Abstract
Topographic organization is a hallmark of sensory cortical organization. Topography is robust at spatial scales ranging from hundreds of microns to centimeters, but can dissolve at the level of neighboring neurons or subcellular compartments within a neuron. This dichotomous spatial organization is especially pronounced in the mouse auditory cortex, where an orderly tonotopic map can arise from heterogeneous frequency tuning between local neurons. Here, we address a debate surrounding the robustness of tonotopic organization in the auditory cortex that has persisted in some form for over forty years. Drawing from various cortical areas, cortical layers, recording methodologies, and species, we describe how auditory cortical circuitry can simultaneously support a globally systematic, yet locally heterogeneous representation of this fundamental sound property.
A history of progress and controversy
The first evidence for a spatially organized representation of sound frequency at the level of the cerebral cortex (see Glossary) came from 19th century lesion experiments in dogs, in which specific behavioral deficits in discriminating low, middle, or high pitch sounds were attributed to the location of focal ablations along the posterior-anterior extent of perisylvian cortex [1,2]. A neurophysiological demonstration of cochleotopy was provided decades later by recording evoked potentials from the surface of the cortex while electrically stimulating a restricted set of auditory nerve fibers that innervated apical (low frequency) versus basal (high frequency) regions of the cochlea [3]. These experiments revealed an apical-to-basal organization along the posterior-to-anterior extent of the middle ectosylvian area of the cat that was subsequently matched to a tonotopic organization when electrical stimulation was replaced with airborne tone burst stimuli [4].
The advent of the microelectrode in the latter half of the 20th century (see Box 1) ushered in a period of great productivity – as well as controversy – for early efforts to characterize the functional organization of auditory cortex (ACX) (see Glossary). Most research labs gravitated towards an approach that involved systematic sampling of multiunit or single unit activity from the middle cortical layers of anesthetized animals at spatial densities ranging from 0.1 to 1 mm. These early efforts were successful in identifying the organization of multiple tonotopic and non-tonotopic cortical fields in the cat and primate and were also able to pinpoint locations of interest, such as boundaries between tonotopic and non-tonotopic fields or circumscribed modules with particular tuning properties, which were then used to guide the placement of neuroanatomical tracers [5–10]. These initial studies established the contemporary framework for how cortical fields are parceled, where they receive inputs from and send outputs to, and where they might sit within a distributed network of auditory information processing. On the other hand, careful study of the same cortical regions by other laboratories during this period found only weak evidence for a tonotopic organization, arguing instead that frequency tuning at the level of the auditory cortex was heterogeneous or strongly modulated by cognitive factors such as attention [11–13].
Box 1. Methods of measuring brain activity.
Extracellular electrophysiological recordings are conducted by inserting a microelectrode into the area of interest. The uninsulated contact measures currents that are produced by neuronal activity in its neighborhood. The signal recorded by such electrode can be filtered to reveal relatively slow fluctuations, called local field potentials (LFP) that represent mostly synaptic currents, and fast fluctuations (<1 ms) that are caused by spikes in nearby neurons. Electrodes can be engineered to record spikes from many neurons (multiunit activity) or only currents from very close neurons, in which case it corresponds to the activity of a single neuron. While extracellular recordings are considered to be the ‘ground truth’ for understanding neuronal responses, they are limited by being blind – the experimenter cannot select the neurons from which to record, and has relatively coarse control about the layer in which the neuronal activity is measured. In addition, microelectrode recordings are informative only about the activity in small extents of the cortical area, and in order to generate a tonotopic map in auditory cortex, multiple electrode penetrations are necessary (a few tens to hundreds of penetrations, depending on the species). Thus, optical imaging methods have been developed in order to either increase the cellular resolution (calcium imaging) or to gain information about large extents of the cortex (intrinsic signal imaging).
Calcium imaging is based on the fact that calcium concentration in neurons is extremely low, and that calcium entry invariably follows the generation of an action potential in neuronal cell bodies, through the activation of voltage-sensitive calcium channels. Calcium indicators are molecules that fluoresce in the presence of calcium. Two important technological advances underlie the use of calcium imaging in-vivo. On the one hand, modern techniques make it possible to introduce calcium indicators into multiple neurons in the tissue. Such techniques include the use of the so-called AM-dyes, which are injected into the extracellular space and are taken by the cells in the tissue; and the use of genetically-encoded calcium indicators, introduced through viral injection or by using transgenic techniques. The second advance is the use of 2-photon scanning microscopy, which made it possible to spatially resolve calcium signals to subcellular levels. Using both methods in conjunction, it became possible to image tens to hundreds of neurons simultaneously in small fields. The main drawbacks of calcium imaging is the fact that calcium signals reflect electrical activity only indirectly, with relatively slow dynamics that make it difficult to resolve single spikes; and the limitations imposed by light scattering, that currently limit calcium imaging to the superficial layers of the cortex.
At the other end of the range of useful spatial resolutions, intrinsic signal imaging uses the changes in reflectivity associated with the oxygenation level of the blood and its amount in the tissue. By shining a light of a particular wavelength (540–700μm, depending on what component of the hemodynamic signal is emphasized in a particular experiment) on the cortex and imaging the reflection, it is possible to observe territories of the cortex that are active. Intrinsic signal imaging reflects neuronal activity even more indirectly than calcium imaging, since it depends on the changes in tissue oxygenation that accompany large cortical activations.
The discrepancies in these early findings, which likely stemmed from basic differences in experimental methodologies, were never fully resolved at the time. The heterogeneous frequency organization reported by the minority of these early studies gradually faded from view as auditory cortex research in the latter years of the 20th century became increasingly reliant upon microelectrode recordings from the thalamorecipient layers (see Glossary) of primary auditory areas in anesthetized animals, conditions that likely favor the appearance of precise tonotopy. However the debate over the degree of tonotopic mapping precision has reappeared in recent years, perhaps reflecting a shift toward experimental approaches that enable measurements at finer spatial scales, from other cortical layers, and other states of vigilance. The purpose of this review is to provide a foundation for understanding how this issue has been studied historically and then highlight very recent findings that may reconcile these differences and point the way toward new directions for auditory cortex research. This controversy in its different reincarnations also carries important lessons for the study of other cortical areas.
General principles of auditory cortex organization
Primary auditory areas are distinguished from secondary areas according to three criteria: First, they receive heavy input from the lemniscal, tonotopically organized (see Glossary) subdivision of the auditory thalamus, named the ventral subdivision of the medial geniculate body (MGBv) based on its anatomical location in cats; Second, they exhibit anatomical or neurochemical features consistent with primary sensory cortex such as koniocellular cytoarchitecture, dense myelination, and elevated expression levels of various molecules such as parvalbumin, cytochrome oxidase, and acetylcholinesterase; Lastly, they are tonotopically organized. Beginning with Woolsey and Rose’s seminal work in the cat [3, 14, 15], the existence and relative positioning of primary and secondary auditory areas have been identified according to one or more of these criteria in over twenty mammalian species (see [16] for review).
Three primary auditory areas have been identified in non-human primates, each separated from one another by mirror reversals in tonotopy: the primary auditory cortex (A1), the rostral area, and the rostrotemporal area (for review see [17]). Most rodents, carnivores and bats also have three primary areas separated by frequency reversals in the tonotopic gradients: A1, the anterior auditory field (AAF), and a posterior auditory field. In the auditory pallidum of birds, Field L exhibits many of the same features as A1, including a prominent input from nucleus ovoidalis, the presumed homolog of MGBv, [18], and a tonotopic organization[19–21].
While all workers in the field agree about the existence of a tonotopic organization in the primary fields of auditory cortex, how tight this organization is has been questioned in the past [22–24]. In the following sections, we will review the evidence for and against tonotopic order based on techniques that characterize the auditory cortex using a variety of neural signal types at various spatial scales and cortical depths.
Low resolution optical imaging reveals tonotopic order
Imaging methods (see Box 1) make it possible to visualize correlates of neural activity, such as hemodynamic responses, over large areas (many mm2) of the brain and thus enable the investigation of the functional representation of relevant stimulus features.
Compared to the successful application of intrinsic signal imaging in the visual cortex, its successful application in ACX has proven more difficult possibly due to the poor driven rates in superficial layers of ACX under deep barbiturate anesthesia [25–28], different hemodynamic responses in ACX because of different spatial layout of blood vessels [29], or due to more variable response properties of neurons within cortical columns in ACX. Interestingly, these difficulties mirrored early investigations of ACX organization with 2-deoxyglucose [30, 31], which had been utilized to great effect in visual cortex [32,33].
A number of approaches have been used to improve intrinsic signal quality in ACX and with these modifications, optical imaging of ACX demonstrated the presence of large-scale tonotopic maps in cats and a variety of rodent species [34–43]. In particular, using tone sequences and analyzing the timing of the resulting activations (a technique pioneered in visual cortex by Kalatsky and Stryker) turned out to be a useful tool for delineating tonotopic organization in core ACX [39, 44].
While intrinsic imaging has the advantage to reveal relatively quickly large-scale maps, the technique has several drawbacks. First, imaging hemodynamic responses biases the signal towards areas containing highly responsive cells that share similar tuning and that are located close to each other. Thus, areas where cells might be tuned very selectively but respond with only few spikes will not show strong optical activations using hemodynamic responses. Moreover, since the hemodynamic signal integrates across a volume and is biased towards superficial layers, laminar differences in processing cannot be resolved. Nevertheless, intrinsic optical imaging studies to a large degree confirmed the presence of tonotopic maps, within their limited resolution (which is not better than that of electrophysiological mapping using electrode penetrations).
The case of the mouse
Compared to humans, the hearing range of the mouse is significantly higher and nearly half as wide (in octaves, approximately 3 kHz to 100 kHz, about 5 octaves, as compared to 20 Hz to 16kHz, about 10 octaves). Despite these differences, the mouse is becoming an increasingly popular model for studies of the auditory cortex. Many of the newer imaging and optogenetic techniques have been pioneered in the mouse, and the availability of genetically modified mouse strains makes it possible to apply a large arsenal of molecular manipulations.
The tonotopic organization of fields A1 or AAF as well as several secondary auditory fields have been identified in the mouse auditory cortex using conventional microelectrode mapping of multiunit spiking in the thalamorecipient layers [26, 45–49] or low-resolution optical imaging of voltage-sensitive dye [50], flavoprotein autofluorescence [51, 52], and intrinsic signals [53]. Tonotopic organization in the middle layers of mouse A1 likely arises from topographically organized feedforward projections from the MGBv [45]; Fig. 1a). Point-to-point thalamocortical connectivity has also been demonstrated in an acute thalamocortical slice preparation that preserves synaptic connections between MGBv and A1[54–57]. For example, by bathing the brain slice in a voltage-sensitive dye, it has also been possible to demonstrate a point-to-point functional mapping between a discrete stimulation site within the MGBv and a focus of activity within the tonotopically aligned region of A1[58, 45].
Figure 1. Evidence for order: Large-scale tonotopy within the middle cortical layers of the mouse auditory cortex.
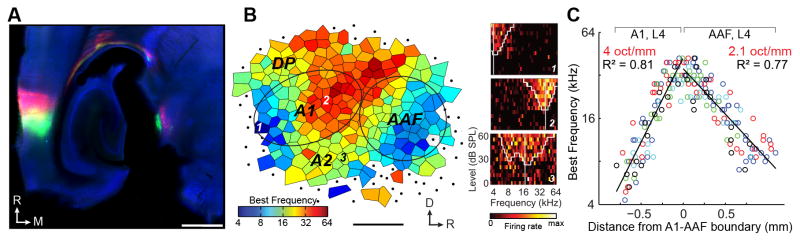
A) Auditory thalamocortical slice immunoreacted for parvalbumin (blue). Retrograde tracers (Cholera toxin β subunit) conjugated to a green or red fluorophore were injected into a low- (7 kHz) or mid-frequency (22.6 kHz) region of the A1 map, respectively. The A1 injection sites appear at the left of the image, the labeled thalamocortical axons in the middle of the image, and the retrogradely labeled MGBv cell bodies to the right of the image. Scale bar = 0.25 mm. B) A tessellated best frequency (frequency that elicits the most spikes across all levels) map delineated from 300 multiunit recording sites in the middle layers of the area identified in (A). Note the clear tonotopic gradient within A1 and AAF compared to the non-tonotopic organization of the remaining fields. Right, Tonal receptive fields from A1 (top and middle) and A2 (bottom) measured at the numbered locations shown on the tessellated map. C) Best frequency distribution along the caudal-to-rostral axis through A1 and AAF. Distance is relative to the mirror reversal in best frequency that indicates the boundary between each field. Data from individual mice are represented by different colors. Solid black lines indicate the linear fit of the A1 and AAF data. See [25] for further details regarding data in A–C
Thus, recent efforts to characterize the functional organization of the thalamorecipient layers through in vivo mapping (Fig. 1b–c), low-resolution in vivo imaging, in vitro functional connectivity studies, and anatomical connectivity studies all point towards a precisely organized gradient of sound frequency in A1 that arises from tonotopically organized projections from MGBv. The feedforward thalamocortical connectivity and tonotopic organization of the mouse auditory cortex are in close agreement with extensively studied model systems such as the rat [59] and the cat [60]. However, it is critical to note that all of these techniques are limited to a relatively coarse spatial resolution of 0.1 mm or more. As such, they can be used to reconstruct the macroscopic organization of the auditory cortex but offer very little insight into to finer scales of spatial organization that may exist between neighboring neurons.
High resolution imaging: beyond smooth tonotopy
While the results surveyed up to this point seems to have settled the issue of the existence of a tonotopic map in ACX, the picture has been muddled again when Ca2+ indicators such as Oregon Green Bapta-1 (OGB-1) have been introduced into neurons in live animals and in vivo Ca2+ signals have been measured with 2-photon imaging [61–64] (see Box 1). The Ca2+ signals are due to voltage-activated currents, and when measured from neuronal somata they reflect action potentials generation. The Ca2+ signals are somewhat slow (with a rise time of a few tens of ms and a decay of hundreds of ms), although they are much faster than intrinsic signals. While Ca2+ signals are typically too slow to document the occurrence of single action potentials, in ACX they are roughly proportional to the number of action potentials that occurred within a window with a duration of 50–100 ms [65], and can therefore be used to document frequency selectivity in ACX neurons. The fluorescence evoked by Ca2+ entry into the neurons can be read in a number of different ways. The highest spatial resolution is achieved by using 2-photon scanning microscopy, which sequentially illuminates only a small voxel (~1 femtoliter, depending on illumination wavelength and the numerical aperture of the objective) of brain tissue. In vivo, the technique makes it possible to record transients from single neurons and even from subcellular structures (dendrites, [66], and even spines, [67]). Furthermore, the sparse illumination prevents bleaching of out of focus focal planes [61, 62]. By sequentially imaging many voxels in one imaging plane rapidly, it is possible to sample the Ca2+ signals from many neurons essentially simultaneously and therefore create activity maps with single cell resolution. Because of light scattering in brain tissue, initial implementation of this technique was focused on imaging activity in supragranular layers [61–64].
A highly influential application of this technique to primary visual cortex (V1) revealed species differences in its micro-organization. While cats had orientation columns, rodents showed a salt and pepper organization of single-neuron orientation preference [63, 68] and substantial heterogeneity of spatial receptive fields both within a tangential imaging plane [69] and across a cortical column [70]. Similar studies in rodent S1 revealed that at the neuronal population level whisker selectivity varied smoothly over the cortical surface but that whisker selectivity of neighboring neurons could differ considerably [71, 72].
In vivo 2-photon imaging in the supragranular layers of mouse A1 [65, 73] demonstrated that sound evoked Ca2+ transients could be reliably measured from single neurons. Individual neurons responded to sounds and were frequency- and sound level- selective. Unexpectedly, the frequency selectivity of neighboring neurons was often very different (Figs. 2a, b), and over spatial scales of <200 μm no reliable tonotopic gradient could be observed, despite the fact that in the best published maps of mouse auditory cortex [47] the frequency gradient of A1 is about 2–4 oct/mm. However, when multiple fields separated by more than about 200 μm were imaged in the same animal, the expected shift in average frequency preference was observed [65, 73] (Fig. 2a). Best frequency was the only response property that showed even approximate spatial order in these experiments. Other stimulus properties, such as bandwidth, produced highly heterogeneous distributions of preferences [73].
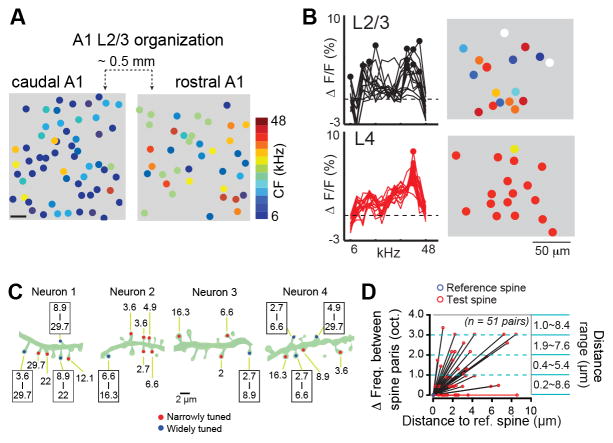
Figure 2. Evidence for disorder: Single cell imaging shows heterogeneity in supragranular layers.
A) Reconstruction of two imaging sites from layer 2/3 in one mouse. Characteristic frequency (CF, frequency tuning at threshold) for each cell illustrates both the local heterogeneity at local scales and a coarse tonotopic organization at larger spatial scales (for further details, see [7]). Scale bar 10um. B) Fractional changes in fluorescence measured from a single imaging site in layer 2/3 and a second imaging site from layer 4 of the same column illustrates the shift from homogeneous to heterogenous frequency tuning between the thalamic input layers and superficial layers (for further details, see [75]. The precise low-resolution tonotopy observed with microelectrode recordings from layer 4 (Fig. 1c) is therefore well matched with the coarse tonotopy over large spatial scales in layer 2/3 (Fig. 2a) and the similar frequency tuning organization within local layer 4 ensembles (Fig. 2a, bottom). C) The bandwidth and center frequency of Ca2+ response-based sound tuning between neighboring spines on a single dendrite are highly heterogenous. Cartoons depict dendritic segments from four layer 2/3 neurons, with numbers indicating the effective range of tone frequencies for each spine. Narrowly tuned and widely tuned spines are indicated by red and blue dots, respectively. D) Plot of the distance between neighboring sound-responsive spines versus their best frequency (for further details, see [67]).
These data, representing the integrated somatic responses, have receive support from in vivo imaging experiments that measured the frequency tuning of single spines of layer 2/3 neurons using in fast 2 photon Ca2+-imaging. These experiments showed that individual spines on single layer 2/3 neurons could be tuned to very different frequencies [67] (2c, d). Moreover these differences were observed even among neighboring spines on the same dendritic segment, suggesting a salt and pepper organization of synaptic tuning on a single layer 2/3 neurons.
This is not to say that there is no evidence for significant local order in ACX when using Ca2+ imaging. Three types of evidence to that effect emerged from these studies. First, while responses of simultaneously imaged cells were heterogeneous, neurons did show high noise correlation suggesting that they might form interconnected networks [65, 73, 74]. These noise correlations decreased with distance between neurons, with a spatial scale of about 100 microns [65, 75] (see also [76]), suggesting that neuronal interactions are organized within anatomical columns. Second, different Ca2+ dyes have different affinity for Ca2+ and thus can report different aspects of the neural response. The widely used indicator OGB-1 is a high affinity indicator and can report both subthreshold and suprathreshold signals, at least under in vitro conditions [73]. Thus a fraction of the imaged fluorescence signal can reflect synaptic inputs to neurons, rather than their spiking activity. Moreover since in ACX spike rates are relatively low compared to V1, the fraction of subthreshold responses in the OGB-1 signal is higher than in V1. On the other hand Fluo-4 has a low Ca2+ affinity, and thus does not report subthreshold responses [72, 73]. When the spatial distribution of frequency selectivity was studied by using either dye it was observed that the responses were more spatially homogenous with OGB-1 than with Fluo-4, suggesting that the additional subthreshold contribution increased spatial homogeneity possibly due to the wide frequency range of synaptic inputs to layer 2/3 [67]. Since a large fraction of inputs to supragranular layer 2/3 neurons originates in layer 4, these results suggested that layer 4 might be more homogeneously organized in frequency than layers 2/3. The third type of evidence for local order in A1 comes from recent in vivo 2-photon Ca2+ imaging of layer 4 and layer 2/3 neurons in the same animal. This study demonstrated directly that the representation of frequency is much more homogeneous in layer 4 than in layer 2/3 [75] (Fig. 2b).
Comparing different methodologies
Which picture of the tonotopic organization of ACX is the valid one? Is it the smooth tonotopic organization that emerges from low resolution imaging and microelectrode mapping, or is it the heterogeneous organization that emerges from 2-photon imaging? Or may both pictures be different approximations to the same reality?
Many of the differences between the different methodologies are likely due to increased spatial resolution of 2-photon imaging over electrophysiological methods as well as different sampling biases. 2-photon imaging has a spatial resolution of ~1 μm, while electrophysiological mapping experiments sample at 50–100 μm and intrinsic optical signals represent activity on even coarser spatial scales. Thus a lot of the heterogeneity that is seen on very small spatial scales is not readily accessible to electrophysiological methods. The cortical layer from which the neurons have been recorded is difficult to identify from electrode depth readings, and essentially no modern mapping study in ACX attempted to precisely localize the recording sites using lesions. This factor is of concern, especially in small rodents, as evidence of layer-specificity of the neuronal responses in ACX accumulates fast [75, 77–82]. Of particular relevance is the observation that sound-evoked spiking responses of ACX are sparse [79, 83–84], and that responses of layer 2/3 neurons are weaker driven and sparser than layer 4 neurons [75, 79], and are more likely to have irregular tuning for pure tone bursts, the stimuli used to characterize tonotopic organization [26]. Thus, electrophysiological mapping is presumably biased towards the responses of layer 4 neurons, with this bias possibly increasing due to anesthesia or state changes [84].
Comparing the biases of the different methods is more difficult. Electrophysiological mapping experiments are most often done using multiunit recordings, without special attempt to separate the activity of single neurons. They are therefore biased towards the most robustly driven neurons, which dominate the multiunit signal. In consequence, weakly driven cells or neurons that do not generate large extracellular potentials will be less represented. 2-photon imaging on the other hand introduces other biases. Since inhibitory cells buffer Ca2+, these cells will not generate large Ca2+-transients. Moreover, when using synthetic dyes there can be loading differences over an imaged area as well as intermingled loading of neurons and astrocytes. To compensate for the difference in baseline fluorescence typically the fractional change in fluorescence is often used. However, potential differences in loading could lead to different recording qualities in different cells. Imaging data have to be interpreted carefully, as calcium transients reliably reported action potentials only when the optical plane intersected with the center of the soma [65, 72]. These factors could artificially increase the variability of the neuronal response areas measured with calcium imaging techniques.
The olive branch
Because of these methodological issues, we currently favor a view that integrates both sets of results into a common framework. This framework should be considered as a working hypothesis, to guide and be refined by future experiments. In this framework, tonotopy is the major organizational principle of the input to A1, even in mice.
There is clear evidence for a tonotopically organized forebrain region in mammals and birds, in which the auditory transduction organ convert sound frequency into a cochleotopic gradient of electrical activity. In this sense, tonotopy may be an epiphenomenon of an ancient organizing principle that predates the evolutionary split between mammals and birds; namely, neurons make topographic projections to other neurons. In the case of tonotopy, the spatial frequency gradient constructed by the auditory periphery is largely preserved throughout the lemniscal divisions of the ascending central auditory pathways, ultimately culminating in tonotopically organized MGv projections to middle layers of the primary auditory areas, where it can be reconstructed through microelectrode recordings.
However, as in other cortices, notably somatosensory cortex, the projections from layer 4 to layer 2/3 are divergent, and the same neuron may receive very different frequency-specific inputs [67] (Fig. 2c, d). Under the appropriate conditions, such neurons may still be reasonably narrowly tuned to frequency (see [85] for orientation selectivity in visual cortex under similar conditions), but now neighboring neurons may show very different frequency tunings, even though they share substantial amount of input (as indexed by their noise correlations) [65, 74, 75]. This diversity is specifically reflected in the 2-photon studies of neuronal responses in the supragranular layers [75].
A transition from precise, homogenous frequency organization in layer 4 to coarse, diffuse organization in layer 2/3 has also been described in recent low-resolution imaging and microelectrode recording studies. In the thalamocortical slice preparation, moving a stimulating electrode from low- to high-frequency areas of the MGBv reveals an orderly march of voltage-sensitive dye response peaks across the low-to-high frequency extent of A1 in layer 4, yet the topography is significantly degraded in layer 2/3 [45]. Moreover, the precisely organized frequency gradient commonly observed with microelectrode mapping from layer 4 (Fig. 1c) is substantially degraded when tonotopy is reconstructed from layer 2/3 recording sites [26]. Thus, approaches to characterize functional organization at low and high spatial resolution have converged on a laminar transformation from homogenous frequency tuning in the thalamorecipient layers to distributed, heterogenous frequency tuning in superficial layers.
Lessons to other sensory systems
The rapidly increasing information about fine structure of the representations in a number of sensory cortices suggest that all sensory cortices share many similarities, but also show significant differences. Studies in mouse V1 showed that while retinotopy was quite robust on large scales, it was heterogeneous on small scales [86]. This heterogeneity with respect to the organization of the periphery receptor might be an organizing feature of at least mouse layer 2/3 [87]. Nevertheless, the functional heterogeneity in layer 2/3 of ACX seems to be more pronounced than in V1. This could be due to the fact that in contrast to visual objects, auditory objects often co-activate distant frequency channels and are thus less likely to be adequately represented by narrowly-tuned, tonotopically-organized sheet. This difference between the physics of auditory objects on the one hand and visual objects on the other hand may be crucial for understanding A1, as well as in directing our attempt to elucidate its function. It has been suggested that the relatively short intra-cortical connectivity length is an important organizational principle of the brain [88, 89]. One possible consequence of this principle is that locally interconnected neurons code for the ethologically relevant entities (‘auditory objects’) that arise from auditory processing. Thus, elucidating the functional properties of neighboring neurons and of the interactions between them is a way to identify what features A1 encodes. For example, neighboring interconnected neurons may be individually activated by sound frequencies with certain frequency relationships; as an ensemble, they could then encode a complex sound feature. Thus by investigating the relationship of tuning properties of local populations, taking into account both order and heterogeneity of these properties, we might be able to infer what A1 can encode.
Lessons to other species
Much of the tonotopy controversy in its most recent reincarnation was centered around the mouse model of auditory cortex. It could be that the small brain size of mice does not support homogeneous organization by sensory maps. While the cortical micro-organization of small carnivores has not been examined, both small and large rodents lack orientation maps in V1 [63, 90] suggesting that rodents and carnivores might have evolved different cortical processing strategies. However, local diversity in V1 response properties may not be restricted to smaller brains. Paired extracellular or intracellular recordings in cat V1 have also shown considerable receptive field heterogeneity between neighboring neurons [91, 92].
The picture in the ACX is less clear. Early electrophysiological evidence in cats [22, 24] as well as more recent data in ferrets [93] suggest the presence of local disorder in carnivore ACX as well. On the other hand, a recent study of micro-organization based in A1 of cats observed that neighboring neurons, particularly in the supragranular layers, were precisely synchronized with highly similar receptive field properties for stimulus features related to sound frequency [77]. Thus, more work is required to determine whether the mixture of homogeneity and heterogeneity in early sensory cortices is a general principle of mammalian processing or might be exaggerated in small rodents either as an evolutionary adaptation or as a byproduct of cortical wiring constraints in a physically smaller brain.
Conclusions
As spatial resolution of experimental techniques allow us to observe more neurons in small areas of the brain a level of heterogeneity becomes obvious that has not been appreciated with traditional low resolution techniques. While the smooth cortical organization uncovered at low resolution scales has provided an essential framework for understanding the organization and plasticity of primary sensory cortex, dynamic interactions between local cortical assemblies await discovery with approaches that reconstruct cortical circuits with cellular resolution. In particular, the interplay between homogeneity and heterogeneity in the organization of primary auditory cortex may give rise to a combined picture that demonstrate how the two can co-exist, and how the interplay between the two is crucial for understanding hearing, sensory processing in general, and possibly other brain functions as well.
Box 2. Outstanding questions.
What circuits give rise to the heterogeneous organization in layer 2/3?
What are the functional relationships between neighboring cells in layer 2/3?
What is encoded by layer 2/3 neurons?
What is the relationship between tuning properties and local circuit connectivity?
What are the specific roles of identified classes of neurons in shaping order and disorder in A1?
Are there species-specific differences in A1 organization?
Highlights.
Topographic organization is a hallmark of sensory cortical organization.
Topography of sound frequency, tonotopy, in auditory cortex robust at large spatial scales ranging from hundreds of microns to centimeters
Tonotopy is not robust at the level of neighboring neurons or subcellular compartments within a neuron.
Auditory cortical circuitry can simultaneously support globally systematic, yet locally heterogeneous representations of fundamental sound properties.
Acknowledgments
POK is supported by NIH R01DC009607. DBP is supported by NIH R01DC009836. IN is supported by grants from the Israel Science Foundation (ISF), the US-Israel Binational Science Foundation (BSF), and the European Research Council (ERC Grant Agreement RATLAND-340063).
Glossary
- Cortex
The cortex (latin “bark”, “rind”) is the thin (about 1–2 mm thick) layer of neurons that cover the mammalian forebrain. Most of the cortex, including auditory cortex (ACX), is composed of multiple layers (up to 6) with different cellular morphology and connections. Cortical layers are grouped into the middle layer (the main thalamorecipient layer; often called also layer 4) that separates the supragranular and infragranular layers (above and below the thalamorecipient layer). The supragranular layers include layer 1, which is usually neuron-poor, and layers 2 and 3 which, in rodent ACX, are often referred to together as layer 2/3. The infragranular layers (5 and 6) have the major cortical projections to subcortical stations, including the thalamus
- Cortical fields; Core and Secondary areas
Differences between different parts of the cortex include differences in architecture, in connectivity, and in function. The part of the cortex which is dominated by auditory responses is named ‘auditory cortex’ (ACX). The auditory cortex can be subdivided again based on a number of criteria (see main text) into core areas (e.g., A1 and AAF) surrounded by secondary areas. Core areas are densely interconnected with the lemniscal division of the auditory thalamus although they get inputs from other subdivisions of the auditory thalamus as well. Secondary areas receive their predominant input from non-lemniscal, subdivisions of the auditory thalamus
- The thalamus and thalamocortical connections
The thalamus is a large forebrain nucleus with many subdivisions, and is the main gateway to the cortex. Sensory nuclei of the thalamus process input from lower parts of the brain and project to the sensory cortices. The main thalamic input usually reaches the middle cortical layers, although auditory thalamic axons branch in layer 6 as well (the ‘thalamorecipient layers’). Neurons from all layers may receive thalamic input, as long as their dendrites reach the thalamorecipient layers. The cortex projects back to the thalamus, usually to the same subnuclei of the thalamus that project to it. The auditory thalamus is composed mostly of the medial geniculate body (MGB), which itself has three major subdivisions, the ventral, medial and dorsal. The ventral subdivision (MGBv) is part of the core ascending auditory pathway, and is the major input to A1
- Topographic organization and tonotopy
In the cochlea, the auditory sensory organ of the inner ear, the sound is mechanically filtered into narrow frequency bands by the basilar membrane. The resulting sensitivity to a narrow frequency band is inherited by the hair cells that sit on the basilar membrane and by the auditory nerve fibers that innervate them. Most of the brainstem auditory structures are composed of neurons that inherit the narrow tuning of the cochlear input. Furthermore, neurons that have similar frequency tuning are grouped together, and the progression of best frequencies of the neurons is continuous along one axis of the structure. This organization is referred to as tonotopic organization or tonotopy. Tonotopy is kept in the core ascending auditory pathway, including in particular the MGBv
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larionow W. Ueber die musikalischen Centren des Geirns. Pflugers Archiv European Journal of Physiology. 1899;76:608–625. [Google Scholar]
- 2.Munk H. #x000DC;ber die Funktionen der Grosshirnrinde. Hirschwald; 1881. [Google Scholar]
- 3.Woolsey CN, Walzl EM. Topical projection of nerve fibers from local regions of the cochlea to the cerebral cortex of the cat. Bull Johns Hopkins Hosp. 1942;71:315–344. [Google Scholar]
- 4.Erulkar SD, Rose JE, Davies PW. Single unit activity in the auditory cortex of the cat. Bull Johns Hopkins Hosp. 1956;99:55–86. [PubMed] [Google Scholar]
- 5.Andersen RA, Snyder RL, Merzenich MM. The topographic organization of corticocollicular projections from physiologically identified loci in the AI, AII, and anterior auditory cortical fields of the cat. J Comp Neurol. 1980;191:479–494. doi: 10.1002/cne.901910310. [DOI] [PubMed] [Google Scholar]
- 6.Imig TJ, Brugge JF. Sources and terminations of callosal axons related to binaural and frequency maps in primary auditory cortex of the cat. J Comp Neurol. 1978;182:637–660. doi: 10.1002/cne.901820406. [DOI] [PubMed] [Google Scholar]
- 7.Imig TJ, Ruggero MA, Kitzes LM, Javel E, Brugge JF. Organization of auditory cortex in the owl monkey (Aotus trivirgatus) The Journal of comparative neurology. 1977;171:111–128. doi: 10.1002/cne.901710108. [DOI] [PubMed] [Google Scholar]
- 8.Merzenich MM, Brugge JF. Representation of the cochlear partition of the superior temporal plane of the macaque monkey. Brain Res. 1973;50:275–296. doi: 10.1016/0006-8993(73)90731-2. [DOI] [PubMed] [Google Scholar]
- 9.Merzenich MM, Knight PL, Roth GL. Representation of cochlea within primary auditory cortex in the cat. J Neurophysiol. 1975;38:231–249. doi: 10.1152/jn.1975.38.2.231. [DOI] [PubMed] [Google Scholar]
- 10.Reale RA, Imig TJ. Tonotopic organization in auditory cortex of the cat. J Comp Neurol. 1980;192:265–291. doi: 10.1002/cne.901920207. [DOI] [PubMed] [Google Scholar]
- 11.Evans EF, Whitfield IC. Classification of unit responses in the auditory cortex of the unanaesthetized and unrestrained cat. J Physiol. 1964;171:476–493. doi: 10.1113/jphysiol.1964.sp007391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein MH, Jr, Abeles M, Daly RL, McIntosh J. Functional architecture in cat primary auditory cortex: tonotopic organization. J Neurophysiol. 1970;33:188–197. doi: 10.1152/jn.1970.33.1.188. [DOI] [PubMed] [Google Scholar]
- 13.Hubel DH, Henson CO, Rupert A, Galambos R. Attention units in the auditory cortex. Science. 1959;129:1279–1280. doi: 10.1126/science.129.3358.1279. [DOI] [PubMed] [Google Scholar]
- 14.Rose JE, Woolsey CN. Organization of the mammalian thalamus and its relationships to the cerebral cortex. Electroencephalography and clinical neurophysiology. 1949a;1:391–403. discussion 403–394. [PubMed] [Google Scholar]
- 15.Rose JE, Woolsey CN. The relations of thalamic connections, cellular structure and evocable electrical activity in the auditory region of the cat. The Journal of comparative neurology. 1949b;91:441–466. doi: 10.1002/cne.900910306. [DOI] [PubMed] [Google Scholar]
- 16.Kaas JH. The evolution of auditory cortex: the core areas. In: Winer JA, Schreiner CE, editors. The Auditory Cortex. Springer; 2011. pp. 407–427. [Google Scholar]
- 17.Hackett TA. Information flow in the auditory cortical network. Hear Res. 2011;271:133–146. doi: 10.1016/j.heares.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karten HJ. The ascending auditory pathway in the pigeon (Columba livia). II. Telencephalic projections of the nucleus ovoidalis thalami. Brain research. 1968;11:134–153. doi: 10.1016/0006-8993(68)90078-4. [DOI] [PubMed] [Google Scholar]
- 19.Heil P, Scheich H. Quantitative analysis and two-dimensional reconstruction of the tonotopic organization of the auditory field L in the chick from 2-deoxyglucose data. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1985;58:532–543. doi: 10.1007/BF00235869. [DOI] [PubMed] [Google Scholar]
- 20.Muller CM, Leppelsack HJ. Feature extraction and tonotopic organization in the avian auditory forebrain. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1985;59:587–599. doi: 10.1007/BF00261351. [DOI] [PubMed] [Google Scholar]
- 21.Zaretsky MD, Konishi M. Tonotopic organization in the avian telencephalon. Brain research. 1976;111:167–171. doi: 10.1016/0006-8993(76)91058-1. [DOI] [PubMed] [Google Scholar]
- 22.Abeles M, Goldstein MH., Jr Functional architecture in cat primary auditory cortex: columnar organization and organization according to depth. J Neurophysiol. 1970;33:172–187. doi: 10.1152/jn.1970.33.1.172. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein MH, Jr, Abeles M. Note on tonotopic organization of primary auditory cortex in the cat. Brain Res. 1975;100:188–191. doi: 10.1016/0006-8993(75)90258-9. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein MH, Jr, Abeles M, Daly RL, McIntosh J. Functional architecture in cat primary auditory cortex: tonotopic organization. J Neurophysiol. 1970;33:188–197. doi: 10.1152/jn.1970.33.1.188. [DOI] [PubMed] [Google Scholar]
- 25.deCharms RC, Merzenich MM. Primary cortical representation of sounds by the coordination of action-potential timing. Nature. 1996;381:610–613. doi: 10.1038/381610a0. [DOI] [PubMed] [Google Scholar]
- 26.Guo W, Chambers AR, Darrow KN, Hancock KE, Shinn-Cunningham BG, Polley DB. Robustness of cortical topography across fields, laminae, anesthetic states, and neurophysiological signal types. J Neurosci. 2012;32:9159–9172. doi: 10.1523/JNEUROSCI.0065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 28.Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Harrison RV, Harel N, Panesar J, Mount RJ. Blood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortex. Cereb Cortex. 2002;12:225–233. doi: 10.1093/cercor/12.3.225. [DOI] [PubMed] [Google Scholar]
- 30.Hungerbuhler JP, Saunders JC, Greenberg J, Reivich M. Functional neuroanatomy of the auditory cortex studied with [2–14C] Deoxyglucose. Exp Neurol. 1981;71:104–121. doi: 10.1016/0014-4886(81)90074-1. [DOI] [PubMed] [Google Scholar]
- 31.Webster WR, Serviere J, Batini C, Laplante S. Autroradiographic demonstration with 2-[(14)C]deoxyglucose of frequency selectivity in the auditory system of cats under conditions of functional activity. Neurosci Lett. 1978;10:43–48. doi: 10.1016/0304-3940(78)90009-5. [DOI] [PubMed] [Google Scholar]
- 32.Hubel DH, Wiesel TN, Stryker MP. Orientation columns in macaque monkey visual cortex demonstrated by the 2-deoxyglucose autoradiographic technique. Nature. 1977;269:328–330. doi: 10.1038/269328a0. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy C, Des Rosiers MH, Sakurada O, Shinohara M, Reivich M, Jehle JW, Sokoloff L. Metabolic mapping of the primary visual system of the monkey by means of the autoradiographic [14C]deoxyglucose technique. Proc Natl Acad Sci U S A. 1976;73:4230–4234. doi: 10.1073/pnas.73.11.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakin JS, Kwon MC, Masino SA, Weinberger NM, Frostig RD. Suprathreshold auditory cortex activation visualized by intrinsic signal optical imaging. Cereb Cortex. 1996;6:120–130. doi: 10.1093/cercor/6.2.120. [DOI] [PubMed] [Google Scholar]
- 35.Dinse HR, Godde B, Hilger T, Reuter G, Cords SM, Lenarz T, von Seelen W. Optical imaging of cat auditory cortex cochleotopic selectivity evoked by acute electrical stimulation of a multi-channel cochlear implant. Eur J Neurosci. 1997;9:113–119. doi: 10.1111/j.1460-9568.1997.tb01359.x. [DOI] [PubMed] [Google Scholar]
- 36.Harel N, Mori N, Sawada S, Mount RJ, Harrison RV. Three distinct auditory areas of cortex (AI, AII, and AAF) defined by optical imaging of intrinsic signals. Neuroimage. 2000;11:302–312. doi: 10.1006/nimg.1999.0537. [DOI] [PubMed] [Google Scholar]
- 37.Harrison RV, Harel N, Kakigi A, Raveh E, Mount RJ. Optical imaging of intrinsic signals in chinchilla auditory cortex. Audiol Neurootol. 1998;3:214–223. doi: 10.1159/000013791. [DOI] [PubMed] [Google Scholar]
- 38.Hess A, Scheich H. Optical and FDG mapping of frequency-specific activity in auditory cortex. Neuroreport. 1996;7:2643–2647. doi: 10.1097/00001756-199611040-00047. [DOI] [PubMed] [Google Scholar]
- 39.Kalatsky VA, Polley DB, Merzenich MM, Schreiner CE, Stryker MP. Fine functional organization of auditory cortex revealed by Fourier optical imaging. Proc Natl Acad Sci U S A. 2005;102:13325–13330. doi: 10.1073/pnas.0505592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langner G, Dinse HR, Godde B. A map of periodicity orthogonal to frequency representation in the cat auditory cortex. Front Integr Neurosci. 2009;3:27. doi: 10.3389/neuro.07.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulze H, Hess A, Ohl FW, Scheich H. Superposition of horseshoe-like periodicity and linear tonotopic maps in auditory cortex of the Mongolian gerbil. Eur J Neurosci. 2002;15:1077–1084. doi: 10.1046/j.1460-9568.2002.01935.x. [DOI] [PubMed] [Google Scholar]
- 42.Spitzer MW, Calford MB, Clarey JC, Pettigrew JD, Roe AW. Spontaneous and stimulus-evoked intrinsic optical signals in primary auditory cortex of the cat. J Neurophysiol. 2001;85:1283–1298. doi: 10.1152/jn.2001.85.3.1283. [DOI] [PubMed] [Google Scholar]
- 43.Versnel H, Mossop JE, Mrsic-Flogel TD, Ahmed B, Moore DR. Optical imaging of intrinsic signals in ferret auditory cortex: responses to narrowband sound stimuli. J Neurophysiol. 2002;88:1545–1558. doi: 10.1152/jn.2002.88.3.1545. [DOI] [PubMed] [Google Scholar]
- 44.Nelken I, Bizley JK, Nodal FR, Ahmed B, Schnupp JW, King AJ. Large-scale organization of ferret auditory cortex revealed using continuous acquisition of intrinsic optical signals. J Neurophysiol. 2004;92:2574–2588. doi: 10.1152/jn.00276.2004. [DOI] [PubMed] [Google Scholar]
- 45.Hackett TA, Barkat TR, O’Brien BM, Hensch TK, Polley DB. Linking topography to tonotopy in the mouse auditory thalamocortical circuit. J Neurosci. 2011;31:2983–2995. doi: 10.1523/JNEUROSCI.5333-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H, Gibboni R, Kirkhart C, Bao S. Impaired critical period plasticity in primary auditory cortex of fragile X model mice. J Neurosci. 2013;33:15686–15692. doi: 10.1523/JNEUROSCI.3246-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stiebler I, Neulist R, Fichtel I, Ehret G. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. J Comp Physiol A. 1997;181:559–571. doi: 10.1007/s003590050140. [DOI] [PubMed] [Google Scholar]
- 48.Yang S, Zhang LS, Gibboni R, Weiner B, Bao S. Impaired Development and Competitive Refinement of the Cortical Frequency Map in Tumor Necrosis Factor-alpha-Deficient Mice. Cereb Cortex. 2013 doi: 10.1093/cercor/bht053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Dyck RH, Hamilton SE, Nathanson NM, Yan J. Disrupted tonotopy of the auditory cortex in mice lacking M1 muscarinic acetylcholine receptor. Hear Res. 2005;201:145–155. doi: 10.1016/j.heares.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Sawatari H, Tanaka Y, Takemoto M, Nishimura M, Hasegawa K, Saitoh K, Song WJ. Identification and characterization of an insular auditory field in mice. Eur J Neurosci. 2011;34:1944–1952. doi: 10.1111/j.1460-9568.2011.07926.x. [DOI] [PubMed] [Google Scholar]
- 51.Horie M, Tsukano H, Hishida R, Takebayashi H, Shibuki K. Dual compartments of the ventral division of the medial geniculate body projecting to the core region of the auditory cortex in C57BL/6 mice. Neurosci Res. 2013;76:207–212. doi: 10.1016/j.neures.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Kubota Y, Kamatani D, Tsukano H, Ohshima S, Takahashi K, Hishida R, Shibuki K. Transcranial photo-inactivation of neural activities in the mouse auditory cortex. Neurosci Res. 2008;60:422–430. doi: 10.1016/j.neures.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Moczulska KE, Tinter-Thiede J, Peter M, Ushakova L, Wernle T, Bathellier B, Rumpel S. Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall. Proc Natl Acad Sci U S A. 2013;110:18315–18320. doi: 10.1073/pnas.1312508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- 55.de la Rocha J, Marchetti C, Schiff M, Reyes AD. Linking the response properties of cells in auditory cortex with network architecture: cotuning versus lateral inhibition. J Neurosci. 2008;28:9151–9163. doi: 10.1523/JNEUROSCI.1789-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaur S, Rose HJ, Lazar R, Liang K, Metherate R. Spectral integration in primary auditory cortex: laminar processing of afferent input, in vivo and in vitro. Neuroscience. 2005;134:1033–1045. doi: 10.1016/j.neuroscience.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 57.Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci. 2007;27:9417–9426. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–1194. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- 60.Lee CC, Imaizumi K, Schreiner CE, Winer JA. Concurrent tonotopic processing streams in auditory cortex. Cereb Cortex. 2004;14:441–451. doi: 10.1093/cercor/bhh006. [DOI] [PubMed] [Google Scholar]
- 61.Denk W, Delaney KR, Gelperin A, Kleinfeld D, Strowbridge BW, Tank DW, Yuste R. Anatomical and functional imaging of neurons using 2-photon laser scanning microscopy. J Neurosci Methods. 1994;54:151–162. doi: 10.1016/0165-0270(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 62.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 63.Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 64.Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rothschild G, Nelken I, Mizrahi A. Functional organization and population dynamics in the mouse primary auditory cortex. Nat Neurosci. 2010;13:353–360. doi: 10.1038/nn.2484. [DOI] [PubMed] [Google Scholar]
- 66.Jia H, Rochefort NL, Chen X, Konnerth A. In vivo two-photon imaging of sensory-evoked dendritic calcium signals in cortical neurons. Nat Protoc. 2011;6:28–35. doi: 10.1038/nprot.2010.169. [DOI] [PubMed] [Google Scholar]
- 67.Chen X, Leischner U, Rochefort NL, Nelken I, Konnerth A. Functional mapping of single spines in cortical neurons in vivo. Nature. 2011;475:501–505. doi: 10.1038/nature10193. [DOI] [PubMed] [Google Scholar]
- 68.Ohki K, Chung S, Kara P, Hubener M, Bonhoeffer T, Reid RC. Highly ordered arrangement of single neurons in orientation pinwheels. Nature. 2006;442:925–928. doi: 10.1038/nature05019. [DOI] [PubMed] [Google Scholar]
- 69.Bonin V, Histed MH, Yurgenson S, Reid RC. Local diversity and fine-scale organization of receptive fields in mouse visual cortex. J Neurosci. 2011;31:18506–18521. doi: 10.1523/JNEUROSCI.2974-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andermann ML, Gilfoy NB, Goldey GJ, Sachdev RN, Wolfel M, McCormick DA, Levene MJ. Chronic cellular imaging of entire cortical columns in awake mice using microprisms. Neuron. 2013;80:900–913. doi: 10.1016/j.neuron.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kerr JN, de Kock CP, Greenberg DS, Bruno RM, Sakmann B, Helmchen F. Spatial organization of neuronal population responses in layer 2/3 of rat barrel cortex. J Neurosci. 2007;27:13316–13328. doi: 10.1523/JNEUROSCI.2210-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sato TR, Gray NW, Mainen ZF, Svoboda K. The functional microarchitecture of the mouse barrel cortex. PLoS Biol. 2007;5:e189. doi: 10.1371/journal.pbio.0050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bandyopadhyay S, Shamma SA, Kanold PO. Dichotomy of functional organization in the mouse auditory cortex. Nat Neurosci. 2010;13:361–368. doi: 10.1038/nn.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rothschild G, Cohen L, Mizrahi A, Nelken I. Elevated correlations in neuronal ensembles of mouse auditory cortex following parturition. J Neurosci. 2013;33:12851–12861. doi: 10.1523/JNEUROSCI.4656-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winkowski DE, Kanold PO. Laminar transformation of frequency organization in auditory cortex. J Neurosci. 2013;33:1498–1508. doi: 10.1523/JNEUROSCI.3101-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levy RB, Reyes AD. Spatial profile of excitatory and inhibitory synaptic connectivity in mouse primary auditory cortex. J Neurosci. 2012;32:5609–5619. doi: 10.1523/JNEUROSCI.5158-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atencio CA, Schreiner CE. Laminar diversity of dynamic sound processing in cat primary auditory cortex. J Neurophysiol. 2010;103:192–205. doi: 10.1152/jn.00624.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oviedo HV, Bureau I, Svoboda K, Zador AM. The functional asymmetry of auditory cortex is reflected in the organization of local cortical circuits. Nat Neurosci. 2010;13:1413–1420. doi: 10.1038/nn.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64:404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun YJ, Kim YJ, Ibrahim LA, Tao HW, Zhang LI. Synaptic mechanisms underlying functional dichotomy between intrinsic-bursting and regular-spiking neurons in auditory cortical layer 5. J Neurosci. 2013;33:5326–5339. doi: 10.1523/JNEUROSCI.4810-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Y, Liu BH, Wu GK, Kim YJ, Xiao Z, Tao HW, Zhang LI. Preceding inhibition silences layer 6 neurons in auditory cortex. Neuron. 2010;65:706–717. doi: 10.1016/j.neuron.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou Y, Mesik L, Sun YJ, Liang F, Xiao Z, Tao HW, Zhang LI. Generation of spike latency tuning by thalamocortical circuits in auditory cortex. J Neurosci. 2012;32:9969–9980. doi: 10.1523/JNEUROSCI.1384-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hromadka T, Deweese MR, Zador AM. Sparse representation of sounds in the unanesthetized auditory cortex. PLoS Biol. 2008;6:e16. doi: 10.1371/journal.pbio.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakata S, Harris KD. Laminar-dependent effects of cortical state on auditory cortical spontaneous activity. Front Neural Circuits. 2012;6:109. doi: 10.3389/fncir.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hansel D, van Vreeswijk C. The mechanism of orientation selectivity in primary visual cortex without a functional map. J Neurosci. 2012;32:4049–4064. doi: 10.1523/JNEUROSCI.6284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith SL, Hausser M. Parallel processing of visual space by neighboring neurons in mouse visual cortex. Nat Neurosci. 2010;13:1144–1149. doi: 10.1038/nn.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaschube M. Neural maps versus salt-and-pepper organization in visual cortex. Current opinion in neurobiology. 2014;24:95–102. doi: 10.1016/j.conb.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 88.Braitenberg V, Schuetz A. Cortex: Statistics and Geometry of Neuronal Connectivity. Springer; 1991. [Google Scholar]
- 89.Chen BL, Hall DH, Chklovskii DB. Wiring optimization can relate neuronal structure and function. Proc Natl Acad Sci U S A. 2006;103:4723–4728. doi: 10.1073/pnas.0506806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Hooser SD, Heimel JA, Chung S, Nelson SB, Toth LJ. Orientation selectivity without orientation maps in visual cortex of a highly visual mammal. J Neurosci. 2005;25:19–28. doi: 10.1523/JNEUROSCI.4042-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DeAngelis GC, Ghose GM, Ohzawa I, Freeman RD. Functional micro-organization of primary visual cortex: receptive field analysis of nearby neurons. J Neurosci. 1999;19:4046–4064. doi: 10.1523/JNEUROSCI.19-10-04046.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin KA, Schroder S. Functional heterogeneity in neighboring neurons of cat primary visual cortex in response to both artificial and natural stimuli. J Neurosci. 2013;33:7325–7344. doi: 10.1523/JNEUROSCI.4071-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bizley JK, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cereb Cortex. 2005;15:1637–1653. doi: 10.1093/cercor/bhi042. [DOI] [PubMed] [Google Scholar]