Highlights
-
•
We used hybrid fusion bc1 complex to test inter-monomer electron transfer in vivo.
-
•
Cross-inactivated complexes were able to sustain photoheterotrophic growth.
-
•
Inter-monomer electron transfer supports catalytic cycle in vivo.
-
•
bc1 dimer is functional even when cytochrome b subunits come from different species.
Keywords: Cytochrome bc1, Mitochondrial complex III, Hybrid fusion protein, Electron transfer, Energy conversion
Abstract
Electronic connection between Qo and Qi quinone catalytic sites of dimeric cytochrome bc1 is a central feature of the energy-conserving Q cycle. While both the intra- and inter-monomer electron transfers were shown to connect the sites in the enzyme, mechanistic and physiological significance of the latter remains unclear. Here, using a series of mutated hybrid cytochrome bc1-like complexes, we show that inter-monomer electron transfer robustly sustains the function of the enzyme in vivo, even when the two subunits in a dimer come from different species. This indicates that minimal requirement for bioenergetic efficiency is to provide a chain of cofactors for uncompromised electron flux between the catalytic sites, while the details of protein scaffold are secondary.
1. Introduction
Cytochrome bc1 (ubihydroquinone:cytochrome c oxidoreductase or mitochondrial complex III) is a multi-subunit enzyme which transfers electrons between quinone molecules and cytochrome c and couples this electron transfer to proton translocation across the membrane. This way cytochrome bc1 contributes to generation of the protonmotive force used for cellular ATP production [1]. The enzyme operates according to the Q cycle, in which the net translocation of protons is a result of the joint action of two quinone oxidation/reduction sites (named the Qo and Qi sites) located at two opposite sides of the membrane [2]: the Qo site oxidizes hydroquinone (quinol) and releases protons, the Qi site reduces quinone and uptakes protons.
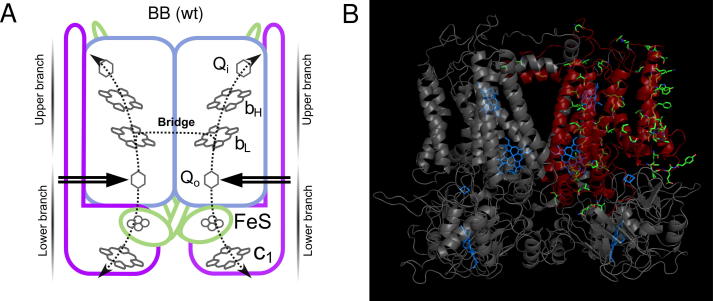
The Qo and Qi sites and cofactor chains are embedded within three subunits: cytochrome b (harbors Qo, Qi, heme bH and heme bL), cytochrome c1 (harbors heme c1) and the FeS subunit (harbors 2Fe–2S iron–sulfur cluster (FeS)) [1,3]. The Qo and Qi sites are connected by a chain composed of two hemes (heme bL and heme bH), while a chain composed of FeS and heme c1 connects the Qo site with cytochrome c pool. In Fig. 1A, these two chains are depicted as parts of the upper and lower branches, respectively. The scheme also shows that cytochrome bc1 is a homodimer.
Fig. 1.
Catalytic core of cytochrome bc1. (A) H-shaped electron transfer system formed by cofactor chains of: cytochrome b (blue), cytochrome c1 (purple) and FeS subunits (green). Electron entry sites (double arrow), electron paths (dotted lines). (B) Crystal structure of R. capsulatus cytochrome bc1 dimer (PDB: 1ZRT). Heme and FeS cofactors are blue. Cytochrome b subunit of one monomer is shown in red and depicts those amino acids that are different in R. sphaeroides (green sticks) to illustrate the heterogeneity of hybrid fusion protein used in this study.
Each monomer contains all structural elements necessary to perform the catalytic Q cycle: the Qo and Qi sites and cofactors of the upper and lower branches. Intriguingly, crystal structures of the dimer revealed short distance between two hemes bL, each coming from a different monomer [4,5]. This creates a possibility for inter-monomer electron transfer [6]. The idea of electron exchange between the monomers was adopted in mechanistic concepts describing operation of the dimer [7–11]. Recent experiments have indicated that this reaction is an integral part of ensemble of electron transfer reactions that take place within the dimer [12–16]. The existence of this connection converts the cofactor chains of a dimer into an H-shaped electron transfer system that connects all four catalytic sites [12].
At present, the inter-monomer electron transfer in cytochrome bc1 is a matter of intense debate [11,14,17,18]. In particular, its physiological significance is not clear. This is because in the case of the fully operational dimer, the inter-monomer electron transfer can in principle be considered as an alternative to the intra-monomer electron transfer. In this context an important question that needs addressing is whether the inter-monomer electron transfer can secure energetic efficiency of the enzyme at level that allows supporting the cytochrome bc1-dependent growth of cells. The results shown here not only confirm the physiologic competence of inter-monomer electron transfer, but also demonstrate that electronic connections in this system are generally robust over structural alterations and can sustain in vivo the catalysis in the non-native heterogeneous assemblies, when two cytochrome b parts of the dimer core come from different species.
2. Methods
2.1. Mutant strains
A genetic system for expression of hybrid BS complex and hybrid fusion BS–B complex in Rhodobacter capsulatus was described in [15]. Details of construction of expression vectors for mutant derivatives of BSBS and BS–B is described in the Supplementary material.
2.2. Bacterial growth, genes and proteins
R. capsulatus strains were grown under semiaerobic/dark or photoheterotrophic conditions as described in [19,20]. When describing the type of growth, the term “aerobic growth” referred to aerobic growth under semiaerobic conditions (at relatively low oxygen concentration), while “photoheterotrophic growth” referred to growth under anaerobic conditions in light as a source of energy. For isolation of proteins from photoheterotrophically grown cultures, 100 μl of overnight 2 ml liquid aerobic culture was spread on each of 14 MPYE plates which then were incubated in anaerobic jars (GasPak, BD) for 48 h.
2.3. Membrane proteins isolation and purification
Preparations of protein complexes from semiaerobic liquid cultures were as described in [21] with modifications reported in [19]. Complexes were isolated by affinity chromatography, Strep-tag purification was performed as described in [19]. SDS–PAGE of purified complexes was performed as described in [22]. To prepare the membranes from the photoheterotrophically grown cultures, cells from 14 MPYE plates were scraped to the MPYE medium and centrifuged (6000g, 20 min). The obtained pellet was subjected to the same purification steps as above.
2.4. Genetic analyses
The size and DNA sequence of the fusion genes present in cells grown photoheterotrophically were verified by restriction analyzes and DNA sequencing of plasmids isolated from R. capsulatus and amplified in Escherichia coli HB101. The double restriction digestion was performed with BstXI (New England Biolabs) and SfuI (Roche Diagnostics) enzymes, yielding shorter DNA fragment (1950 bp) in case of cytochrome b gene or longer DNA fragment (3325 bp) for cytochrome bSb gene.
3. Results
Photoheterotrophic growth of R. capsulatus requires obligatory presence of the functional cytochrome bc1, thus the most straightforward way to verify in vivo functionality of specific mutant derivatives of this complex is to test the capability of the mutant cells to grow under the photoheterotrophic conditions [20]. In this work, we used this approach in combination with the asymmetric mutagenesis to assess physiological relevance of the inter-monomer electron transfer in cytochrome bc1. The asymmetric mutagenesis was accomplished using the system based on expression, in R. capsulatus cells, of cytochrome bc1-like complexes in which two separate cytochrome b subunits of the dimer core were replaced with a hybrid fusion of Rhodobacter sphaeroides and R. capsulatus cytochromes b (this protein complex is named BS–B) [15]. The structures of these two cytochromes b are similar, but not identical [3,23]. In fact, the 10% difference in amino acid composition makes the fusion protein heterogeneous, offering a unique opportunity to test electron transfers in vivo in an enzyme in which the asymmetry does not concern just the point mutations knocking out parts of individual branches of the system (see below), but also several other amino acid positions randomly spread throughout the protein (Fig. 1B).
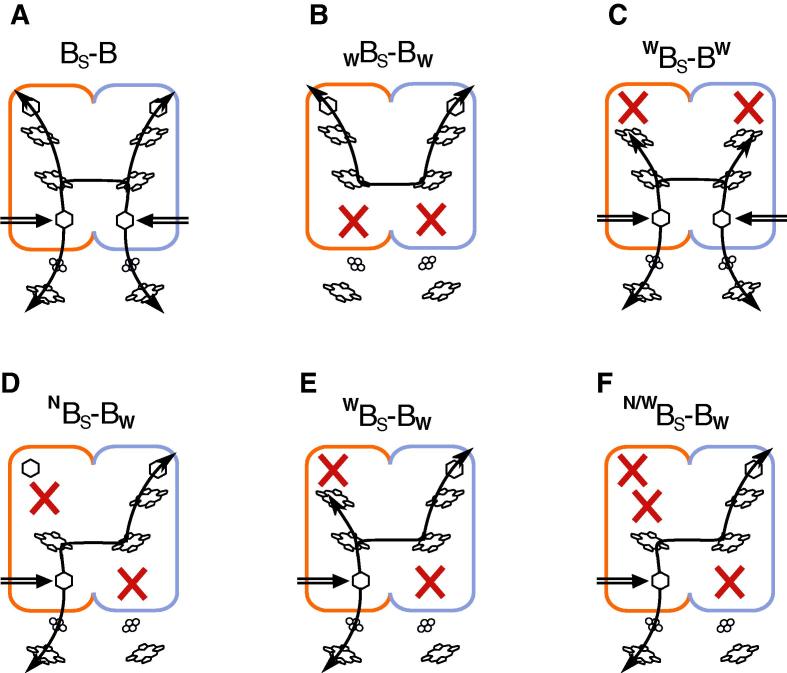
Fig. 2 depicts the cofactor knockout patterns that have been tested using BS–B as the protein template for mutagenesis. Without any additional mutations, all the cofactors and the catalytic sites of BS–B form an H-shaped electron transfer system. This system, characteristic also for the native cytochrome bc1 dimer, consists of two upper and two lower branches connected together by the two-heme bridge (Fig. 2A). All the remaining patterns shown in Fig. 2 refer to the derivatives of BS–B containing specific mutations that knock out individual branches in various combinations (Fig. 2B–F).
Fig. 2.
Electron paths in hybrid fusion complexes with various cofactor knockout patterns. (A) BS–B complex contains all electronic connections characteristic of native cytochrome bc1 (see Fig. 1A). (B and C) Symmetric patterns disrupt all connections between the Qo and Qi sites. (D–F) Three variants of asymmetric patterns cross-inactivate the complex and leave one possible connection between the Qo and Qi sites (involving the inter-monomer electron transfer). Orange and blue show two halves of the fusion protein corresponding to cytochromes b of R. sphaeroides and R. capsulatus, respectively. Red crosses indicate cofactor knockouts.
WBS–BW (Fig. 2B) contains mutations knocking out the Qo site in both halves of the fusion protein, thus inactivating the two lower branches of the system (the two subscripts “W” refer to equivalents of mutation G158W in R. capsulatus cytochrome b [24]). WBS–BW (Fig. 2C) contains mutations knocking out the Qi site in both halves of the fusion protein, thus inactivating the two upper branches (the two superscripts “W” refer to equivalents of H217W mutation in R. capsulatus cytochrome b, which has knockout effects similar to those described earlier for H217L [25]. WBS–BW and WBS–BW are the two control forms of cytochrome bc1-like complexes containing the fused cytochrome b inactivated in such a way that a complete turnover of the enzyme involving the joint action of the Qo and the Qi sites is not possible.
Lower panel of Fig. 2 depicts three variants of the cross-inactivated complex: NBS–BW, WBS–BW and N/WBS–BW. The cross-inactivation refers to inactivation of the Qi site (upper branch) in one half of the fusion protein and the Qo site (lower branch) in the other half. The annotations “W” and “W” refer to the same mutations that were described above (for the forms of Fig. 2B and C), while superscript “N” refers to a mutation that knocks out heme bH (“N” is an equivalent of H212N in R. capsulatus cytochrome b [6]). H212N inactivates the upper branch as H217W, but in a different manner: while H217W blocks just the Qi site allowing electrons to advance as far as to heme bH, H212N eliminates the electron transfer from heme bL to heme bH. “N/W” combines both ways of inactivating the upper branch in one monomer. All three cross-inactivated forms preserve the electronic communication between the active Qo site in one half and the active Qi site of the other half. This communication is possible due to the presence of the intact heme bL–bL bridge.
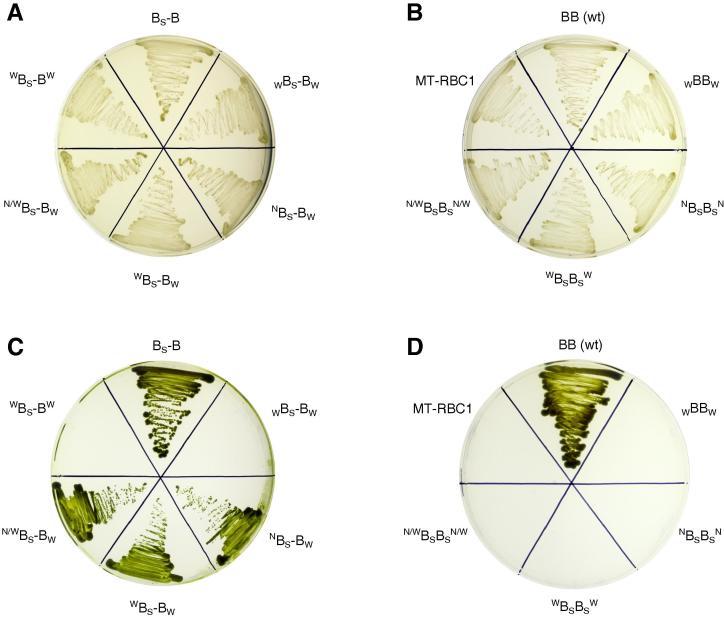
Fig. 3A shows aerobic growth in dark of the R. capsulatus strains transformed with mutated genes coding for the BS–B derivatives described above. In all cases the cells under these growing conditions expressed the complexes with the fusion protein. This was confirmed genetically by verifying the size and the sequence of the plasmids isolated from the respective R. capsulatus strains and also by analyzing the subunit composition of complexes isolated by Strep-tag affinity chromatography. SDS–PAGE profiles of BS–B and its four derivatives were compared with the profile of wild-type cytochrome bc1 (Fig. 4A). The derivatives include all three variants of cross-inactivated complex and one symmetrically inactivated WBS–BW. The profile of WBS–BW is omitted here as it was already shown in [15]. It is clear that in lanes 3–7 the predominant band of ∼100 kDa corresponds to the fusion protein (cytochrome bSb) that replaces the native cytochrome b (lane 2). The SDS profiles (high intensity of the 100 kDa band comparing to the bands of cytochrome c1 and the FeS subunit) reflect an increased probability of dissociation of subunits not-containing Strep-tag during the purification using the affinity chromatography. In these profiles a band corresponding in size to cytochrome b is also detectable, which possibly comes as a result of partial degradation of a fusion protein upon expression and/or isolation of proteins [15]. We note that this cannot be a result of genetic recombination, as established in [15]. We also note that the background of cytochrome b would not affect the meaning of the results of photoheterotrophic growth tests of the cross-inactivated forms as any complex containing identical halves of the fusion protein would be inactive, thus not able to support the photoheterotrophic growth of the cells.
Fig. 3.
Testing in vivo functionality of inter-monomer connection between the catalytic sites of cytochrome bc1. Aerobic growth of R. capsulatus expressing (A) hybrid fusion BS–B and its derivatives or (B) homodimeric cytochrome bc1: BB or BSB and their derivatives. (C and D) Photoheterotrophic growth of the strains depicted in (A and B), respectively. MT-RBC1 is a strain devoid of cytochrome bc1[20].
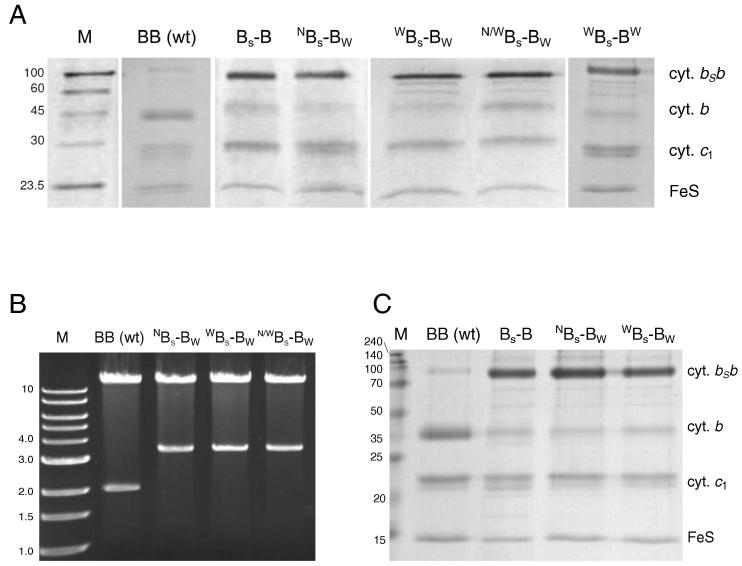
Fig. 4.
Protein and gene analysis for strains expressing the fusion complexes. (A) SDS–PAGE profiles of the complexes purified from cells grown under semiaerobic conditions. (B) Restriction analysis of plasmid DNA isolated from cells grown under photoheterotrophic conditions. (C) SDS–PAGE profiles of the complexes purified from cells grown under photoheterotrophic conditions. M – mass marker (kDa or kb), cyt. bSb – hybrid fusion of cytochromes b in BS–B complex, cyt. b – monomeric cytochrome b, remaining lanes descriptions correspond to the names of the mutants shown in Figs. 2 and 3.
We report a success in obtaining WBS–BW as complex containing the fused cytochrome bSb. In view of the results from our earlier experiments, introducing mutations symmetrically to the fusion protein (i.e., the same mutation present in both halves of the protein) does not always guarantee the proper expression of the fusion protein [15,19]. Indeed, WBS–BW appears as the first example of the fusion protein with successful inactivation of the two upper branches (so far the symmetric inactivation was only possible with the two lower branches in WBS–BW [15]).
Fig. 3B shows aerobic growth of other R. capsulatus strains that were used as additional controls in further photoheterotrophic growth tests. MT-RBC1 is devoid of genes coding for cytochrome bc1. All other strains expressed the homodimeric cytochrome bc1 (containing two cytochrome b subunits). BB refers to native cytochrome bc1 of R. capsulatus, WBBW is its derivative – a mutant with G158W in cytochrome b. The remaining three mutants are derivatives of R. capsulatus cytochrome bc1 having a native cytochrome b replaced with cytochrome b of R. sphaeroides (BSBS). NBSBSN and WBSBSW are mutants with H212N and H217W in cytochrome b, respectively, while N/WBSBSN/W is a double mutant H212N/H217W.
Panels C and D in Fig. 3 show the results of photoheterotrophic growth tests performed with the same mutants as in Fig. 3A and B, respectively. As expected, the strains expressing BB or BS-B grow photoheterotrophically, while all the strains expressing the symmetrically-inactivated forms of the complexes – do not. This includes two complexes with the fusion protein, i.e., derivatives of BS–B (WBS–BW and WBS–BW), the mutant of cytochrome bc1 (WBBW), as well as derivatives of BSBS (NBSBSN, WBSBSW, and N/WBSBSN/W). Photosynthetic incompetence of these strains is correlated with a lack of functional cytochrome bc1; the symmetric inactivation effectively disrupts the electronic connections between all the Qo and the Qi sites (Fig. 2B and C).
Remarkably, all three strains expressing the cross-inactivated forms of BS–B (NBS–BW, WBS–BW, N/WBS–BW) can grow photoheterotrophically similarly to the cells expressing native cytochrome bc1 (Fig. 3C). To confirm that expression of the BS–B complexes containing the appropriately mutated fusion protein did take place under photoheterotrophic conditions in these strains, we performed analyzes of both the DNA and the protein complexes isolated from the cells grown photoheterotrophically. At the DNA level, restriction analysis of isolated expression plasmids amplified in E. coli confirmed the presence of the fused gene of the expected length (Fig. 4B), Moreover, the DNA sequencing confirmed that the mutations originally introduced to the gene (N, W, N/W and W) were retained at original positions during the photoheterotrophic growth.
At the protein level, the SDS–PAGE profiles of the complexes isolated from the membranes showed a prominent band of ∼100 kDa corresponding to the fusion protein (Fig. 4C). This band was accompanied by the bands corresponding to the two other subunits: cytochrome c1 and FeS. It is of note, that the results of Fig. 4B and C provide first verification of expression of asymmetrically mutated complexes isolated from cultures grown under photoheterotrophic conditions.
4. Discussion
To efficiently translocate protons across the bioenergetic membrane, cytochrome bc1 must have functional Qo and Qi sites and those two sites must be electronically connected together. In the H-shaped electron transfer system of the native cytochrome bc1 dimer, the Qo and the Qi sites are connected not only by the cofactor chains of the same monomer (upper and lower branches making connections with the Qo and Qi sites), but also by the chain built of the lower branch of one monomer and the upper branch of the other [12]. The latter connection requires electron transfer between the two monomers which occurs through the bridge formed by two hemes bL. This additional link increases a number of available electron paths and allows the quinone catalytic sites to communicate across the dimer. This engineering principle provides one testable prediction: the disruption of the catalytic Q-cycle and consequent loss of bioenergetic function of the enzyme will not occur unless all possible connections linking the Qo with the Qi sites are blocked. Our work confirmed this principle at in vivo level with a series of mutants that exposed various electron paths for functional testing.
The loss of the cytochrome bc1-dependent photoheterotrophic growth of the cells was observed in all symmetrically-mutated derivatives of cytochrome bc1 in which either both upper or both lower branches were inactivated (Fig. 3C and D). Such inactivations cut all possible connections linking the Qo with the Qi sites and thus the enzyme was not functional in vivo under the conditions used.
All three cross-inactivated forms of BS–B (NBS–BW, WBS–BW and N/WBS–BW) effectively supported photoheterotrophic growth of the cells, in spite of the fact that the connection between the Qo and Qi sites at the level of the same monomer was blocked. In those forms, the intact bridge allowed the inter-monomer electron transfer to connect the lower branch and the Qo site of one monomer with the upper branch and the Qi site of the other. In fact, the cross-inactivations forced the enzyme to use the path involving the inter-monomer electron transfer if all reactions of the catalytic Q-cycle were to be completed. Our results clearly demonstrate that enzymes relying just on this path perform their bioenergetic function in vivo.
These results are fully consistent with the results of our previous kinetic experiments performed in vitro with asymmetrically-mutated variants of the fusion complexes. The measurements of flash-induced electron transfer in membranes demonstrated that inter-monomer electron transfer occurs in milliseconds or less and thus is a catalytically-relevant event [12,15]. Furthermore, the measured activities of isolated complexes confirmed the competence of inter-monomer electron transfer in supporting the multiple enzymatic turnovers [14].
Our results are also fully consistent with the in vitro and in vivo results obtained independently with the two-plasmid system developed for the R. capsulatus to probe the inter-monomer electron transfer through the asymmetrically-mutated heterodimers of cytochrome bc1 [13,16]. In this case the cross-inactivation was achieved by compiling F144R or Y147A mutation (inactivating the Qo site) present in one copy of specifically-tagged cytochrome b gene with H217L or H212N mutation (inactivating the Qi site) present in another copy of differently-tagged cytochrome b gene. The expression of the mutated heterodimers in the cells grown under semiaerobic conditions was confirmed biochemically and the occurrence of inter-monomer electron transfer was supported by kinetic measurements. Furthermore, the mutated strains grew photoheterotrophically indicating that the inter-monomer electron transfer can support the function of cytochrome bc1 in vivo.
It is clear that both systems (i.e., the system based on fusion protein used in this work and the two-plasmid system) consistently indicate that cytochrome bc1 complexes modified specifically so that the only route connecting the Qo site with the Qi site involves electron transfer between the two hemes bL, are enzymatically active and efficient to sustain the cytochrome bc1-dependent photoheterotrophic growth of the cells. This further rules out the arguments against the existence of inter-monomer electron transfer [17] (detailed discussion dealing with these arguments can be found in [15,26]).
The in vivo demonstration of the enzymatic competence of electron path involving the cross-communication of Qo and Qi sites can be considered as a step forward towards understanding the physiological significance of the inter-monomer electron transfer in cytochrome bc1. This still remains an open issue. Clearly, the tests conducted so far concerned specific conditions where the inter-monomer electron transfer was imposed to the system by eliminating other possible connections present in the native dimer. Considering living cells, this could correspond to the situation when parts of the enzyme are damaged by mutations (a case specifically relevant to mitochondria, where mutations, involving those in cytochrome b subunit of complex III, appear to accumulate with age [27]). In such cases, the advantage of the apparent redundancy of the components of the H-shaped electron transfer system is to allow the function of the partly damaged enzyme. In cases when all parts of the enzyme are operational, the inter-monomer electron transfer, constituting an integral part of the entire electron transfer system, is expected to compete kinetically with the intra-monomer electron transfer. How this translates into the physiological operation of the enzyme, in particular in the context of its possible regulatory function and the role in reactive oxygen species generation, is currently unknown and requires further study.
All known cytochrome bc1 complexes are homodimers characterized by high structural symmetry between the monomers [3,28]. While the symmetry concerns both the architecture of cofactor chains and the amino-acid composition/structure of proteins, its mechanistic and structural roles are not clear. Our results show that functional in vivo complex assembles not only when the symmetry in the cofactor chains is broken, but also when the protein core is heterogeneous. This implies a key principle and minimal requirement to secure bioenergetic efficiency: the chains of cofactors must support catalytically-competent electron transfer between the quinone binding sites located on the opposite sides of the membrane. Once this is achieved, the enzyme appears to be able to use in vivo any of the paths that are available (intra- or inter-monomer electron transfer) in a manner robustly tolerant to structural alterations.
Acknowledgments
This work was supported by The Wellcome Trust International Senior Research Fellowship (to AO, grant number 095078/Z/10/Z) and by funding from the Jagiellonian University within the SET project co-financed by the European Union (to RE).
Appendix A. Supplementary data
This document contains supplementary information.
References
- 1.Berry E.A., Guergova-Kuras M., Huang L., Crofts A.R. Structure and function of cytochrome bc complexes. Annu. Rev. Biochem. 2000;69:1005–1075. doi: 10.1146/annurev.biochem.69.1.1005. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P. The protonmotive Q cycle: a general formulation. FEBS Lett. 1975;59:137–139. doi: 10.1016/0014-5793(75)80359-0. [DOI] [PubMed] [Google Scholar]
- 3.Berry E.A., Huang L.-S., Saechao L.K., Pon N.G., Valkova-Valchanova M., Daldal F. X-ray structure of Rhodobacter capsulatus cytochrome bc1: comparison with its mitochondrial and chloroplast counterparts. Photosynth. Res. 2004;81:251–275. doi: 10.1023/B:PRES.0000036888.18223.0e. [DOI] [PubMed] [Google Scholar]
- 4.Hunte C., Koepke J., Lange C., Rossmanith T., Michel H. Structure at 2.3Å resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure. 2000;8:669–684. doi: 10.1016/s0969-2126(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 5.Xia D., Yu C.A., Kim H., Xia J.Z., Kachurin A.M., Zhang L. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PubMed] [Google Scholar]
- 6.Osyczka A., Moser C.C., Daldal F., Dutton P.L. Reversible redox energy coupling in electron transfer chains. Nature. 2004;427:607–612. doi: 10.1038/nature02242. [DOI] [PubMed] [Google Scholar]
- 7.Gopta O.A., Feniouk B.A., Junge W., Mulkidjanian A.Y. The cytochrome bc1 complex of Rhodobacter capsulatus: ubiquinol oxidation in dimeric Q-cycle? FEBS Lett. 1998;431:291–296. doi: 10.1016/s0014-5793(98)00768-6. [DOI] [PubMed] [Google Scholar]
- 8.Covian R., Trumpower B.L. Regulatory interactions in the dimeric cytochrome bc1 complex: the advantages of being a twin. Biochim. Biophys. Acta. 2008;1777:1079–1091. doi: 10.1016/j.bbabio.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooley J.W., Lee D.-W., Daldal F. Across membrane communication between the Qo and Qi active sites of cytochrome bc1. Biochemistry. 2009;48:1888–1899. doi: 10.1021/bi802216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong X., Yu L., Xia D., Yu C.-A. Evidence for electron equilibrium between the two hemes bL in the dimeric cytochrome bc1 complex. J. Biol. Chem. 2005;280:9251–9257. doi: 10.1074/jbc.M409994200. [DOI] [PubMed] [Google Scholar]
- 11.Shinkarev V.P., Wraight C.A. Intermonomer electron transfer in the bc1 complex dimer is controlled by the energized state and by impaired electron transfer between low and high potential hemes. FEBS Lett. 2007;581:1535–1541. doi: 10.1016/j.febslet.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Świerczek M., Cieluch E., Sarewicz M., Borek A., Moser C.C., Dutton P.L. An electronic bus bar lies in the core of cytochrome bc1. Science. 2010;329:451–454. doi: 10.1126/science.1190899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanciano P., Lee D.-W., Yang H., Darrouzet E., Daldal F. Intermonomer electron transfer between the low-potential b hemes of cytochrome bc1. Biochemistry. 2011;50:1651–1663. doi: 10.1021/bi101736v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czapla M., Borek A., Sarewicz M., Osyczka A. Enzymatic activities of isolated cytochrome bc1-like complexes containing fused cytochrome b subunits with asymmetrically inactivated segments of electron transfer chains. Biochemistry. 2012;51:829–835. doi: 10.1021/bi2016316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czapla M., Cieluch E., Borek A., Sarewicz M., Osyczka A. Catalytically-relevant electron transfer between two hemes bL in the hybrid cytochrome bc1-like complex containing a fusion of Rhodobacter sphaeroides and capsulatus cytochromes b. Biochim. Biophys. Acta. 2013;1827:751–760. doi: 10.1016/j.bbabio.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanciano P., Khalfaoui-Hassani B., Selamoglu N., Daldal F. Intermonomer electron transfer between the b hemes of heterodimeric cytochrome bc1. Biochemistry. 2013;52:7196–7206. doi: 10.1021/bi400561e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong S., Victoria D., Crofts A. Inter-monomer electron transfer is too slow to compete with monomeric turnover in bc1 complex. Biochim. Biophys. Acta Bioenerg. 1817;2012:1053–1062. doi: 10.1016/j.bbabio.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalfaoui-Hassani B., Lanciano P., Lee D.-W., Darrouzet E., Daldal F. Recent advances in cytochrome bc1: inter monomer electronic communication? FEBS Lett. 2012;586:617–621. doi: 10.1016/j.febslet.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czapla M., Borek A., Sarewicz M., Osyczka A. Fusing two cytochromes b of Rhodobacter capsulatus cytochrome bc1 using various linkers defines a set of protein templates for asymmetric mutagenesis. Protein Eng. Des. Sel. 2012;25:15–25. doi: 10.1093/protein/gzr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atta-Asafo-Adjei E., Daldal F. Size of the amino acid side chain at position 158 of cytochrome b is critical for an active cytochrome bc1 complex and for photosynthetic growth of Rhodobacter capsulatus. Proc. Natl. Acad. Sci. U.S.A. 1991;88:492–496. doi: 10.1073/pnas.88.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valkova-Valchanova M.B., Saribas A.S., Gibney B.R., Dutton P.L., Daldal F. Isolation and characterization of a two-subunit cytochrome b–c1 subcomplex from Rhodobacter capsulatus and reconstitution of its ubihydroquinone oxidation (Qo) site with purified Fe–S protein subunit. Biochemistry. 1998;37:16242–16251. doi: 10.1021/bi981651z. [DOI] [PubMed] [Google Scholar]
- 22.Osyczka A., Dutton P.L., Moser C.C., Darrouzet E., Daldal F. Controlling the functionality of cytochrome c1 redox potentials in the Rhodobacter capsulatusbc1 complex through disulfide anchoring of a loop and a β-branched amino acid near the heme-ligating methionine. Biochemistry. 2001;40:14547–14556. doi: 10.1021/bi011630w. [DOI] [PubMed] [Google Scholar]
- 23.Esser L., Elberry M., Zhou F., Yu C.-A., Yu L., Xia D. Inhibitor-complexed structures of the cytochrome bc1 from the photosynthetic bacterium Rhodobacter sphaeroides. J. Biol. Chem. 2008;283:2846–2857. doi: 10.1074/jbc.M708608200. [DOI] [PubMed] [Google Scholar]
- 24.Ding H., Moser C.C., Robertson D.E., Tokito M.K., Daldal F., Dutton P.L. Ubiquinone pair in the Qo site central to the primary energy conversion reactions of cytochrome bc1 complex. Biochemistry. 1995;34:15979–15996. doi: 10.1021/bi00049a012. [DOI] [PubMed] [Google Scholar]
- 25.Gray K.A., Dutton P.L., Daldal F. Requirement of histidine 217 for ubiquinone reductase activity (Qi site) in the cytochrome bc1 complex. Biochemistry. 1994;33:723–733. doi: 10.1021/bi00169a014. [DOI] [PubMed] [Google Scholar]
- 26.Khalfaoui-Hassani B., Lanciano P., Daldal F. A robust genetic system for producing heterodimeric native and mutant cytochrome bc1. Biochemistry. 2013;52:7184–7195. doi: 10.1021/bi400560p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kujoth G.C., Bradshaw P.C., Haroon S., Prolla T.A. The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet. 2007;3:161–173. doi: 10.1371/journal.pgen.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia D., Esser L., Tang W.-K., Zhou F., Zhou Y., Yu L. Structural analysis of cytochrome bc1 complexes: implications to the mechanism of function. Biochim. Biophys. Acta. 2013;1827:1278–1294. doi: 10.1016/j.bbabio.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document contains supplementary information.