Abstract
Most adult mosquito surveillance in Maryland is performed using dry ice-baited or unbaited Centers for Disease Control (CDC) miniature light traps suspended ≈1.5 m above the ground. However, standardized trapping methods may miss mosquito species involved in disease transmission cycles. During a 2-yr study, the effectiveness of the olfactory attractant 1-octen-3-ol alone and in combination with carbon dioxide was evaluated for collecting mosquito vector species. In addition, trap heights were examined to determine the optimal vertical placement to target various species. We evaluated the results during the second year by targeting selected species by using various habitat–height–bait combinations. Although Culex erraticus Dyar & Knab and Anopheles quadrimaculatus Say were not successfully targeted, Culex salinarius Coquillett, Aedes vexans Meigen, Anopheles bradleyi/crucians King, Coquillettidia perturbans Walker, Aedes sollicitans Walker, and Aedes taeniorhynchus Wiedemann were preferentially captured using targeted trapping schemes.
Keywords: octenol, carbon dioxide, light trap, height, trapping scheme
Vector-borne diseases exact high costs on affected populations through expenditures on surveillance, control, and human health (Reiter 2001). This is especially true where little research has been focused or for introduced pathogens where the biology may have shifted and the vectors, reservoirs, and ecology of the pathogen may not be fully understood. Thus, tools that help to provide information to fill some of these knowledge gaps will reduce the disease burden on affected populations.
Although human body odor is considered to be the most important cue in the host-seeking behavior of anthropophilic mosquitoes (Takken and Knols 1999), several researchers have investigated other chemical compounds as potential olfactory attractants. These include carbon dioxide (CO2), acetone, lactic acid, butanone, 1-octen-3-ol (octenol), and mixed phenols (Kline et al. 1990b). The results from these studies conclude that the responses to these scents are highly species specific because they can act as kairomones or allomones. Moreover, combinations of these odorants can have very different effects than the individual chemicals alone. For example, catches of Aedes vigilax Skuse and Aedes funereus Theobald were considerably enhanced when octenol was combined with CO2 (Kemme et al. 1993).
Since its discovery as an attractant for tsetse flies (Hall et al. 1984), octenol has been widely studied for many other insect species, including mosquitoes (Kline 1994). Several studies have investigated the efficacy of octenol in conjunction with CO2 to attract mosquitoes, either to enhance vector-borne disease surveillance, or as a potential control method (Vaidyanathan and Edman 1997a, b; Kline and Lemire 1998; Rueda et al. 2001; van den Hurk et al. 1997; Shone et al. 2003). Various Culex, Anopheles, and Aedes species are attracted to octenol, especially when combined with CO2. Many of these species are also important vectors of mosquito-borne diseases, including West Nile encephalitis (family Flaviviridae, genus Flavivirus, WNV), Eastern equine encephalitis (family Togaviridae, genus Alphavirus, EEE), St. Louis encephalitis (family Flaviviridae, genus Flavivirus, SLE), Venezuelan equine encephalitis (family Flaviviridae, genus Flavivirus, VEE), canine heartworm, and malaria. Therefore, trapping systems that incorporate combinations of these attractants may enhance surveillance for these diseases and provide a better estimate of disease prevalence in individual vector species.
In addition to olfactory attractants, the vertical placement of traps and habitat selection greatly influence the species that are captured (Snow and Wilkes 1977, Denke et al. 1996, Bellini et al. 1997). For example, traps placed in the tree canopy often capture primarily ornithophilic mosquitoes (Lepore et al. 2004). Therefore, trapping schemes in which trap height is modified may improve the accuracy of distribution and density estimates for mosquitoes in an area as well as the prevalence of a mosquito-borne disease in that region.
Most of the adult mosquito surveillance conducted in Maryland is performed using dry ice baited or unbaited Centers for Disease Control (CDC) miniature light traps (model 512, John W. Hock, Co., Gainesville, FL) suspended ≈1.5 m above the ground. Bidlingmayer (1985) described how this method of adult surveillance selects for or against some mosquito species. Furthermore, and most importantly, standardized trapping methods such as these may miss or even repel mosquito species involved in disease transmission cycles (Chadee 1991). In Maryland, there is endemic transmission of WNV, EEE, and Dirofilaria im-mitis (Leidy) with sporadic occurrences of airport malaria. Some of the major vector species that transmit these pathogens in the state have previously been found to respond to light traps baited with CO2, octanol, or both (Kline et al. 1990a, b; Becker et al. 1995; Beavers et al. 1998; Kline and Mann 1998).
This study focused on selected mosquito species for targeted collection by modifying existing trapping methods and surveying over a wide range of habitats around the Chesapeake Bay. Preference in species selection was given to those considered to be important potential vectors of disease in the region. Different bait combinations and trap heights were chosen and evaluated for their ability to selectively trap vectors through two mosquito seasons (2002 and 2003). Traps baited with CO2 and octenol either alone or in combination were compared with standard trapping protocols.
Materials and Methods
Mosquito Collection Techniques
CDC miniature light traps (model 512, John W. Hock, Co.) were used because they are the most commonly used trap by mosquito control agencies. The standard attractant on these traps is a 1-W light bulb; however, these traps can be baited with additional attractants.
CO2 baiting was accomplished by suspending a 3.785-liter (1-gal) Igloo container filled with 1.4 kg of dry ice above the trap. The container had a 3-cm hole drilled in the bottom so that CO2 gas was released at a rate of ≈42 g/h. Gas released from the cooler fell over the trap and was spread by the trap’s fan and existing air currents. Octenol baiting was accomplished using a 5-ml glass reaction vial (Supelco, Belle-fonte, PA) attached to the side of the trap’s motor housing. The reaction vial was capped with a plastic lid exposing a neoprene septum. A 3-mm hole was bored through the septum from which a pipe cleaner wick protruded ≈1.5 cm. As the volatile mixture was drawn up the wick, it aerosolized, and dissipated from the source either alone or as a mixture with the CO2 gas released from the dry ice container. The actual release rate of octenol was not calculated but based on Kline and colleagues wick out method with a rate of ≈41 mg/h (Kline et al. 1990a, b).
A tripod apparatus was assembled from polyvinyl-chloride (PVC) pipe and outfitted with a rope and pulley system from which the trap was suspended at variable heights up to 5 m above the ground. All heights were measured from the ground to the motor/ light assembly.
Study Sites
Year 1
Adult mosquito surveys were performed at three sites in Maryland on the west side of the Chesapeake Bay. These sites included three different mosquito habitats: freshwater swamp, flooded woodland, and salt marsh. The sites were chosen as representative of the major mosquito breeding areas throughout eastern Maryland. These habitat types are widely distributed throughout the Chesapeake Bay region and ecologically account for some of the richest, most sensitive, and most important natural areas in the state. The sites selected did not participate in mosquito control activities and had little ground light present.
The freshwater swamp and flooded woodland sites were located ≈1.45 km apart at the Jug Bay Wetlands Sanctuary (Lothian, MD). The swamp site was an area permanently flooded by the Patuxent River with emergent vegetation (National Wetlands Inventory [NWI] code: PSS1). This vegetation included arrow arum, arrowhead, pickerel weed, spatterdock, rose mallow, and skunk cabbage. The light traps were operated on moderately dry land directly adjacent to the swamp.
The flooded woodland (NWI code: PFO1A) trap site was located adjacent to a creek on a floodplain that flooded during periods of rain. The predominant vegetation in the woodland area was oak, hickory, sweet gum, American beech, poplar, red maple, sassafras, and Virginia pine. The subcanopy contained American holly, sweet bay, musclewood, flowering dogwood, witch hazel, and black gum. Groundcover included partridge-berry, spotted wintergreen, spring beauty, and mayapple. The traps were operated directly over the flooded area.
Salt marsh traps were operated at the Parker’s Creek Wetlands Sanctuary (Port Republic, MD). The salt marsh flooded with the tides of the Chesapeake Bay and had a measured salinity of 13 ppt (NWI code: E2EM5P). The predominant vegetation was Spartina patens (Aiton) Muhl. and phragmites. The traps were operated within the marsh boundaries.
Year 2
To evaluate the results from year 1, 15 sites representing the same habitat types used during year one (five each of freshwater swamp, flooded woodland, and salt marsh) were selected. Again, the sites did not participate in mosquito control activities and had little ground light present.
The woodland sites selected were located near Bacontown Park (Laurel, MD), in three areas of the Patuxent River Park (Bowie and Croom, MD), and at the Jug Bay Wetlands Sanctuary. All five sites flooded after rain and had vegetation characteristic of a flooded woodland habitat, as described previously. The traps were operated directly over the flooded area.
The swamp sites selected were located near the Oxbow Nature Preserve (Laurel, MD), in three areas of the Patuxent River Park, and at the Jug Bay Wetlands Sanctuary. All five sites were permanently flooded and had vegetation characteristic of a swamp habitat, as described previously.
The salt marsh sites selected were located at the Smithsonian Environmental Research Center (Edge-water, MD), Thomas Point State Park (Annapolis, MD), Sandy Point State Park (Annapolis, MD), and two areas in the Horsehead Wetlands Sanctuary at the Chesapeake Bay Environmental Center (Grasonville, MD). All five sites were flooded by the tidal activity of the Chesapeake Bay and the vegetation was predominantly Spartina grasses and phragmites.
Experimental Design
Year 1
A 4 by 4 Latin square design with CDC light traps placed at 20-m intervals in the grid (Cochran and Cox 1957) was used to test the bait treatments: no bait, CO2, octenol, or CO2 and octenol. The traps were operated for four consecutive nights. To test the effect of vertical trap placement, each night a different height (0.5, 1.5, 3.0, and 5.0 m) was chosen, and all traps were suspended at that height such that all four trap heights were used over the 4 days. In addition, the baiting scheme at each location in the grid was randomly selected each night. The traps were operated from May through September 2002 and ran for 24 h each trapping period. After 4 d of trapping in one habitat, the setup was transferred to a different habitat. Thus, the sampling returned to the original habitat every third week.
Collected female mosquitoes were morphologically identified to species (Darsie and Ward 1981, Slaff and Apperson 1989) on a chill table and counted. Mosquitoes of the same species were pooled into tubes containing no >40 individuals and stored at −80°C for future arbovirus testing.
Year 2
Targeted trapping schemes designed using the results from year one were evaluated during the second year of the study. To test the efficacy of the scheme to target a particular species, a test trap was suspended from a natural or artificial structure (i.e., our tripods from year 1) at the best height, measured from the ground to the fan/motor assembly, determined in year 1 with the best bait determined in year 1. A control trap was suspended at 1.5 m with no olfactory attractant. Traps were operated 50 m apart for two consecutive nights at all five sites of a particular habitat type. On the second night, the trap positions were rotated. At the end of the 2-d study, the traps were moved to a different set of five sites, and a different species was targeted with the appropriate bait and vertical placement. The species targeted was determined by the seasonality pattern seen in the results from year 1. For example, Aedes sollicitans Walker was targeted in year 2 during the same weeks as it was most prevalent in the previous year. Trap collections were retrieved each morning and kept cool until arrival in the laboratory. Similar to year 1, the traps were operated from May through September 2003. Female mosquitoes were morphologically identified to species on a chill table by using dichotomous keys (Darsie and Ward 1981, Slaff and Apperson 1989) and enumerated.
Because of the size of several collections of Culex salinarius Coquillett, a system was designed to closely approximate the number of individuals present in these samples by mass. To establish this system, 12,000 female Cx. salinarius mosquitoes were separated into pools of 200. These pools were removed from storage at −80°C and held at 4°C for 15 min. Two hundred mosquitoes at time were added to an Ohaus electronic balance (model GA 200-D, Ohaus Corp., Florham Park, NJ), and the mass was recorded to the nearest 1/10,000 of a gram. The 60 measurements were used to calculate a best fit line: y = 2137.9x, R2 = 0.999. Thus, when Cx. salinarius collections were too large to count, the sample was held at 4°C for 15 min, mass taken to the nearest 1/10,000 of a gram, and the total number of mosquitoes was calculated using the above best fit line.
Data Analysis
Year 1
The counts of all specimens collected were transformed to loge (n + 1) for analysis of variance (ANOVA) to determine the effect of the attractant and height. The transformed data were analyzed by two-way ANOVA by using SigmaStat (SPSS Inc., Chicago, IL).
Year 2
An odds ratio (OR) was calculated for each target species to determine the efficacy of the targeted trapping scheme for that species: (no. female of target species in test trap × no. female of nontarget species in control trap) ÷ (no. female of nontarget species in test trap × no. female of target species in control trap). The 95% confidence interval for each odds ratio was calculated using the SigmaStat (SPSS Inc.). For traps where more than one species responded to the same height and bait combination as the target, the counts of these additional species were added to counts of the target species such that an OR was calculated for all species which responded to the same bait/height combination.
Results
Year 1
During the 2002 mosquito season (May to September 2002), light traps were operated for a total of 1,212 nights: 444 nights in the flooded woodland, 443 nights at the freshwater swamp, and 325 nights at the salt marsh. A total of 39,328 female mosquitoes were collected at all three sites. The reduction in trap nights for the salt marsh is because of a prolonged flooding of the marsh that prevented access to the trap site.
At the flooded woodland site, 4,035 female mosquitoes were collected representing 18 species. Six species (Coquillettidia perturbans Walker [24.1%], Culex erraticus Dyab & Knab [14.0%], Anopheles quadrimaculatus Say [11.6%], Cx. salinarius [10.3%], Uranotaenia sapphirina Osten Sacken [7.5%], and Aedes vexans Meigen [5.8%]) were predominant in the collections. In total, 6,281 female mosquitoes were collected at the freshwater swamp site representing 17 species, including Ur. sapphirina (30.8%), Cq. perturbans (18.0%), An. quadrimaculatus (9.1%), Cx. erraticus (9.0%), Cx. salinarius (8.3%), and An. bradleyi/ crucians King (7.6%). The salt marsh site yielded 29,012 female mosquitoes representing 12 species. Four species (Cx. salinarius [56.0%], Ae. sollicitans [28.7%], An. bradleyi/crucians [11.5%], and Ae. taeniorhynchus Weidemann [2.1%]) were predominant in the collection.
Regardless of habitat, no mosquito species was most commonly attracted to an unbaited CDC light trap. Moreover, for most species, the addition of octenol alone did not increase the number of mosquitoes collected. In contrast, the addition of CO2 or both CO2 and octenol resulted in a significant increase in the number of mosquitoes collected (Table 1). For example, Cq. perturbans, An. bradleyi/crucians, Ae. sollicitans, Ae. taeniorhynchus, and Cx. salinarius were more attracted to traps baited with CO2 and octenol than traps baited with the other attractants. Furthermore, Cx. erraticus, Ae. vexans, and An. quadrimaculatus were more attracted to traps baited with CO2 or CO2 with octenol than traps with no bait or octenol alone. However, there was no difference in the counts of these species between traps with CO2 or CO2 with octenol.
Table 1.
Mean (SE) response of eight mosquito vector species to unbaited, carbon dioxide (CO2)-baited, 1-octen-3-ol (octenol)-baited, or CO2- and octanol-baited CDC miniature light traps suspended at 0.5, 1.5, 3.0, or 5.0m. Results are combined over all habitats
| 0.5 m | 1.5 m | 3.0 m | 5.0 m | |
|---|---|---|---|---|
| Ae. vexans | ||||
| None | 0.06 (0.03) | 0.43 (0.11) | 0.22 (0.07) | 0.53 (0.18) |
| CO2 | 0.42 (0.10) | 0.54 (0.10) | 0.54 (0.15) | 0.70 (0.18) |
| Octenol | 0.21 (0.10) | 0.30 (0.07) | 0.29 (0.09) | 0.52 (0.16) |
| CO2+ octenol | 0.35 (0.10) | 0.65 (0.13) | 0.49 (0.17) | 0.58 (0.18) |
| Cx. erraticus | ||||
| None | 0.21 (0.07) | 0.27 (0.08) | 0.28 (0.08) | 0.39 (0.17) |
| CO2 | 1.55 (0.31) | 1.92 (0.37) | 2.63 (0.58) | 0.68 (0.15) |
| Octenol | 0.38 (0.12) | 0.64 (0.29) | 0.31 (0.08) | 0.26 (0.09) |
| CO2+ octenol | 0.99 (0.20) | 1.83 (0.35) | 1.55 (0.34) | 0.64 (0.19) |
| Cx. salinarius | ||||
| None | 0.40 (0.18) | 1.16 (0.28) | 0.76 (0.34) | 0.56 (0.19) |
| CO2 | 23.19 (6.65) | 24.24 (7.02) | 7.64 (2.60) | 2.41 (0.81) |
| Octenol | 1.33 (0.55) | 6.61 (2.71) | 2.31 (0.76) | 1.37 (0.61) |
| CO2+ octenol | 50.45 (15.66) | 66.78 (32.78) | 25.92 (10.07) | 5.64 (2.85) |
| Oc. sollicitans | ||||
| None | 0.38 (0.19) | 0.58 (0.22) | 0.63 (0.25) | 0.13 (0.07) |
| CO2 | 19.63 (10.86) | 15.73 (6.56) | 3.08 (1.95) | 0.18 (0.07) |
| Octenol | 0.44 (0.17) | 1.22 (0.49) | 0.67 (0.19) | 0.23 (0.09) |
| CO2+ octenol | 33.66 (18.25) | 23.77 (10.34) | 6.17 (4.06) | 0.48 (0.22) |
| Oc. taeniorhynchus | ||||
| None | 0.22 (0.12) | 0.06 (0.03) | 0.12 (0.07) | 0.17 (0.11) |
| CO2 | 0.46 (0.17) | 1.33 (0.52) | 0.26 (0.14) | 0.07 (0.06) |
| Octenol | 1.33 (0.92) | 0.18 (0.09) | 0.15 (0.06) | 0.22 (0.15) |
| CO2+ octenol | 1.40 (0.61) | 1.63 (0.63) | 0.28 (0.13) | 0.18 (0.11) |
| An. bradleyi/crucians | ||||
| None | 0.64 (0.30) | 0.83 (0.24) | 0.17 (0.04) | 0.38 (0.13) |
| CO2 | 5.91 (3.51) | 4.55 (1.92) | 0.77 (0.19) | 0.41 (0.10) |
| Octenol | 2.53 (1.38) | 1.47 (0.50) | 0.46 (0.10) | 0.36 (0.11) |
| CO2+ octenol | 25.17 (20.31) | 5.82 (2.46) | 1.54 (0.65) | 0.36 (0.13) |
| An. quadrimaculatus | ||||
| None | 0.76 (0.23) | 1.09 (0.33) | 0.46 (0.10) | 0.64 (0.30) |
| CO2 | 1.55 (0.39) | 1.54 (0.28) | 0.44 (0.13) | 0.53 (0.13) |
| Octenol | 0.62 (0.18) | 1.06 (0.28) | 0.33 (0.09) | 0.44 (0.13) |
| CO2+ octenol | 0.91 (0.25) | 2.24 (0.55) | 0.53 (0.13) | 0.49 (0.18) |
| Cq. perturbans | ||||
| None | 0.51 (0.25) | 1.39 (0.47) | 1.36 (0.54) | 1.50 (0.80) |
| CO2 | 1.56 (0.35) | 3.19 (0.80) | 1.85 (0.48) | 1.22 (0.44) |
| Octenol | 0.24 (0.10) | 1.13 (0.32) | 0.95 (0.39) | 0.78 (0.37) |
| CO2+ octenol | 1.84 (0.86) | 5.97 (1.82) | 2.33 (0.58) | 1.32 (0.59) |
Over all habitats, the largest numbers of mosquitoes were collected at a trap height of 0.5 or 1.5 m. A greater number of Cx. salinarius, An. bradleyi/crucians, Ae. sollicitans, Ae. taeniorhynchus were collected at 0.5 and 1.5 m than at any other height. An. quadrimaculatus and Cq. perturbans were collected more at 1.5 m than at any other height. Additionally, An. bradleyi/crucians was most commonly collected at 0.5 m. The only species which was collected more often at a height >1.5 m was Cx. erraticus.
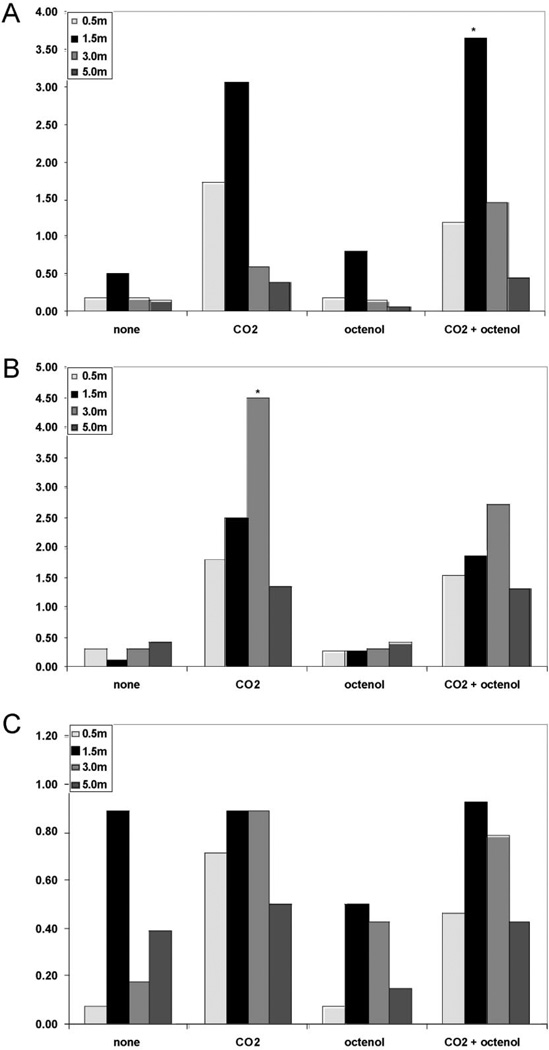
There were significant differences when the results are categorized by habitat. In the flooded woodland, Cx. erraticus was significantly more attracted to a trap baited with CO2 alone suspended at 3.0 m above the ground, and Cx. salinarius was significantly more attracted to a light trap baited with CO2 and octenol set at 1.5 m above the ground. Conversely, there was no statistical difference in the attraction of Ae. vexans to any of the attractants or trap heights (Fig. 1).
Fig. 1.
Mean response of Cx. salinarius (A), Cx. erraticus (B), and Ae. vexans (C) to unbaited, carbon dioxide (CO2)-baited, 1-octen-3-ol (octenol)-baited, or CO2 and octanol-baited CDC miniature light traps suspended at 0.5,1.5,3.0, or 5.0 m in the woodland habitat.
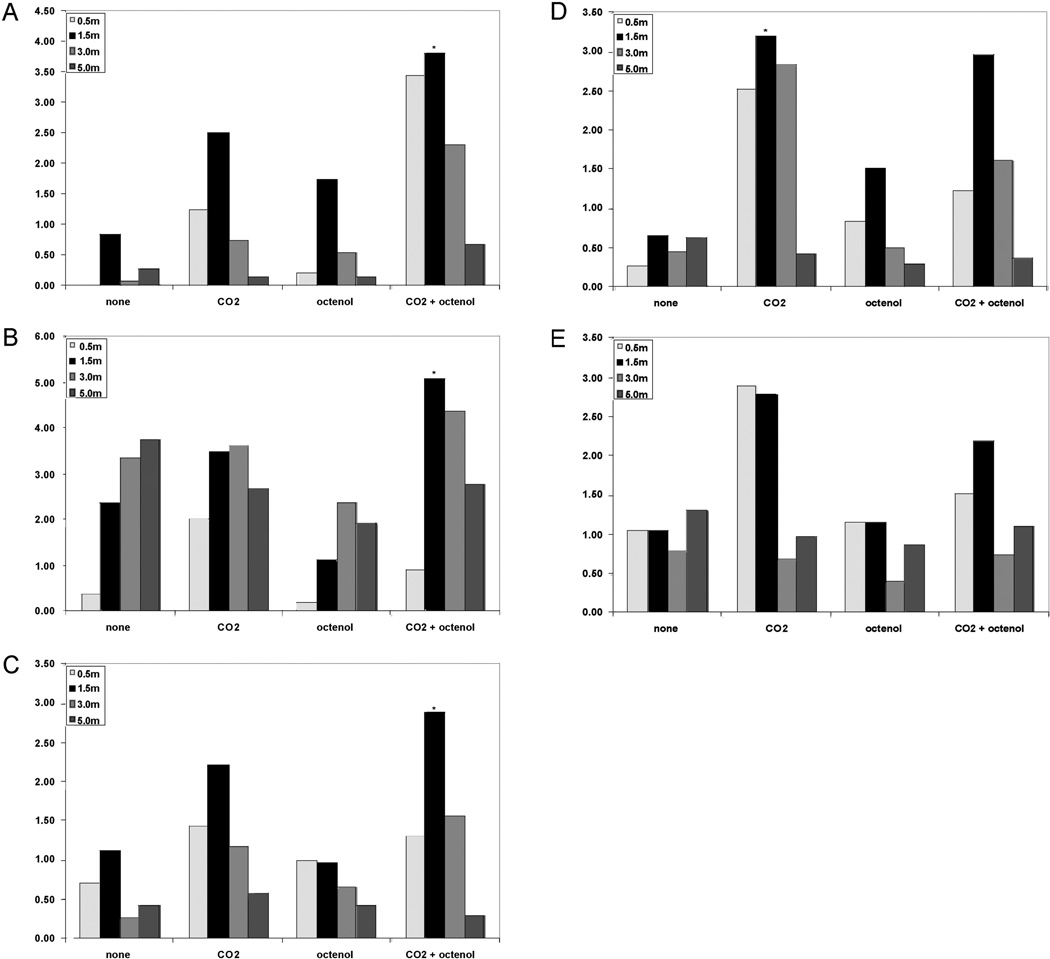
At the swamp; Cq. perturbans, An. bradleyi/crucians, and Cx. salinarius were all significantly more attracted to CO2 and octanol-baited traps suspended at 1.5m that any other trap set-up (P < 0.05). Cx. erraticus and An. quadrimaculatus also were most attracted to traps at 1.5 m but baited with CO2 only (Fig. 2).
Fig. 2.
Mean response of Cx. salinarius (A), Cq. perturbans (B), An. bradleyi/crucians (C), Cx. erraticus (D), and An. quadrimaculatus (E) to unbaited, carbon dioxide (CO2)-baited, 1-octen-3-ol (octenol)-baited, or CO2 and octanol-baited CDC miniature light traps suspended at 0.5, 1.5, 3.0, or 5.0 in the freshwater swamp habitat.
The species most commonly collected at the salt marsh site—Ae. sollicitans, Ae. taeniorhynchus, Cx. salinarius, and An. bradleyi/crucians—were significantly more attracted to traps baited with CO2 and octenol than any other bait. Furthermore, although Ae. sollicitans, Ae. taeniorhynchus, and Cx. salinarius were most attracted to a trap suspended at 1.5 m, An. bradleyi/crucians was most attracted to traps hung at 0.5 m (Fig. 3).
Fig. 3.
Mean response of Cx. salinarius (A), Ae. sollicitans (B), An. bradleyi/crucians (C), and Ae. taeniorhynchus (D) to unbaited, carbon dioxide (CO2)-baited, 1-octen-3-ol (octenol)-baited, or CO2 and octanol-baited CDC miniature light traps suspended at 0.5, 1.5, 3.0, or 5.0 m in the salt marsh habitat.
Using these results, trapping schemes were developed to target eight mosquito vector species during year 2. These species included Cq. perturbans, An. quadrimaculatus, An. bradleyi/crucians, Ae. sollicitans, Ae. taeniorhynchus, Cx. erraticus, Cx. salinarius, and Ae. vexans. The factors involved in determining the targeted trapping scheme were habitat, height, bait, and time of year at which the majority of each species was collected (Table 2).
Table 2.
Targeted trapping scheme for year 2 developed using the habitat, height, bait, and time of year at which the majority of each species was collected in year 1
| Species | Height (m) | Bait | Habitat | Yr 2002 (week no.) |
Yr 2003 (week no.) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Start | Peak | End | ||||||||
| Cx. salinarius | 1.5 | CO2+ octenol | Marsh | 22 | 22/31/37 | 37 | 23 | 37 | ||
| Oc. taeniorhynchus | 1.5 | CO2+ octenol | Marsh | 22 | 31 | 34 | 25 | 31 | 35 | |
| Oc. sollicitans | 1.5 | CO2+ octenol | Marsh | 22 | 37 | 37 | 22 | 28 | 32 | 39 |
| An. bradleyi/crucians | 0.5 | CO2+ octenol | Marsh | 25 | 34/37 | 37 | 29 | 35 | 38 | |
| Cq. perturbans | 1.5 | CO2+ octenol | Swamp | 23 | 27 | 33 | 22 | 32 | ||
| Cx. salinarius | 1.5 | CO2+ octenol | Swamp | 24 | 27/33 | 33 | 24 | 26 | ||
| An. bradleyi/crucians | 1.5 | CO2+ octenol | Swamp | 24 | 27/30 | 33 | 25 | 29 | 37 | |
| An. quadrimaculatus | 1.5 | CO2 | Swamp | 22 | 27/28 | 35 | 21 | 24 | 36 | |
| Cx. erraticus | 1.5 | CO2 | Swamp | 24 | 30 | 33 | 28 | 30 | ||
| Cx. salinarius | 1.5 | CO2+ octenol | Woods | 20 | 20 | 26 | 20 | 23 | 38 | |
| Cx. erraticus | 3.0 | CO2 | Woods | 26 | 32 | 35 | 31 | 33 | 36 | |
| Ae. vexans | 1.5 | CO2 | Woods | 20 | 27/32/38 | 39 | 21 | 26 | 30 | 39 |
Year 2
During the 2003 mosquito season (May–September 2003), light traps were operated for 578 nights: 168 nights in the flooded woodland, 230 nights at the freshwater swamp, and 180 nights at the salt marsh. In total, 502,538 female mosquitoes and 29 species were collected at all three sites.
At the flooded woodland site, 6,766 female mosquitoes were collected representing 22 species. Four species (Cx. salinarius [21.0%], Cx. erraticus [18.1%], Aedes canadensis Theobald [7.6%], and Ae. vexans [7.3%]) were predominant in the collections. In total, 106,244 female mosquitoes were collected at the freshwater swamp site representing 23 species including Cx. salinarius (90.3%), Ae. vexans (1.5%), An. bradleyi/ crucians (1.5%), An. punctipennis (1.2%), Cq. perturbans (1.1%), and An. quadrimaculatus (1.0%). The salt marsh site yielded 389,528 female mosquitoes representing 27 species. Three species (Cx. salinarius [91.8%], An. bradleyi/crucians [4.9%], and Ae. sollicitans [0.4%]) were predominant in the collection.
The test traps, which evaluated the efficacy of the targeted trapping schemes, were significantly more successful at collecting Ae. vexans, An. bradleyi/crucians, Cq. perturbans, Cx. salinarius, Ae. sollicitans, and Ae. taeniorhynchus than the control traps at all habitats sampled. The ORs ranged from 1.32 to 67.23 (Table 3). Conversely, there was no significant difference between the test traps and control traps for An. quadrimaculatus or Cx. erraticus.
Table 3.
Odds ratios and 95% confidence intervals for seven vector species targeted in year 2
| Habitat | Target | OR | 95% CI |
|---|---|---|---|
| Woods | Ae. vexans | 1.32 | 1.06–1.64 |
| Cx. erraticus | 0.64 | 0.5–0.82 | |
| Cx. salinarius | 6.53 | 2.82–15.11 | |
| Swamp | An. quadrimaculatus | 0.48 | 0.39–0.6 |
| Cx. erraticus | |||
| Cq. perturbans | 67.23 | 62.56–72.25 | |
| Cx. salinarius | |||
| An. bradleyi/crucians | |||
| Marsh | Cx. salinarius | 2.75 | 2.62–2.88 |
| Oc. sollicitans | |||
| Oc. taeniorhynchus | |||
| An. bradleyi/crucians | 2.4 | 1.88–3.07 |
Counts of species that were targeted using the same bait–height combination were combined for OR calculations.
Discussion
For decades, the most common trapping technique used by mosquito control and mosquito-borne disease surveillance programs has been CDC light traps suspended ≈1.5 m above the ground either unbaited or baited with CO2. Although this technique collects various mosquito species with moderate success, this study demonstrates that minor modifications to the height at which the trap is operated or the olfactory attractants supplied with the trap can significantly augment a collection for an individual vector species of interest.
As with previous studies, the results of this investigation reveal species-specific responses to both olfactory attractants and to trap height. Similar to earlier research that investigated salt marsh mosquito species (Takken and Kline 1989; Kline et al. 1990b, 1991b; Kline 1994; Rueda et al. 2001), all of the salt marsh species collected in this study—Cx. salinarius, An. bradleyi/crucians, Ae. sollicitans, and Ae. taeniorhynchus—were most attracted to traps baited with both CO2 and octenol. Furthermore, with the exception of the anopheline species, they were captured most in traps suspended at 1.5 m. These results are not surprising as all four species are potential bridge vectors of several pathogens including West Nile virus, Eastern equine encephalitis virus, and Venezuelan equine encephalitis virus; and feed on various hosts found at these heights with these odorants. When compared with control traps, the test trapping scheme was significantly more successful at collecting the target species.
The only species collected in the woodland habitat that was attracted most to CO2 and octanol-baited light traps was Cx. salinarius. This species was collected most with this trapping scheme at the other two habitats as well, a finding which supports previous research (Kline et al. 1991a, Kline and Mann 1998, Rueda et al. 2001). Similar to the findings of Rueda et al. (2001), Ae. vexans and Cx. erraticus were both most attracted to traps baited with CO2 alone. Traps suspended at 1.5 m collected Ae. vexans most, whereas traps suspended at 3.0 m collected Cx. erraticus most. The test traps were significantly more successful at targeting Cx. salinarius and Ae. vexans than the control traps; however, there was no difference between the test and control traps in the ability to target Cx. erraticus. The failure of the test trap to target Cx. erraticus may be because of a shift in the weather pattern from year 1 to year 2 that affected population sizes of this mosquito species. The temperature stayed cooler longer into the summer in the second year and disrupted the seasonal trapping pattern. Thus, although Cx. erraticus were present in trap collections, they may not have been at their peak activity when they were targeted.
Two species at the swamp, Cx. erraticus and An. quadrimaculatus, were most attracted to traps baited with CO2 hung at 1.5 m. The test traps for these species were no more successful at collecting the target species than the control trap. Again, the failure to target Cx. erraticus may be because of the change in weather from year 1 to year 2. Previous research has yielded mixed results for An. quadrimaculatus. Some studies report that CO2 and octanol-baited light traps are most attractive (Takken and Kline 1989, Kline et al. 1991a, Kline and Mann 1998), whereas others, including this study, suggest a CO2-baited trap would best target this species (Kline et al. 1990a, Rueda et al. 2001). Therefore, our inability to successfully target An. quadrimaculatus may be because of the use of the incorrect olfactory attractant. Cq. perturbans, An. bradleyi/crucians, and Cx. salinarius were attracted most to traps baited with CO2 and octenol suspended at 1.5 m, and the test traps for these three species were significantly better at collecting the target species than the control traps.
Cx. salinarius, Ae. vexans, An. bradleyi/crucians, Cq. perturbans, Ae. sollicitans, and Ae. taeniorhynchus have been implicated in the transmission of WNV, EEE, SLE, VEE, canine heartworm, and malaria. The ability to target these species could be used to more efficiently estimate the prevalence of disease in these mosquitoes or to understand the role these mosquitoes might play in the ecology and spread of the pathogens. This knowledge could be used to focus control and surveillance efforts and potentially limit occurrences of these diseases.
Acknowledgments
We thank Chris Swarth and the employees of the Jug Bay Wetlands Sanctuary, Dan Sampson at the American Chestnut Land Trust, Kenny Hartman at Sandy Point State Park, Greg Lewis at the Patuxent River Park, Peter Marra at the Smithsonian Environmental Research Center, and Judy Wink at the Chesapeake Bay Environmental Center for allowing us to trap on the land. We also thank Zirui Song and Sarah Singson for assistance with identifying and counting all of the mosquitoes. Finally, we appreciate all of the colleagues of S.M.S. who helped assemble the PVC tripods. This work was supported by a CDC Cooperative Agreement Award U50/ CCU320520 to D.E.N. and a NOAA Climate Variability and Human Health Program grant NA16GP2631 to G.E.G.
References Cited
- Beavers GM, Hanafi HA, Tetreault GE. Response of mosquitoes (Diptera: Culicidae) to carbon dioxide and octenol in Egypt. J. Egypt Soc. Parasitol. 1998;28:303–312. [PubMed] [Google Scholar]
- Becker N, Zgomba M, Petric D, Ludwig M. Comparison of carbon dioxide, octenol and a host-odour as mosquito attractants in the Upper Rhine Valley, Germany. Med. Vet. Entomol. 1995;9:377–380. doi: 10.1111/j.1365-2915.1995.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Bellini R, Veronesi R, Draghetti S, Carrieri M. Study on the flying height of Aedes caspius and Culex pipiens females in the Po Delta area, Italy. J. Am. Mosq. Control Assoc. 1997;13:356–360. [PubMed] [Google Scholar]
- Bidlingmayer WL. The measurement of adult mosquito population changes–some considerations. J. Am. Mosq. Control Assoc. 1985;1:328–348. [PubMed] [Google Scholar]
- Chadee DD. Seasonal incidence and vertical distribution patterns of oviposition by Aedes aegypti in an urban environment in Trinidad. W.I. J. Am. Mosq. Control Assoc. 1991;7:383–386. [PubMed] [Google Scholar]
- Cochran WG, Cox GM. Experimental designs. Wiley; New York: 1957. [Google Scholar]
- Darsie RF, Ward RA. Identification and geographical distribution of the mosquitoes of North America, North of Mexico. Mosq. Syst. 1981;1(Suppl.):1–313. [Google Scholar]
- Denke PM, Lloyd JE, Littlefield JL. Elevational distribution of mosquitoes in a mountainous area of southeastern Wyoming. J. Am. Mosq. Control Assoc. 1996;12:8–16. [PubMed] [Google Scholar]
- Hall DR, Beevor PS, Cork A, Nesbitt BF, Vale GA. 1-octen-3-ol: a potent olfactory stimulant and attractant for tsetse isolated from cattle odours. Insect Sci. Appl. 1984;5:335–339. [Google Scholar]
- Kemme JA, Essen PHAVan, Ritchie SA, Kay BH. Response of mosquitoes to carbon dioxide and 1-octen-3-ol in southeast Queensland, Australia. J. Am. Mosq. Control Assoc. 1993;9:431–435. [PubMed] [Google Scholar]
- Kline DL. Olfactory attractants for mosquito surveillance and control: 1-octen-3-ol. J. Am. Mosq. Control Assoc. 1994;10:280–287. [PubMed] [Google Scholar]
- Kline DL, Lemire GF. Evaluation of attractant-baited traps/targets for mosquito management on Key Island, Florida, USA. J. Vector Ecol. 1998;23:171–185. [PubMed] [Google Scholar]
- Kline DL, Mann MO. Evaluation of butanone, carbon dioxide, and 1-octen-3-OL as attractants for mosquitoes associated with north central Florida bay and cypress swamps. J. Am. Mosq. Control Assoc. 1998;14:289–297. [PubMed] [Google Scholar]
- Kline DL, Wood JR, Morris CD. Evaluation of 1-octen-3-ol as an attractant for Coquillettidia perturbans, Mansonia spp. and Culex spp. associated with phosphate mining operations. J. Am. Mosq. Control Assoc. 1990a;6:605–611. [PubMed] [Google Scholar]
- Kline DL, Dame DA, Meisch MV. Evaluation of 1-octen-3-ol and carbon dioxide as attractants for mosquitoes associated with irrigated rice fields in Arkansas. J. Am. Mosq. Control Assoc. 1991a;7:165–169. [PubMed] [Google Scholar]
- Kline DL, Wood JR, Cornell JA. Interactive effects of 1-octen-3-ol and carbon dioxide on mosquito (Diptera: Culicidae) surveillance and control. J. Med. Entomol. 1991b;28:254–258. doi: 10.1093/jmedent/28.2.254. [DOI] [PubMed] [Google Scholar]
- Kline DL, Takken W, Wood JR, Carlson DA. Field studies on the potential of butanone, carbon dioxide, honey extract, 1-octen-3-ol, L-lactic acid and phenols as attractants for mosquitoes. Med. Vet. Entomol. 1990b;4:383–391. doi: 10.1111/j.1365-2915.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Lepore TJ, Pollack RJ, Spielman A, Reiter P. A readily constructed lard-can trap for sampling host-seeking mosquitoes. J. Am. Mosq. Control Assoc. 2004;20:321–322. [PubMed] [Google Scholar]
- Reiter P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001;109(Suppl. 1):141–161. doi: 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda LM, Harrison BA, Brown JS, Whitt PB, Harrison RL, Gardner RC. Evaluation of 1-octen-3-ol, carbon dioxide, and light as attractants for mosquitoes associated with two distinct habitats in North Carolina. J. Am. Mosq. Control Assoc. 2001;17:61–66. [PubMed] [Google Scholar]
- Shone SM, Ferrao PN, Lesser CR, Glass GE, Norris DE. Evaluation of carbon dioxide- and 1-octen-3-ol-baited centers for disease control Fay-Prince traps to collect Aedes albopictus. J. Am. Mosq. Control Assoc. 2003;19:445–447. [PMC free article] [PubMed] [Google Scholar]
- Slaff M, Apperson C. A key to the mosquitoes of North Carolina and the mid-Atlantic states. The North Carolina Agriculture Extension Service; Raleigh, NC: 1989. [Google Scholar]
- Snow WF, Wilkes TJ. Age composition and vertical distribution of mosquito populations in The Gambia, West Africa [Anopheles melas, Culex thalassius] J. Med. Entomol. 1977;13:507–513. doi: 10.1093/jmedent/13.4-5.507. [DOI] [PubMed] [Google Scholar]
- Takken W, Kline DL. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J. Am. Mosq. Control Assoc. 1989;5:311–316. [PubMed] [Google Scholar]
- Takken W, Knols BG. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu. Rev. Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R, Edman JD. Sampling with light traps and human bait in epidemic foci for eastern equine encephalomyelitis virus in southeastern Massachusetts. J. Am. Mosq. Control Assoc. 1997a;13:348–355. [PubMed] [Google Scholar]
- Vaidyanathan R, Edman JD. Sampling methods for potential epidemic vectors of eastern equine encephalomyelitis virus in Massachusetts. J. Am. Mosq. Control. Assoc. 1997b;13:342–347. [PubMed] [Google Scholar]
- van den Hurk AF, Beebe NW, Ritchie SA. Responses of mosquitoes of the Anopheles farauti complex to 1-octen-3-ol and light in combination with carbon dioxide in northern Queensland, Australia. Med. Vet. Entomol. 1997;11:177–180. doi: 10.1111/j.1365-2915.1997.tb00310.x. [DOI] [PubMed] [Google Scholar]