained a mosquito abatement program for many years, operated through the Environmental Health Bio-Disease Management Division. In 1986, the City of Albuquerque Environmental Health Department conducted a large-scale survey of mosquito population abundance and distribution in Bernalillo County, New Mexico, identifying 16 species in the area (Schultz 1987). More recently, a statewide collection was carried out in 2001 across 13 counties in New Mexico in response to the WNV threat (Brown 2002). This study was conducted as a 1-time survey, but it identified 25 species of mosquitoes from various parts of the state. Despite these efforts, systematic, ongoing surveys of New Mexico mosquito populations have been rare in recent years.
In 2002, a new initiative was undertaken to monitor and document the appearance and spread of WNV within a portion of the state. This initiative was developed as a collaboration of the New Mexico Department of Health, the Albuquerque Environmental Health Department, the Johns Hopkins Bloomberg School of Public Health, and the University of New Mexico Department of Biology. Although 78 equine cases were reported in early fall 2002, mostly in the southern Great Plains of eastern New Mexico, no positive mosquito pools were found in New Mexico (Ettestad et al. 2004). Only 353 pools were tested in 2002, and it is probable that the sampling was not sensitive enough to detect infected pools at the time. Based on the westward spread of WNV during previous years, it was anticipated that WNV would arrive in central and western New Mexico during summer 2003. Therefore, an intensive surveillance program was started in spring 2003 to monitor the introduction and establishment of WNV in the mosquito populations of the Rio Grande Basin from southern Colorado through New Mexico to western Texas.
A variety of trapping regimens have been used for such surveys, with results often dependent on local environmental variables, such as temperature and humidity, density of vegetation, and proximity of different water sources (Service 1993). The goal of our survey was to provide insight into the mosquito ecology of New Mexico and a more complete picture of the potential for spread of arboviruses in the region. We wanted to capture as many mosquitoes as possible, representing the greatest possible number of species. However, no information was available on the comparative utility of different trapping methods in this geographic region.
In this study, we evaluate the relative trapping success of 3 different approaches for mosquito collection in the semiarid conditions of New Mexico. The majority of the region is typified by scrub vegetation in a semiarid, high-elevation desert, with a concentration of prolific cottonwoods and other vegetation along the banks of the Rio Grande. The Rio Grande valley bosque bisects New Mexico, running from southern Colorado, into western Texas, where it defines the U.S.–Mexico border until it runs into the Gulf of Mexico. We chose to focus our efforts specifically on this corridor of woodland, because it serves as a migratory corridor for avian reservoirs of WNV as well as an optimal habitat that concentrates local wildlife and the mosquitoes that feed upon them (Yong and Finch 2002). We hypothesized that the bosque would yield the greatest success in mosquito collection. Results relating to the presence of WNV will be published separately, whereas the results presented here pertain to the efficacy of the 3 different trapping approaches used.
Materials and Methods
As part of an extended West Nile virus surveilvlance program (DiMenna et al. in press), mosquito traps were set along the Rio Grande in New Mexico from mid-May to late October and in early November 2003. Sites were selected at intervals of approximately 25–30 km along the river basin, based on proximity to the river and apparent suitability as mosquito habitat. Suitable mosquito habitat was considered to be sites within the riparian forest bordering the river (bosque), rarely extending past 400 m from the river or other nearby permanent water sources (e.g., marsh, irrigation channel, or runoff ditch). Thirteen sites along the Rio Grande were included in this analysis. The transect area extended approximately 560 km from Taos, New Mexico, in the north to San Marcial, New Mexico, in the south (Fig.1).
Fig. 1.
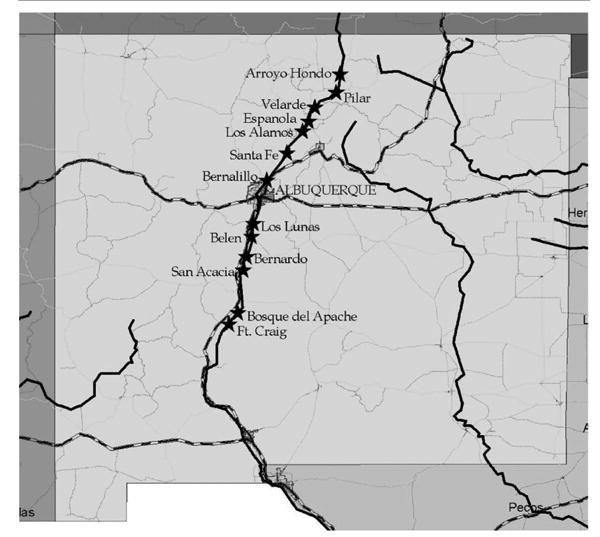
Map of New Mexico showing the study area along the Rio Grande River. Sites are categorized by habitat type as described in the text: type I-U, Espanola, Bernalillo, Los Lunas, Belen; type I-R, Velarde, Bernardo, San Acacia; type II, Santa Fe, Bosque del Apache, Ft. Craig; and type III, Arroyo Hondo, Pilar, Los Alamos.
Each site was sampled for 1 night every other week, beginning in mid-May and continuing until the number of captures had dropped to near 0. Sites north of Albuquerque, at higher latitude and elevation, were sampled through early October. Sites to the south were sampled until mid-November. Three trapping methods were used at each site. The 1st trap was a standard CDC light trap (John Hock Co., Gainesville, FL), baited with dry ice canisters. This trap was typically suspended from a tree branch approximately 1.5 m from the ground (ground trap). The 2nd trap, also a CO2-baited CDC light trap, was suspended by rope in the cottonwood canopy, typically at a height of 10 – 15 m, depending on local forest growth (canopy trap). Each CO2 canister was baited with approximately 1.5 kg of dry ice/night. The final trap used was a gravid trap baited with a standard fermentation mixture of nonchlorinated water with horse manure, grass clippings, and bacterial culture (Pro-Pump® Liquid Live Bacteria High Count, Ecological Laboratories, Freeport, NY). The gravid trap was set in the undergrowth, in the vicinity of the other traps at each site. One trap of each type was set up at each site, out of the line of sight from one another to prevent trap interaction. All traps were set in the mid-afternoon to early evening, left operating overnight, and collected the next morning. The nets from the traps were removed and placed in a cooler filled with dry ice to preserve and kill the captured specimens.
Mosquitoes were killed and maintained at – 82°C in the laboratory until being sorted to species by using a dissecting microscope. Dichotomous keys provided the basis for mosquito identification (Pratt and Barnes 1959, Darsie and Ward 1981). All collection data, including mosquito identifications, number of specimens, and trap type and location were recorded into a database. Mosquito data were analyzed using MINITAB® version 14 statistical software. A 2-factor analysis of variance (ANOVA) was performed, using site and trap type as the 2 factors, and the numbers of individuals caught (during each of the 10 trapping periods from May through October) as the response variables. All mosquito counts were based on a log(n + 1) transformation of the capture numbers to reduce skewness in the data. A least significant difference test (LSD) was subsequently used to identify which of the sites and trap types resulted in significantly different numbers of captures. The analysis was applied to each of the 3 Culex species captured (Cx. tarsalis Coq., Cx. salinarius Coq., and Cx. quin quefasciatus Say) as well as Aedes vexans (Meigen), because these were the most abundant species caught. The total catch numbers of all species also were evaluated, as an indication of the overall abundance of mosquitoes caught at each site, and in each trap type. For interpretation, sites were categorized based on vegetation type and structure approximately within the surrounding kilometer. Categories are broadly based on a visual qualitative assessment of general habitat characteristics. The categories are described as follows:
Type I
Type I sites are characterized as cottonwood bosque. The major vegetation consisted of cottonwood (Populus deltoides ssp. wizlizenii S. Wats.) forest and nonnative Russian olive (Elaeagnus angustifolia L.) trees and salt cedar (Tamarix spp.). Undergrowth consisted of mixed grasses, forbs, and shrubs. These areas were generally dry, open woodland with interspersed areas of dense salt cedar growth immediately away from the river. Soils were typically dry and sandy. This vegetation was found in 2 areas, urban (type I-U) and rural (type I-R). Urban sites with this vegetation type occurred along levy roads where development had occurred near riverbanks. Homes or other types of human structures, such as water treatment facilities, schools, and parks, were common in these areas. Rural sites tended to be relatively undisturbed by regular human activity. These sites also tended to be wooded areas near open-range pasture, refuge land, or simply unvisited areas well away from settlements.
Type II
Vegetation in type II areas was similar to that in type I areas but differed by the presence of a significant water source in addition to the river itself. These water sources were commonly marshy or swampy bodies of permanent standing water. Reeds and other emergent plants at the edges of the water were associated with this habitat. In addition, the cottonwood forest tended to be open, with lower densities of trees and invasive salt cedar. Such sites were only found in rural areas.
Type III
Type III sites were localized to the rocky canyon areas of the river valley and were typified by steep, rocky shores with clear, fast-moving water in the riverbed and sparse vegetation. Isolated cottonwood or olive trees occurred in these sites, but they tend to be much smaller and found only in stands of 2 or 3 individual trees. Grasses and thorny shrubs were also present, but the density of understory vegetation was low. These sites were exclusively rural in setting.
Results
There was little evidence to indicate that the canopy traps or gravid traps were consistently more effective than the ground level CDC traps in capturing individual species or for the overall collection (Fig. 2). For the species we examined in detail, we found significant differences in abundance at different sites, consistent with an influence of habitat characteristics on mosquito populations. In general, the highest numbers of mosquitoes captured were trapped at type I and type II sites, where bosque vegetation is found in greatest density. A type I-U site had the highest captures and was associated with human habitation. Type III sites had the lowest capture success (Fig. 3).
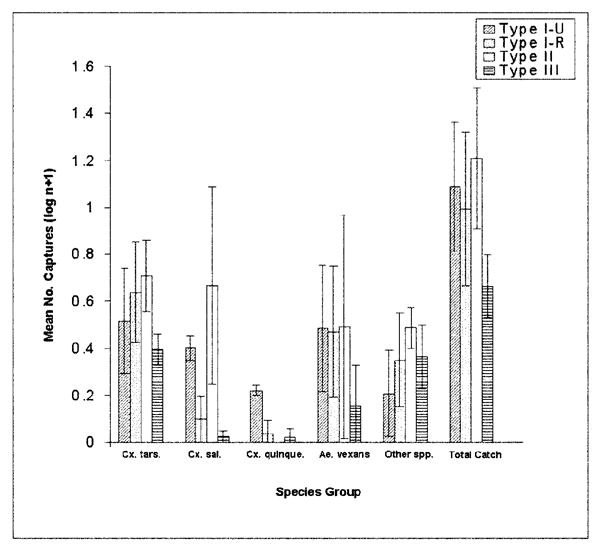
Fig. 2.

Transformed numbers of mosquitoes caught are shown by species for each trap type.
Fig. 3.
Transformed numbers of mosquitoes captured are shown by species. The mean numbers of captures are averaged based into site type category.
Culex tarsalis
Both canopy and ground traps caught significantly more Cx. tarsalis than the gravid traps (mean ± SE = 1.0 ± 0.09) (F = 29.20; df = 2,351; P < 0.001). However, there was not a statistically significant difference in the numbers caught between the canopy (3.3 ± 0.11) and ground traps (4.6 ± 0.12). There was not a significant interaction effect between site and trap type (P = 0.15), indicating that the capture results were consistent for trap type across all sites.
We found a significant difference in trapping results for Cx. tarsalis at different locations (F = 4.06; df = 12,351; P < 0.001). The differences in these results seem to be dependent on habitat characteristics rather than geographic location. The highest numbers captured were at type I (U and R) and type II sites. Generally, the type III sites, with low vegetation densities, had fewer captures of this species; however, the lowest overall capture success was at a type I-U site.
Culex salinarius
Although a significant interaction between trap type and site existed for this species (P < 0.001), it was significantly more likely to be caught in ground traps (1.1 ± 0.15) or gravid traps (1.4 ± 0.10) than it was in the canopy traps (0.6 ± 0.08) (F = 10.23; df = 2,351; P < 0.001). The interaction effect indicates that trap type results are not consistent across all sites. Numbers of Cx. salinarius captured differed by site (F = 21.48; df = 12,351; P < 0.001). In general, type II and type I-U sites had the highest captures of this species. Ground light traps had better results at type II sites. In type I-U habitats, gravid traps were the most effective, and light traps were typically less effective. The highest catches were taken at the 2 sites on the southernmost sites in the study area, whereas the lowest captures occurred at the northernmost sites, suggesting that latitude may play a role in Cx. salinarius distribution. Northern sites were typically cooler and composed of type III vegetation, where as southern sites were warmer and tend to be associated with type II habitat.
Culex quinquefasciatus
The gravid traps (0.4 ± 0.08) performed better at catching Cx. quinquefasciatus than either the ground traps (0.13 ± 0.03) or the canopy traps (0.11 ± 0.03) (F = 6.5; df = 2,351; P = 0.002). There was no significant difference in the effectiveness of the canopy- versus ground-based light traps. No interaction effect existed between trap type and site (P = 0.18), indicating that the trap type results are consistent across all sites.
Location had a significant effect on the numbers of Cx. quinquefasciatus caught (F = 5.5; df = 12,351; P < 0.001). Type I-U sites had the highest numbers of captures by a wide margin compared with all other site types. This trend seems to be independent of latitude. This species was absent from many sites, notably all of the type II sites.
Aedes vexans
Ground level CDC light traps (9.3 ± 0.18) were significantly more effective for collection of Ae. vexans than other trap types (F = 279.9; df = 2,351; P < 0.001). Gravid traps (0.1 ± 0.05) were comparable in effectiveness to the canopy traps (0.4 ± 0.07). This trend was pronounced in sampling. When Ae. vexans were abundant, relatively large numbers were collected in the ground level trap in single trapping periods. An interaction effect was found between site and trap type (P < 0.001). Trapping results were most likely to deviate at sites and during trapping periods with extremely high Ae. vexans abundances.
Despite the interaction effect, a significant difference existed among sites (F = 20.94; df = 12,351; P < 0.001). The differences did not follow a clear trend based on latitude or habitat type, although numbers were generally higher in type I and type II habitats where vegetation was dense. The most important influence of location seemed to have been the presence of flood-prone areas. Population explosions occurred at some sites after rain, and Ae. vexans numbers were consistently high at the Bosque del Apache National Wildlife Refuge where nearby fields were periodically flooded as part of the refuge maintenance. Type III sites generally had the lowest captures for this species.
Total catch
The results of the overall mosquito captures also were analyzed. These captures include all of the species discussed individually above, which made up the majority of the mosquitoes captured, as well as all other species captured. Among the other species collected were Culiseta inornata (Williston) and Cs. incidens (Thomson), Ochlerotatus dorsalis (Meigen), Oc. nigromaculis (Ludlow), and Oc. sollicitans (Walker) as well as several species of Anopheles. Full ecological descriptions of the taxa captured in this study and the seasonal patterns of their abundance will be published elsewhere. A significant effect for trap type was detected (F = 19.75; df = 2,351; P < 0.001), with the ground-based light traps being the most effective (26.6 ± 0.16). Canopy (5.7 ± 0.12) and gravid traps (4.2 ± 0.12) did not have significantly different mean total captures.
A significant interaction effect existed between trap type and site (P < 0.001). However, this finding was expected, given the contribution of this interaction with individual species discussed above.
There were also significant site effects in the overall data (F = 9.08; df = 12,351; P < 0.001). In general, mosquito captures were highest at more densely vegetated sites (types I and II). Both type I-U and I-R habitats had high overall captures. Type III habitats typically had the lowest captures. Density of vegetation and nearby standing water sources seemed to be the most important overall factor in determining the relative abundance of mosquitoes.
To investigate whether a significant pattern existed beyond the influence of the species analyzed above, we also compared the remainder of the total catch excluding the 4 species already discussed. The results for the remaining species showed a significant difference in the effectiveness of trap types (F = 42.26; df = 2,348; P < 0.001). Standard CDC light traps were the most effective (0.60 ± 0.12), with the canopy (0.19 ± 0.07) and gravid traps (0.23 ± 0.07) being less effective, but not significantly different from one another.
No significant interaction effect was shown for the remaining species (F = 1.29; df = 24,348; P = 0.1643). This finding indicates that the standard CDC trap was most effective in collecting these other species at all locations.
Although a significant effect was demonstrated for these remaining species by site (F = 5.23; df = 12; P < 0.001), it was difficult to assign any meaningful explanation to the pattern of these results. Sites of all types and geography were intermixed in terms of the numbers of mosquitoes captured. This finding was expected, given that different species at each site contributed in various ways to the numbers captured, particularly in cases where occasionally large emergences yielded high capture numbers for a particular species at a specific site or time. Further analysis might be of use in determining how these species vary at each site; however, it is sufficient to understand that the standard light trap protocol is most effective in capturing the majority of these species.
Discussion
The mosquito trapping methodologies in the Rio Grande Basin of New Mexico revealed distinct differences in the number and species of individuals captured. These differences were primarily associated with species and general habitat characteristics at different sites, and to a lesser extent, with different trap types. In most cases, the ground trap (the standard CDC light trap baited with CO2 and set at a height of 1.5 m) was the most efficient trap design for sampling a diverse array of mosquito species in this region.
Culex tarsalis was most frequently caught in CDC light traps, either at the standard height or in the canopy. The gravid traps were not as effective for this species, perhaps because they prefer ephemeral pools over artificial containers for oviposition (Carpenter and LaCasse 1955). We used light traps in the canopy in hopes of increasing our capture success with this ornithiphilic mosquito (Tempelis et al. 1965), but we found that our trapping success was no better than identical traps set at the standard height (1.5 m above the ground). Indeed, no species captured in this study showed a preference for the canopy traps. This finding may be in part because of the relatively low and sparse cottonwood canopy. In most areas of New Mexico, the canopy never reaches great height or density and may not be as appealing to mosquitoes as dense canopies that are physically distinct from the surrounding habitat. The indistinct separation between the understory and canopy zones in the Rio Grande valley may allow for significant bird and insect traffic between the treetops and the ground. Because the canopy traps were not particularly effective at increasing numbers of any species captured, and because they are costly to use in terms of time and damage to equipment, we would not recommend their use in this region. Culex tarsalis seems to do well in any habitat throughout the river valley, with very little site effect. The density of vegetation seems to have some influence on population, because the most densely vegetated sites showed the highest success of capture, and the rocky, sparsely vegetated sites had the lowest numbers captured. Being both ubiquitous and prolific in this region, and given the close relationship between this species and bird populations, Cx. tarsalis has probably been a highly influential and important vector species in determining the dynamics of West Nile for the state of New Mexico.
The standard ground-based CDC light traps or gravid traps were equally effective in capturing Cx. salinarius. This species was found in association with more rural areas, with nearby sources of stagnant water and dense vegetation. When these water sources were not present, as was common in type I-U environments, the gravid trap water bait might have been a more potent attractant. In the Rio Grande valley, such areas also were typically highly populated with Tamarix spp., which create heavily salinated marsh conditions. This species was most commonly caught in the southern collection sites and seemed primarily restricted to lower elevations in southern New Mexico. This species will likely prove to be important in maintaining WNV cycles in birds and other wildlife, but it may be of less significance to human transmission in New Mexico, where the preferred habitat tends to be removed from human settlements.
Culex quinquefasciatus was caught almost exclusively in or around areas of human activity. This finding was probably because of this species' affinity for artificial containers in and around human settlements (Carpenter and LaCasse 1955; Clements 1992, 1999). From these data, we also suggest that this species plays a very significant role in WNV transmission in settled areas, and as such, may turn out to be one of the most important vector species leading to human exposure to WNV in this region. The strong bias of this species for gravid traps emphasizes the necessity of stagnant, organic water sources for egg deposition. Such habitats in association with urban areas could be targeted for intervention by public health personnel in New Mexico communities; control and removal of potential breeding sites could help to reduce the successful buildup of these mosquitoes around population centers. Despite being uncommon in nonurban habitat, Cx. quinquefasciatus was the only species that was preferentially caught in the gravid traps, particularly in rural areas. In addition, the use of gravid traps in more rural areas, particularly along the river, where breeding habitat is relatively plentiful was not effective in increasing the likelihood of capturing other mosquitoes. For these reasons, we would recommend restricting the use of gravid traps to virus surveillance in semiurban or urban environments, where Cx. quinquefasciatus is likely to be present.
The standard CDC light trap proved to be the most effective at collecting large numbers of Ae. vexans. In comparison, the canopy traps and gravid traps were not particularly effective in capturing these mosquitoes. Aedes vexans was the most abundant species in our collections, owing to certain sites where mass emergences took place after the onset of the New Mexico rainy season in mid-August. The largest catches were obtained in areas of greater vegetation, and the dense trees and open understory of the cottonwood forest seemed to yield the best results. This finding may in part have been related to better visibility of the light sources in the traps across greater distances. This species does not usually have an affinity for gravid traps, but it might have been collected occasionally in the gravid traps when the abundance was high enough that mosquitoes were caught incidentally during flight. It has not yet been established as to whether these mosquitoes are important in WNV transmission, but if they are found to be involved, there could be a significant risk. In addition to being present in vast numbers across a wide area, these mosquitoes are also aggressive toward humans, even in full daylight, and have a flight range of several kilometers (Carpenter and LaCasse 1955, Service 1993).
Within the Rio Grande valley, several defined habitat types exist, each with different mosquito species abundances and diversity. In general, the use of CDC light traps in the standard method results in a representative collection of the mosquito species present that may be involved in arbovirus transmission. Surveillance of some species, such as Cx. quinquefasciatus, may be more efficacious by using targeted trapping methods (i.e., gravid traps) in selected areas. The use of gravid traps also is recommended for increased success in sampling Cx. salinarius in type I-U environments. Canopy traps were not effective in increasing the catches of any of the species found in this region. Rocky, scrub vegetation areas in northern New Mexico tended to have fewer mosquitoes, both in numbers caught as well as species diversity. Greater amounts of vegetation and standing water were associated with greater numbers of mosquitoes, with dry, open areas of cottonwood forest yielding the highest catches. The relative importance of the various species of mosquito in transmission and maintenance dynamics of West Nile virus in this geographic area will become clearer after continued surveillance and testing.
Acknowledgments
All research was conducted through the Albuquerque Environmental Health Bio-Disease Management Laboratory at Montessa Park, 3600 Los Picaros Road SE, Albuquerque, NM 87105. We express our gratitude to all of the individuals and organizations whose kind permission allowed trapping to be conducted on respective properties: Pueblos and towns throughout the study area, numerous individuals, and the Sevilleta and Bosque del Apache National Wildlife Refuges, the Bureau of Reclamation, the U.S. Army Corps of Engineers, and the Bureau of Land Management. We also thank collaborators at the Alamosa Mosquito Control District, New Mexico State University, and other municipal mosquito programs, and the University of Illinois Medical Entomology Laboratory and the New Mexico Scientific Laboratory Division for WNV testing. Partial funding support for this research was provided by the National Science Foundation and National Institutes of Health (Grant DEB-0328173, Ecology of Infectious Disease Program) and by the National Science Foundation's Undergraduate Mentorships in Environmental Biology (UMEB, Grant DEB-0102773). Additional support was provided from The Johns Hopkins University Bloomberg School of Public Health, W. Harry Feinstone Department of Molecular Microbiology and Immunology, The University of New Mexico Department of Biology, the Valles Caldera Trust and the New Mexico Department of Health, and the Albuquerque Environmental Health Department.
References Cited
- Brown TL. The 2001 New Mexico West Nile virus surveillance project: a regional mosquito survey. New Mexico Department of Health; 2002. [Google Scholar]
- Carpenter SJ, LaCasse WJ. Mosquitoes of North America (North of Mexico) Berkeley: University of California Press; 1955. [Google Scholar]
- Clements AN. The biology of mosquitoes. Vol. 1. New York: CABI Publishing; 1992. [Google Scholar]
- Clements AN. The biology of mosquitoes. Vol. 2. New York: CABI Publishing; 1999. [Google Scholar]
- Darsie RF, Ward RA. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. Mosq Syst. 1981;1(Suppl):1–313. [Google Scholar]
- DiMenna MA, Bueno R, Parmenter RR, Norris DE, Sheyka J, Molina J, LaBeau EM, Hatton E, Glass GE. The emergence of West Nile virus in mosquito (Diptera: Culicidae) communities of the New Mexico Rio Grande Valley. 2006 doi: 10.1603/0022-2585(2006)43[594:eownvi]2.0.co;2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettestad P, Reynolds P, Powers N. West Nile virus in New Mexico 2003. New Mexico Epidemiology Report, New Mexico Department of Health; 2004. [Google Scholar]
- Peterson LR, Roehrig JT. West Nile virus: a reemerging global pathogen. Emerg Infect Dis. 2001;7:611–614. doi: 10.3201/eid0704.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig JT, Layton M, Smith P, Campbell GL, Nasci R, Lanciotti RS. The emergence of West Nile virus in North America: ecology, epidemiology, and surveillance. Curr Top Microbiol Immunol. 2002;267:223–240. doi: 10.1007/978-3-642-59403-8_11. [DOI] [PubMed] [Google Scholar]
- Schultz JH. Occurrence and seasonal distribution of mosquitoes in Bernalillo County, New Mexico, 1986. City of Albuquerque Environmental Health Department in-house; 1987. [Google Scholar]
- Service MW. Mosquito ecology: field sampling methods. 2nd. London: Chapman & Hall; 1993. [Google Scholar]
- Tempelis CH, Reeves WC, Bellamy RE, Lofy MF. A three-year study of the feeding habits of Culex tarsalis in Kern County, California. Am J Trop Med Hyg. 1965;14:170–177. doi: 10.4269/ajtmh.1965.14.170. [DOI] [PubMed] [Google Scholar]
- Yong W, Finch DM. Gen Tech Rep RMRS-GTR-99. Ogden, UT: U.S. Department of Agriculture, Forest Service; 2002. Stopover ecology of landbirds migrating along the middle Rio Grande in spring and fall; p. 52. Rocky Mountain Research Station. [Google Scholar]


