Abstract
Lamins B1 and B2 have a high degree of sequence similarity and are widely expressed from the earliest stages of development. Studies of Lmnb1 and Lmnb2 knockout mice revealed that both of the B-type lamins are crucial for neuronal migration in the developing brain. These observations naturally posed the question of whether the two B-type lamins might play redundant functions in the development of the brain. To explore that issue, Lee and coworkers generated “reciprocal knock-in mice” (knock-in mice that produce lamin B1 from the Lmnb2 locus and knock-in mice that produce lamin B2 from the Lmnb1 locus). Both lines of knock-in mice manifested neurodevelopmental abnormalities similar to those in conventional knockout mice, indicating that lamins B1 and B2 have unique functions and that increased production of one B-type lamin cannot compensate for the loss of the other.
Keywords: lamin B1, lamin B2, nuclear envelope, nuclear lamina
The nuclear lamina is an intermediate-filament meshwork that lies adjacent to the inner nuclear membrane. In somatic cells, the main proteins of the nuclear lamina are lamins A, C, B1, and B2.1,2 Lamins A and C (the A-type lamins) are alternatively spliced isoforms of LMNA; they are expressed late in development and are found in most differentiated cells.3-6 Inactivation of Lmna has little or no impact on embryonic development but causes death from skeletal muscle disease and cardiomyopathy.6 These findings suggest that the A-type lamins are primarily important for differentiated cells. Lamins B1 and B2 (the B-type lamins) are products of separate genes, LMNB1 and LMNB2, and are ~60% identical at the amino acid level.7 Lamins B1 and B2 are expressed in virtually every cell type from the earliest stages of development. For many years, dogma held that B-type lamins played essential functions in the nucleus of eukaryotic cells, including in DNA replication, organization of the heterochromatin, and formation of the mitotic spindle.8-12 Those findings were further supported by cell culture studies showing that RNAi-mediated knockdown of LMNB1 or LMNB2 in HeLa cells leads to impaired cell growth and apoptosis.13
B-type Lamins Are Dispensable in Some Cell Types
The reports that B-type lamins play crucial roles in eukaryotic cells undoubtedly dampened enthusiasm for examining the functions of B-type lamins in mouse models. (If the B-type lamins were essential for DNA replication and formation of the mitotic spindle, efforts to examine protein function in genetically modified mice would likely be unrewarding.) In the end, however, Yang et al.14 decided to use mouse models to test the dogma holding that B-type lamins have essential functions in the cell nucleus. They created conditional knockout alleles for Lmnb1 and Lmnb2 and went on to breed mice lacking both lamin B1 and lamin B2 in keratinocytes (a cell type that proliferates rapidly and undergoes complex differentiation programs). Remarkably, the loss of both B-type lamins had no detectable effect on keratinocyte proliferation or on the development of the epidermis, hair, or nails. The ultrastructural appearance of the nuclear envelope and chromatin was also normal. Moreover, the loss of both B-type lamins in hepatocytes did not lead to histological abnormalities in the liver or defects in liver function.15 In light of these findings, Yang and coworkers14,15 suggested that any truly vital function of B-type lamins must also be performed by lamins A and C (which are expressed highly in keratinocytes and hepatocytes). However, that notion might be only partially correct. Kim et al.16 recently generated mouse embryonic stem cells lacking all nuclear lamins and found that they proliferated normally and formed large teratomas in mice. Those studies obviously undercut the idea that nuclear lamins play vital roles in DNA replication and mitosis but do not exclude roles for the nuclear lamina in maintaining nuclear structure. At this point, more studies are needed to define the function of nuclear lamins and to assess the consequences of a complete loss of nuclear lamins in tissues.
Crucial Roles for B-type Lamins in the Brain
A key insight into the in vivo importance of the B-type lamins came with the discovery, by Coffinier et al.,17,18 that Lmnb2–/– mice have a striking defect in the migration of neurons in the developing brain. The neuronal migration defect, which was documented by BrdU birthdating studies and immunohistochemical studies, led to a striking neuronal layering abnormality in the cerebral cortex (apparent by E16.5). The cerebellum was also hypoplastic and lacked sulci. Aside from the neurodevelopmental abnormalities, Lmnb2–/– mice had no obvious pathology. These observations were later confirmed with an independent line of Lmnb2–/– mice.19 The widespread neurodevelopmental brain defects in Lmnb2–/– mice were incompatible with postnatal life; all of the mice died within an hour after birth. Interestingly, Coffinier et al.20 observed that many of the neurons in the cerebral cortex of Lmnb2–/– embryos had an elongated, comet-shaped nucleus. Coffinier et al.20 proposed that the comet-shaped nuclei were a consequence of the forces imparted on the nucleus during neuronal migration. Neuronal migration is dependent on the movement of the cell nucleus forward into the leading edge of the cell (nucleokinesis). Nucleokinesis is mediated by cytoplasmic motors that “tug” on the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex, composed of the nesprins in the outer nuclear membrane and the Sun proteins in the inner nuclear membrane. The Sun proteins are thought to be anchored to the nuclear lamina.17 In the setting of Lmnb2 deficiency, Coffinier et al.17,18 proposed that the structural integrity of the nuclear lamina is impaired, such that the forces generated by the cytoplasmic motors simply stretch out the nucleus rather than translocating it into the leading edge of the cell. Coffinier et al.20 went on to show that Lmnb1 deficiency also leads to neurodevelopmental abnormalities. Lmnb1–/– mice were smaller than Lmnb2–/– mice, but like Lmnb2–/– mice died shortly after birth with neuronal migration abnormalities in the cerebral cortex and a small cerebellum devoid of sulci. Also, many of the cortical neurons in Lmnb1–/– mice had nuclear shape abnormalities (a large nuclear bleb).
Aside from roles in neuronal migration during brain development, B-type lamins are important for the survival of neurons. Coffinier et al.20 generated forebrain-specific Lmnb2 knockout mice, forebrain-specific Lmnb1 knockout mice, and forebrain-specific Lmnb2/Lmnb1 double-knockout mice. All of these mice survived after birth, very likely because the gene inactivations and the resulting neuropathology was confined to the forebrain. In one-month-old forebrain-specific Lmnb2 knockout mice, the forebrain was very small; some forebrain neurons survived, but their numbers were markedly reduced (more so than in the cortex of newborn Lmnb2–/– mice). The same phenotypes, but more severe, were observed in forebrain-specific Lmnb1 knockout mice. In forebrain-specific Lmnb2/Lmnb1 double-knockout mice, the forebrain was atrophic; no neurons survived.
Why do neurons die in the absence of lamins B1 and B2, while keratinocytes and hepatocytes are “happy” without these proteins? Coffinier, Young, and colleagues20,21 proposed that the answer relates to the fact that Lmna is expressed highly in keratinoyctes and hepatocytes, while it is absent in migrating neurons of the developing brain. According to this interpretation, the absence of all nuclear lamins in Lmnb2/Lmnb1 double-knockout neurons puts that cell type on an inexorable path to cell death. However, the interpretation may not be that simple, particularly in light of the studies by Kim et al.,16 which showed that mouse embryonic stem cells proliferate normally in the absence of all nuclear lamins.
Coffinier et al.17,18,20 showed that B-type lamins are crucial for neuronal migration during development and are also essential for neuronal survival. Remarkably, overexpression of lamin B1 leads to a demyelinating disease, autosomal dominant leukodystrophy (ADLD).22 ADLD is caused by LMNB1 gene duplications and is associated with a reduced capacity of oligodendrocytes to produce myelin.23,24
Assessing Functional Redundancy of Lamin B1 and Lamin B2
The fact that both Lmnb1–/– and Lmnb2–/– mice die soon after birth with neuronal layering defects in the cerebral cortex inevitably prompts simple questions: Are lamins B1 and B2 functionally redundant in brain development? And would the neurodevelopmental defects associated with the loss of one B-type lamin be eliminated by increased synthesis of the other? The proposition that the two B-type lamins might have redundant functions is plausible. The expression patterns are similar and the two proteins are ~60% identical at the amino acid level.7
Perhaps the best way to address functional redundancy of a pair of related proteins is to create knock-in mice in which the coding sequences for one protein are “knocked in” to the gene for the other protein. Wang et al.25 tested the functional redundancy of myogenic factor 5 and myogenin by creating knock-in mice in which the myogenin cDNA (Myg) was inserted into the myogenic factor 5 (Myf5) locus (thereby replacing Myf5 with Myg). Normally, Myf5-deficient mice have an abnormal rib cage and die perinatally due to an inability to breathe. In contrast, the Myg knock-in mice had a normal rib cage and were viable, demonstrating the redundancy of myogenin for rib development. Also, Geng et al.26 used knock-in mice to examine the redundancy of cyclin D1 and cyclin E. Cyclin D1-deficient mice display neurologic abnormalities, hypoplastic retinas, and abnormal development of the mammary epithelial cells. Knocking in a cyclin E cDNA into the Ccnd1 (cyclin D1 gene) locus prevents these disease phenotypes, indicating that cyclin E can functionally replace cyclin D1. Further studies suggested that cyclin E is a downstream target of cyclin D1. Knock-in mice also yielded crucial insights into the molecular basis for different phenotypes of En-1 and En-2 knockout mice.27
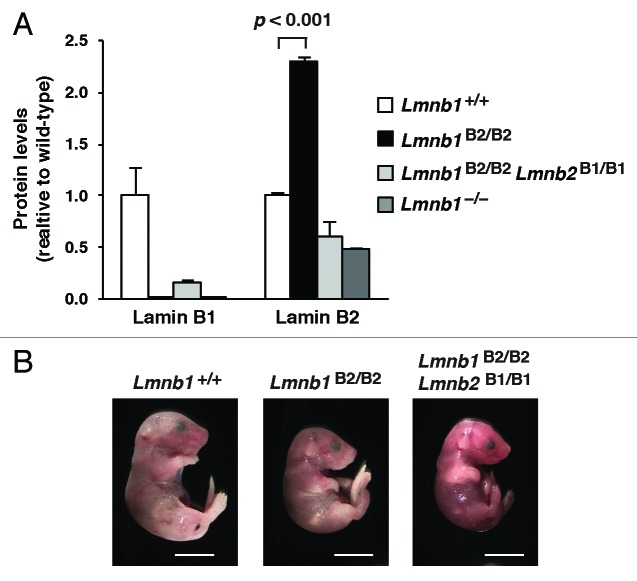
Lee et al.28 reasoned that the best way to test the functional redundancy of lamins B1 and B2 was to create “reciprocal knock-in mice” (i.e., one knock-in line, Lmnb1B2/B2, in which a lamin B2 cDNA was introduced into exon 1 of Lmnb1; and a second knock-in line, Lmnb2B1/B1, in which a lamin B1 cDNA was introduced into exon 1 of Lmnb2). The knock-in alleles worked as planned. Lmnb1B2/B2 mice did not express lamin B1 but had a ~3-fold increase in lamin B2 expression at the RNA and protein levels (the consequence of lamin B2 expression from the endogenous Lmnb2 alleles and two Lmnb1B2 alleles) (Fig. 1A). Of note, the increased expression of lamin B2 in Lmnb1B2/B2 mice did not prevent the neurodevelopmental abnormalities that are associated with Lmnb1 deficiency; Lmnb1B2/B2 mice were small and died soon after birth with neuronal migration defects in the cerebral cortex. Interestingly, however, the abnormalities in Lmnb1B2/B2 mice were less severe than in Lmnb1–/– mice. At E18.5, Lmnb1B2/B2 embryos had significantly higher body and brain weights than Lmnb1–/– embryos (Fig. 1B). Also, the cellularity of the cerebral cortex was greater in Lmnb1B2/B2 embryos than in Lmnb1–/– embryos (Fig. 1C).
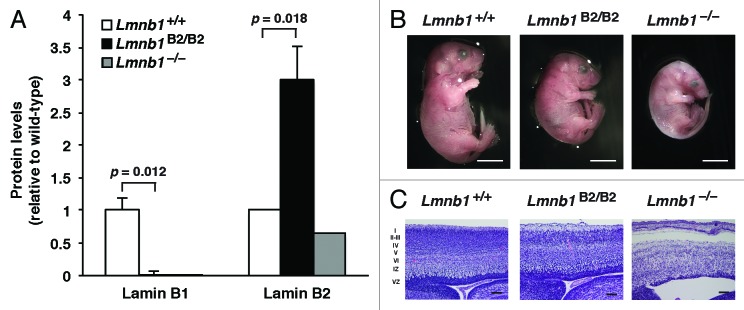
Figure 1. Levels of lamin protein in the cerebral cortex and phenotypes of E18.5 Lmnb1B2/B2 embryos. (A) Lamin protein levels, as judged by quantitative western blots, for E18.5 Lmnb1+/+, Lmnb1B2/B2, and Lmnb1−/− embryos, relative to actin (mean ± SD). Protein levels in wild-type mice were set at 1.0. Lamin B2 protein levels in the cerebral cortex were higher in Lmnb1B2/B2 embryos than in Lmnb1+/+ embryos (P = 0.018). Lamin B1 was undetectable in Lmnb1B2/B2 embryos (P = 0.012). (B) Photographs of E18.5 Lmnb1+/+, Lmnb1B2/B2, and Lmnb1−/− embryos. Scale bar, 2 mm. (C) Hematoxylin and eosin–stained sagittal sections of brains from E18.5 embryos, showing abnormal layering of cortical neurons in Lmnb1B2/B2 mice. Scale bar, 100 µm. Reproduced with permission from Lee et al.28
Lmnb2B1/B1 mice expressed no lamin B2 and had higher Lmnb1 transcript levels, reflecting lamin B1 production from the endogenous Lmnb1 gene and two Lmnb2B1 alleles. However, lamin B1 protein levels were only slightly elevated. Higher levels of lamin B1 expression did not prevent the neurodevelopmental defects associated with the loss of lamin B2; Lmnb2B1/B1 mice died soon after birth and had neuronal layering abnormalities in the cerebral cortex similar to those in Lmnb2–/– mice (Fig. 2A and B).
Figure 2. Phenotypes of Lmnb2B1/B1 mice. (A) Photographs of E18.5 Lmnb1+/+ and Lmnb2B1/B1 embryos. Scale bar, 2 mm. (B) Hematoxylin and eosin–stained sagittal sections of brains from E18.5 embryos. Scale bar, 500 µm. Reproduced with permission from Lee et al.28
Lee et al.28 bred Lmnb1B2/+Lmnb2B1/+ mice and went on to intercross those mice. The goal was to obtain viable Lmnb1B2/B2Lmnb2B1/B1 mice, but all of those mice died soon after birth with neuronal layering abnormalities in the cerebral cortex. Lamin B2 levels in the cortical extracts of Lmnb1B2/B2Lmnb2B1/B1 embryos were essentially normal, but lamin B1 levels were only 16.4% of those in wild-type embryos (Fig. 3A). At E18.5, brain weights in Lmnb1B2/B2Lmnb2B1/B1 embryos were quite low but were nevertheless higher than those in Lmnb1B2/B2 mice, implying that the small amounts of lamin B1 from the Lmnb2B1 allele was sufficient to partially ameliorate disease phenotypes (Fig. 3B). It seems likely that the failure of Lmnb1B2/B2Lmnb2B1/B1 mice to survive relates to the low levels of lamin B1 production from the Lmnb2B1 allele, although there is a formal possibility that the death of these mice relates to subtle differences in the spatial or temporal patterns of Lmnb1 and Lmnb2 expression.
Figure 3. Characterization of Lmnb1B2/B2Lmnb2B1/B1 mice. (A) Quantification of lamin B1 and lamin B2 levels, relative to actin (mean ± SD), in extracts of cerebral cortex from E18.5 Lmnb1B2/B2, Lmnb1B2/B2Lmnb2B1/B1, Lmnb1–/– embryos, and wild-type embryos, as judged by western blots. Protein levels in wild-type controls were set at 1.0. Lamin B1 was undetectable in Lmnb1B2/B2 embryos. Lamin B1 levels in Lmnb1B2/B2Lmnb2B1/B1 embryos were only 16.4% of those in wild-type mice. (B) Photographs of E18.5 wild-type, Lmnb1B2/B2, and Lmnb1B2/B2Lmnb2B1/B1 embryos. Scale bar, 2 mm. Reproduced with permission from Lee et al.28
Intercrosses of Lmnb1B2/+Lmnb2B1/+ mice yielded viable Lmnb1B2/+Lmnb2B1/B1 mice with the expected Mendelian frequency.28 Lmnb1B2/+Lmnb2B1/B1 mice expressed substantial amounts of lamin B1 and lamin B2 in the brain, were healthy and fertile, and were free of brain pathology. The viability of Lmnb1B2/+Lmnb2B1/B1 mice demonstrate that lamin B2 functions normally when it is expressed exclusively from the Lmnb1 locus. In contrast to the health and vitality of Lmnb1B2/+Lmnb2B1/B1 mice, Lmnb1B2/B2Lmnb2B1/+ mice died soon after birth with neurodevelopmental abnormalities, likely because of the low levels of lamin B1 expression from the Lmnb2B1 allele.
The phenotypes of the reciprocal knock-in mice support the idea that lamins B1 and B2 play distinct roles in development.28 Increased production of lamin B2 did not prevent the neurodevelopmental abnormalities associated with Lmnb1 deficiency, nor did increased production of lamin B1 prevent neurodevelopmental abnormalities associated with Lmnb2 deficiency. However, we did find evidence that increased production of one B-type lamin can partially ameliorate the phenotypes associated with the loss of the other. For example, Lmnb1B2/B2 mice expressed ~3-fold more lamin B2 than Lmnb1–/– mice and their developmental abnormalities were less severe than those of Lmnb1–/– mice.
In hindsight, other observations can be viewed as being consistent with the discovery that lamins B1 and B2 play distinct roles in the developing brain. First, the nuclear shape abnormalities in Lmnb1–/– and Lmnb2–/– neurons are distinct. Coffinier et al.20 observed comet-shaped nuclei in the cortex of Lmnb2–/– embryos, but that nuclear abnormality was rarely observed in neurons of Lmnb1–/– embryos. Instead, the cortical neurons of Lmnb1–/– mice often had a large, solitary nuclear bleb.20 Solitary nuclear blebs were also observed in the cerebral cortex of forebrain-specific Lmnb1 knockout embryos, but these blebs disappeared later in life—after the onset of Lmna expression. The fact that deficiencies in the two B-type lamins yielded different nuclear shape abnormalities suggests that the two proteins have different functions. Second, the importance of protein farnesylation is different for lamin B1 and lamin B2. Jung et al.29 generated knock-in mice that produced nonfarnesylated versions of lamin B1 and lamin B2 (by replacing the cysteine of the CaaX motif with a serine). Knock-in mice expressing nonfarnesylated lamin B2 were healthy, fertile, and free of behavioral abnormalities; neither neuropathology nor nuclear shape abnormalities were detected in the cerebral cortex. In contrast, knock-in mice expressing nonfarnesylated lamin B1 died soon after birth with neuronal migration defects in the cerebral cortex. Many of the midbrain neurons in these mice had dumbbell-shaped nuclei, with the nuclear lamina at one end of the dumbbell and the bulk of chromosomal DNA at the other end of the dumbbell. Dumbbell-shaped nuclei were also found in cultured neurons as they migrated away from neurospheres. Jung et al.29 suggested that dumbbell-shaped nuclei were the consequence of defective anchoring of lamin B1 to the inner nuclear membrane and honeycombing of the nuclear lamina. They proposed that, during nucleokinesis, the lamina was pulled into the leading edge of the cell in a normal fashion, but the nuclear chromatin did not “come along for the ride.” Instead, the chromatin simply drained through a porous nuclear lamina into the “potential space” between the lamina and the inner nuclear membrane and was left behind in the trailing edge of the cell. This potential space does not exist in wild-type neurons because lamin B1’s farnesyl lipid anchor firmly attaches the lamina to the inner nuclear membrane. The fact that the farnesyl lipid anchor is so crucial for lamin B1 yet utterly dispensable for lamin B2 lends support to the idea that lamins B1 and B2 have distinct functions in the nuclear envelope.
Summary and Perspective
The fact that Lmnb1–/– and Lmnb2–/– mice manifest similar phenotypes (perinatal death with neuronal migration defects),17,18,20,21 along with the fact that the B-type lamins are ~60% identical at the amino acid level,7 naturally suggested the possibility that the two proteins might have redundant functions in the brain. The “reciprocal knock-in mice” proved that this was not the case; increased expression of one B-type lamin did not prevent the neurodevelopmental abnormalities resulting from the loss of the other.28 With that finding under our belt, the remaining task—and it is a daunting one—is to better define the unique and overlapping functions of the two B-type lamins. Success will require a more complete understanding of interacting proteins and a better understanding of which sequences within the two proteins underlie their unique functions in the cell biology of the developing brain.
The creation and analysis of the reciprocal knock-in mice represented a considerable investment in time and resources. Needless to say, it would have been highly preferable to have been able to glean the same insights from cell culture studies. However, as noted earlier, some of the studies of nuclear lamin function that have relied solely on biochemical and cell-based assays have been problematic. For example, the idea that B-type lamins are crucial for DNA replication and mitosis is inconsistent with the normal skin and hair in keratinocyte-specific Lmnb1/Lmnb2 double-knockout mice. Also, cell culture studies did not allow anyone to foresee a crucial role for B-type lamins in brain development, nor did they allow anyone to predict that protein farnesylation is crucial for lamin B1 but not for lamin B2. In the future, advances in cell biology might eventually make it possible to make accurate predictions about the in vivo functions of different nuclear lamins. In the meantime, genetically modified mice will remain essential. We can foresee many opportunities. For example, knocking in lamin B1/lamin B2 chimeric cDNAs into Lmnb1 or Lmnb2 could be quite helpful in defining unique functional domains within lamin B1 and lamin B2—and in generating clues about unique protein partners. Our understanding of the A-type lamins is also limited. No one knows which domains of lamin A, if any, are functionally interchangeable with those of B-type lamins. Also, no one knows if Lmna expression in the brain—if it were activated early in development—would interfere with the functions of B-type lamins in neuronal migration. Answers to these questions and many others will require investing in genetically modified mice.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This work was supported by the National Institutes of Health Grants [HL089781 (L.G.F.), AG035626 (S.G.Y.), and 5T32HL007895–15 (J.M.L.).
References
- 1.Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119:1825–36. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol. 2013;14:13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- 3.Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–6. [PubMed] [Google Scholar]
- 4.Röber RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989;105:365–78. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- 5.Stewart C, Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987;51:383–92. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–20. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies BSJ, Coffinier C, Yang SH, Jung HJ, Fong LG, Young SG. Posttranslational Processing of Nuclear Lamins. The Enzymes. 2011;29:21–41. doi: 10.1016/B978-0-12-381339-8.00003-2. [DOI] [Google Scholar]
- 8.Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–21. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moir RD, Montag-Lowy M, Goldman RD. Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J Cell Biol. 1994;125:1201–12. doi: 10.1083/jcb.125.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moir RD, Spann TP, Lopez-Soler RI, Yoon M, Goldman AE, Khuon S, Goldman RD. Review: the dynamics of the nuclear lamins during the cell cycle-- relationship between structure and function. J Struct Biol. 2000;129:324–34. doi: 10.1006/jsbi.2000.4251. [DOI] [PubMed] [Google Scholar]
- 11.Tang CW, Maya-Mendoza A, Martin C, Zeng K, Chen S, Feret D, Wilson SA, Jackson DA. The integrity of a lamin-B1-dependent nucleoskeleton is a fundamental determinant of RNA synthesis in human cells. J Cell Sci. 2008;121:1014–24. doi: 10.1242/jcs.020982. [DOI] [PubMed] [Google Scholar]
- 12.Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, Zheng Y. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science. 2006;311:1887–93. doi: 10.1126/science.1122771. [DOI] [PubMed] [Google Scholar]
- 13.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–65. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 14.Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y, de Jong PJ, Fong LG, Young SG. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet. 2011;20:3537–44. doi: 10.1093/hmg/ddr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang SH, Jung HJ, Coffinier C, Fong LG, Young SG. Are B-type lamins essential in all mammalian cells? Nucleus. 2011;2:562–9. doi: 10.4161/nucl.2.6.18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y, Zheng X, Zheng Y. Proliferation and differentiation of mouse embryonic stem cells lacking all lamins. Cell Res. 2013;23:1420–3. doi: 10.1038/cr.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coffinier C, Fong LG, Young SG. LINCing lamin B2 to neuronal migration: growing evidence for cell-specific roles of B-type lamins. Nucleus. 2010;1:407–11. doi: 10.4161/nucl.1.5.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, Fong LG, Young SG. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci U S A. 2010;107:5076–81. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MS, Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334:1706–10. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coffinier C, Jung HJ, Nobumori C, Chang S, Tu Y, Barnes RH, 2nd, Yoshinaga Y, de Jong PJ, Vergnes L, Reue K, et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol Biol Cell. 2011;22:4683–93. doi: 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young SG, Jung HJ, Coffinier C, Fong LG. Understanding the roles of nuclear A- and B-type lamins in brain development. J Biol Chem. 2012;287:16103–10. doi: 10.1074/jbc.R112.354407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, Hogan K, Ptácek LJ, Fu YH. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–23. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 23.Heng MY, Lin ST, Verret L, Huang Y, Kamiya S, Padiath QS, Tong Y, Palop JJ, Huang EJ, Ptáček LJ, et al. Lamin B1 mediates cell-autonomous neuropathology in a leukodystrophy mouse model. J Clin Invest. 2013;123:2719–29. doi: 10.1172/JCI66737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin ST, Huang Y, Zhang L, Heng MY, Ptácek LJ, Fu YH. MicroRNA-23a promotes myelination in the central nervous system. Proc Natl Acad Sci U S A. 2013;110:17468–73. doi: 10.1073/pnas.1317182110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Schnegelsberg PN, Dausman J, Jaenisch R. Functional redundancy of the muscle-specific transcription factors Myf5 and myogenin. Nature. 1996;379:823–5. doi: 10.1038/379823a0. [DOI] [PubMed] [Google Scholar]
- 26.Geng Y, Whoriskey W, Park MY, Bronson RT, Medema RH, Li T, Weinberg RA, Sicinski P. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell. 1999;97:767–77. doi: 10.1016/S0092-8674(00)80788-6. [DOI] [PubMed] [Google Scholar]
- 27.Hanks M, Wurst W, Anson-Cartwright L, Auerbach AB, Joyner AL. Rescue of the En-1 mutant phenotype by replacement of En-1 with En-2. Science. 1995;269:679–82. doi: 10.1126/science.7624797. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Tu Y, Tatar A, Wu D, Nobumori C, Jung HJ, Yoshinaga Y, Coffinier C, de Jong PJ, Fong LG, et al. Reciprocal knock-in mice to investigate the functional redundancy of lamin B1 and lamin B2. Mol Biol Cell. 2014;25:1666–75. doi: 10.1091/mbc.E14-01-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung HJ, Nobumori C, Goulbourne CN, Tu Y, Lee JM, Tatar A, Wu D, Yoshinaga Y, de Jong PJ, Coffinier C, et al. Farnesylation of lamin B1 is important for retention of nuclear chromatin during neuronal migration. Proc Natl Acad Sci U S A. 2013;110:E1923–32. doi: 10.1073/pnas.1303916110. [DOI] [PMC free article] [PubMed] [Google Scholar]