Abstract
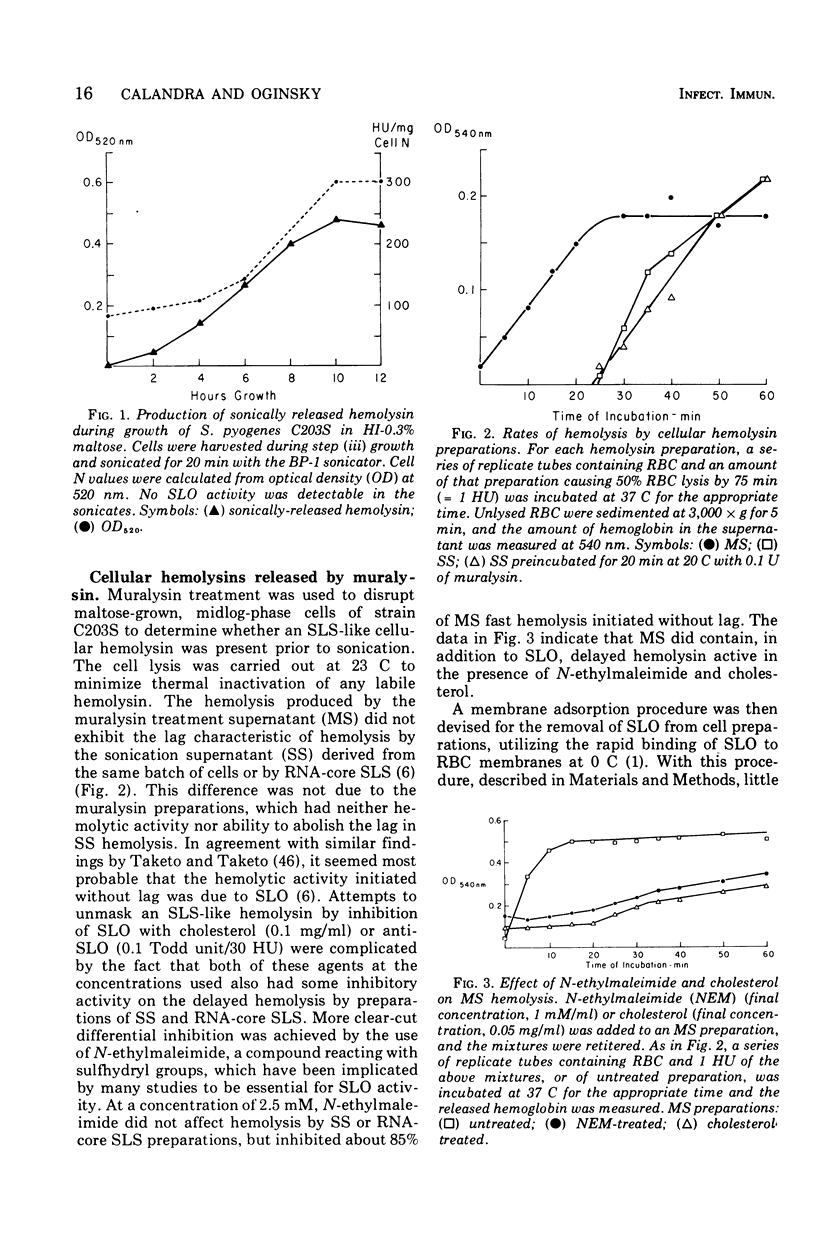
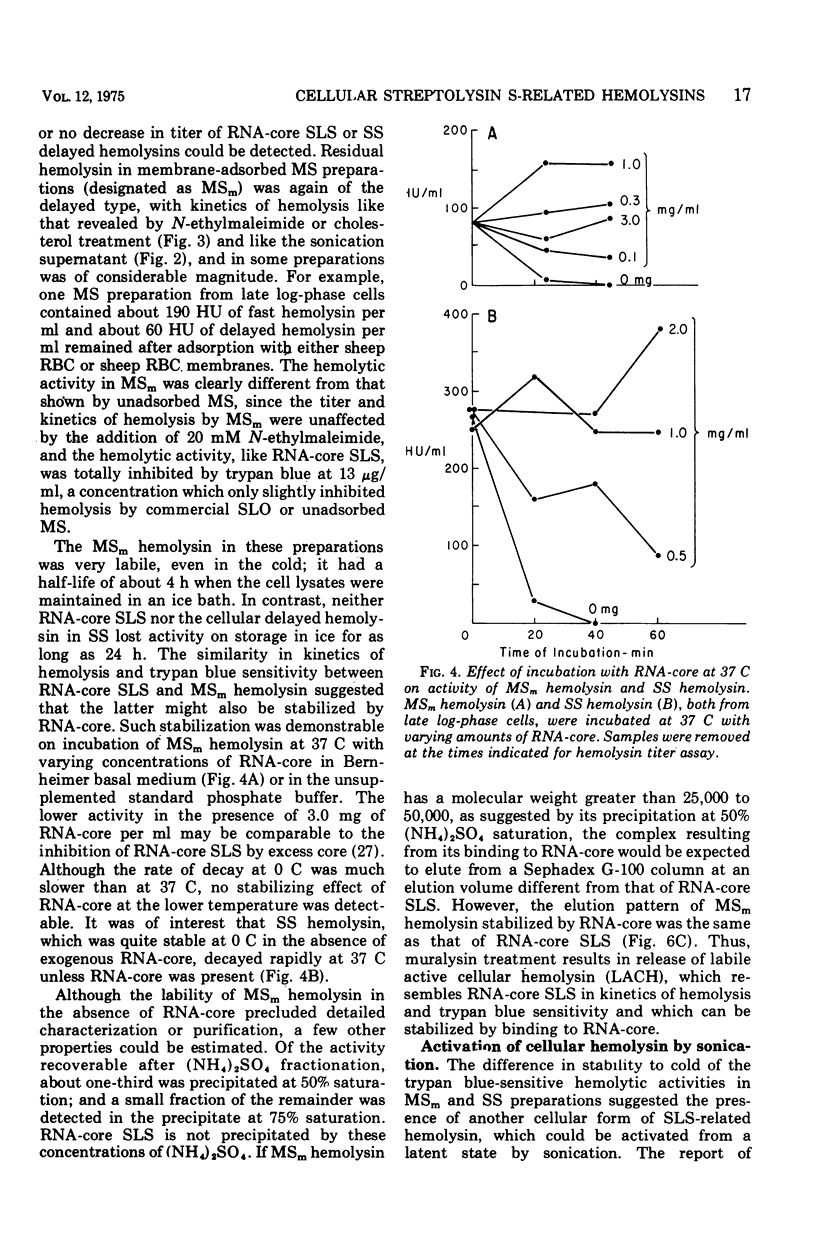
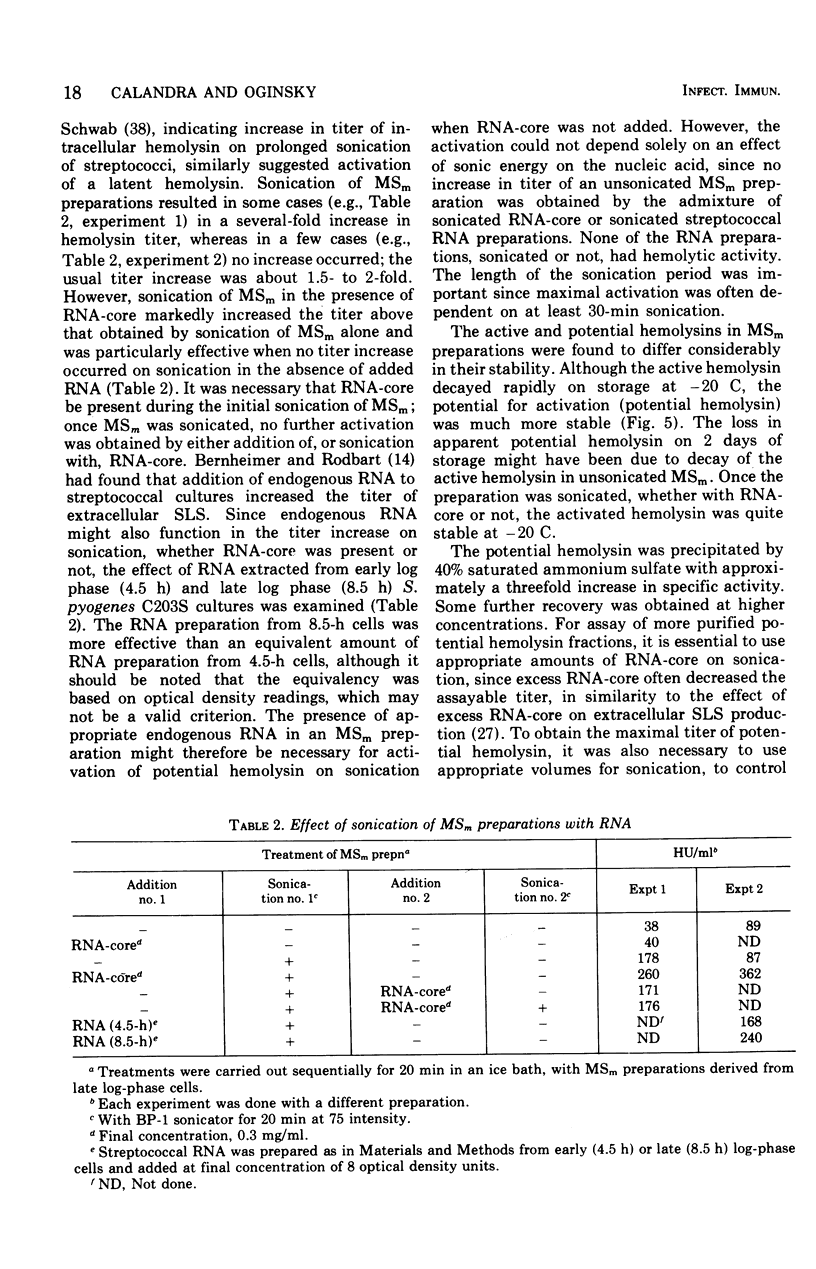
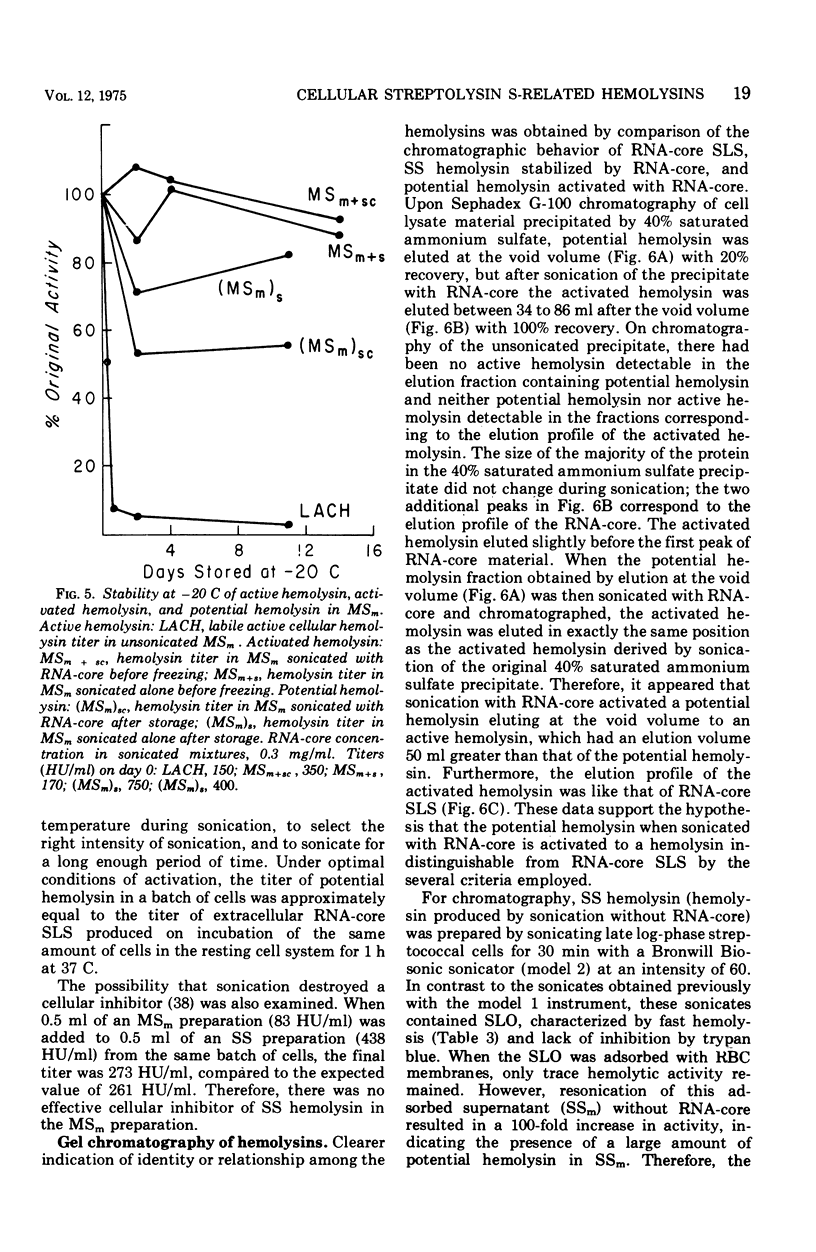
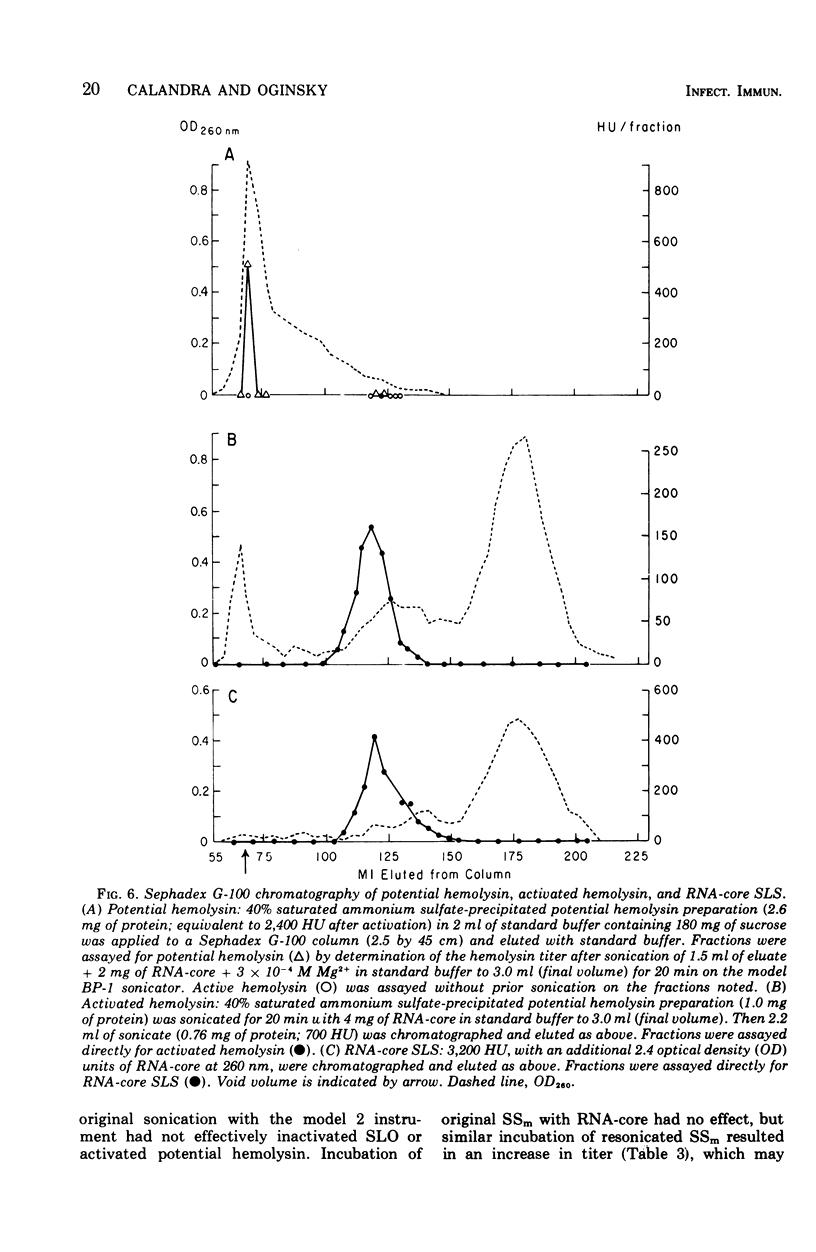
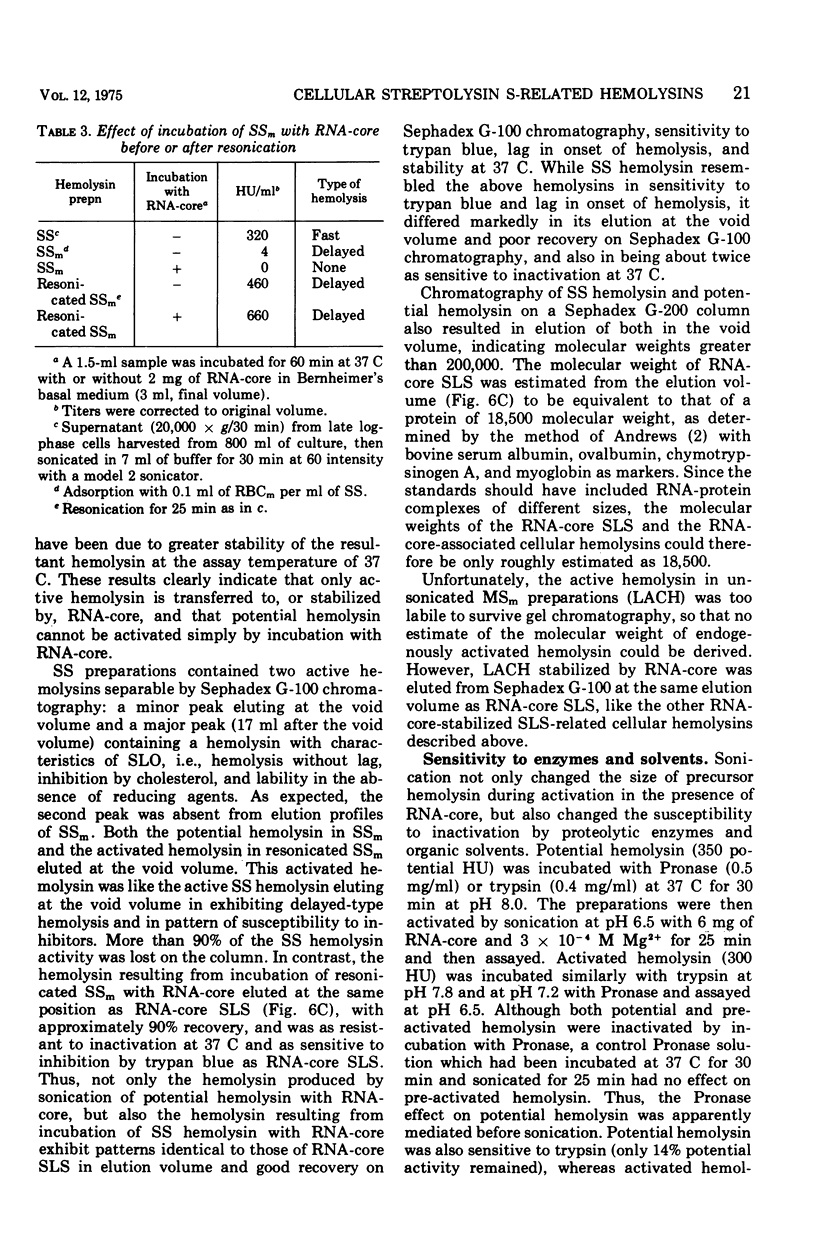
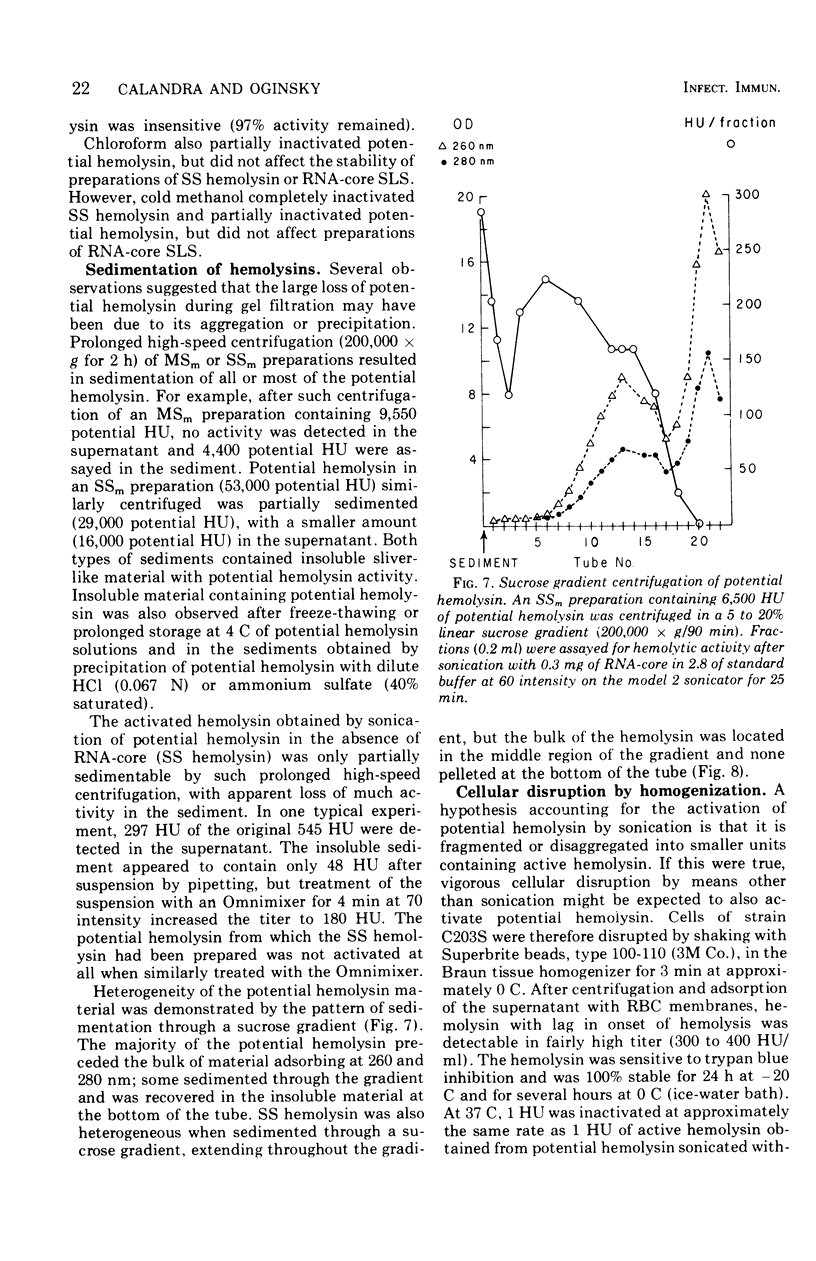
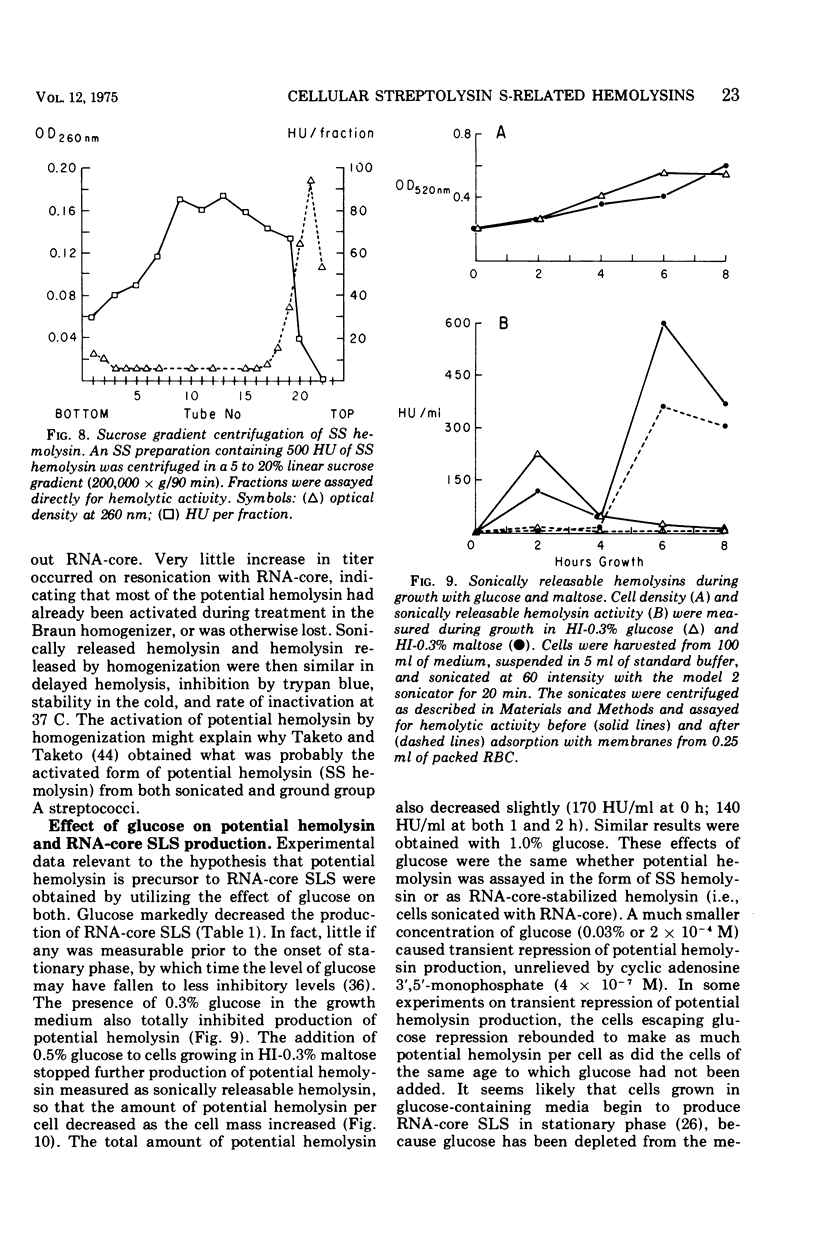
Group A streptococci strain C203S, grown in heart infusion broth with 0.3% maltose, produce two cellular hemolysins related to extracellular streptolysin S (SLS). Enzymatic lysis of the streptococci by group C streptococcal phage-associated lysin results in release of low titer, labile hemolysin, which can be stabilized by ribonucleic acid (RNA)-core (RNA preparation from yeast). This labile hemolysin can be detected only after the higher titer cellular streptolysin O is removed by erythrocyte membranes or inactivated by N-ethylmaleimide. The other cellular SLS-related hemolysin is released in a latent state (potential hemolysin) which can be activated to high-titer hemolysin by sonication with RNA-core. The titer of such activated hemolysin depends upon the intensity of sonic energy, duration of sonication, and amount of RNA-core. RNA obtained from the streptococci is far less effective than RNA-core. When the cocci are disrupted by sonication or grinding, potential hemolysin and/or activated form may be released, depending upon the conditions employed. The potential hemolysin material is large and heterogeneous; activation appears to involve, in part, disaggregation or fragmentation. Labile hemolysin, potential hemolysin, and the activated form of potential hemolysin can all be converted to hemolysin having the same hemolytic and physical properties as RNA-core SLS, suggesting that all have the same hemolytic moiety. The presence of glucose in heart infusion broth prevents formation of both potential hemolysin and RNA-core SLS by log-phase cells, whereas addition of glucose to a culture in heart infusion broth with 0.3% maltose stops accumulation of potential hemolysin but does not affect continuation of RNA-core SLS release. These results suggest that potential hemolysin is a cellular precursor to RNA-core SLS.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott J. P., Freer J. H., Bernheimer A. W. Physical states of staphylococcal alpha-toxin. J Bacteriol. 1967 Oct;94(4):1170–1177. doi: 10.1128/jb.94.4.1170-1177.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Basinger S. F., Jackson R. W. Bacteriocin (hemolysin) of Streptococcus zymogenes. J Bacteriol. 1968 Dec;96(6):1895–1902. doi: 10.1128/jb.96.6.1895-1902.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S. Nature and properties of a cytolytic agent produced by Bacillus subtilis. J Gen Microbiol. 1970 Jun;61(3):361–369. doi: 10.1099/00221287-61-3-361. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W. Cytolytic toxins of bacterial origin. The nature and properties of cytolytic proteins are discussed with emphasis on staphylococcal alpha-toxin. Science. 1968 Feb 23;159(3817):847–851. doi: 10.1126/science.159.3817.847. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Grushoff P. Cereolysin: production, purification and partial characterization. J Gen Microbiol. 1967 Jan;46(1):143–150. doi: 10.1099/00221287-46-1-143. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W. Physical behavior of streptolysin S. J Bacteriol. 1967 Jun;93(6):2024–2025. doi: 10.1128/jb.93.6.2024-2025.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter J. R. Production, purification, and composition of staphylococcal alpha toxin. J Bacteriol. 1966 Dec;92(6):1655–1662. doi: 10.1128/jb.92.6.1655-1662.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Elias N., Heller M., Ginsburg I. Binding of streptolysin S to red blood cell ghosts and ghost lipids. Isr J Med Sci. 1966 May-Jun;2(3):302–309. [PubMed] [Google Scholar]
- FOX E. N. INTRACELLULAR M PROTEIN OF GROUP A STREPTOCOCCUS. J Bacteriol. 1963 Mar;85:536–540. doi: 10.1128/jb.85.3.536-540.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG I., BENTWICH Z., HARRIS T. N. OXYGEN-STABLE HEMOLYSINS OF GROUP A STREPTOCOCCI. 3. THE RELATIONSHIP OF THE CELL-BOUND HOMOLYSIN TO STREPTOLYSIN S. J Exp Med. 1965 Apr 1;121:633–645. doi: 10.1084/jem.121.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG I., GROSSOWICZ N. A cell-bound hemolysin of group A streptococci. Bull Res Counc Isr Sect E Exp Med. 1958 Dec;7E:237–246. [PubMed] [Google Scholar]
- GINSBURG I., HARRIS T. N., GROSSOWICZ N. OXYGEN-STABLE HEMOLYSINS OF GROUP A STREPTOCOCCI. I. THE ROLE OF VARIOUS AGENTS IN THE PRODUCTION OF THE HEMOLYSINS. J Exp Med. 1963 Dec 1;118:905–917. doi: 10.1084/jem.118.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG I., HARRIS T. N. OXYGEN-STABLE HEMOLYSINS OF BETA-HEMOLYTIC STREPTOCOCCI. Ergeb Mikrobiol Immunitatsforsch Exp Ther. 1964;38:198–222. doi: 10.1007/978-3-662-42622-7_6. [DOI] [PubMed] [Google Scholar]
- GINSBURG I., HARRIS T. N. OXYGEN-STABLE HEMOLYSINS OF GROUP A STREPTOCCI. IV. STUDIES ON THE MECHANISM OF LYSIS BY CELL-BOUND HEMOLYSIN OF RED BLOOD CELLS AND EHRLICH ASCITES TUMOR CELLS. J Exp Med. 1965 Apr 1;121:647–656. doi: 10.1084/jem.121.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG I., HARRIS T. N. OXYGEN-STABLE HEMOLYSINS OF GROUP A STREPTOCOCCI. II. CHROMATOGRAPHIC AND ELECTROPHORETIC STUDIES. J Exp Med. 1963 Dec 1;118:919–934. doi: 10.1084/jem.118.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI T., MAEKAWA S., TANAKA K. Studies on the hemolysin of hemolytic streptococci. IV. Antagonistic action of ribonucleic acid on the hemolysis of hemolysin formed with ribonucleic acid. Jpn J Exp Med. 1956 Aug;26(3-4):113–123. [PubMed] [Google Scholar]
- KOYAMA J., SOKAWA Y., EGAMI F. CHEMICAL NATURE AND BIOSYNTHESIS OF STREPTOLYSIN S'. Biochem Z. 1963;338:206–216. [PubMed] [Google Scholar]
- KRAUSE R. M. Studies on the bacteriophages of hemolytic streptococci. II. Antigens released from the streptococcal cell wall by a phage-associated lysin. J Exp Med. 1958 Dec 1;108(6):803–821. doi: 10.1084/jem.108.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOVITZ A., DORFMAN A. Synthesis of capsular polysaccharide (hyaluronic acid) by protoplastmembrane preparations of group A Streptococcus. J Biol Chem. 1962 Feb;237:273–279. [PubMed] [Google Scholar]
- MARUYAMA Y., SUGAI S., EGAMI F. Formation of streptolysin S by streptococcal protoplasts. Nature. 1959 Sep 12;184(Suppl 11):832–833. doi: 10.1038/184832a0. [DOI] [PubMed] [Google Scholar]
- MAXTED W. R. The active agent in nascent phage lysis of streptococci. J Gen Microbiol. 1957 Jun;16(3):584–595. doi: 10.1099/00221287-16-3-584. [DOI] [PubMed] [Google Scholar]
- OKAMOTO H., FUJIMURA A., SHOIN S., BANDO I., KOSHIMURA S., UJIIE T. STUDIES OF THE PHENOMENON OF HIGH PROMOTION BY NUCLEIC ACID OF THE PRODUCTION OF STREPTOLYSIN-S OF HEMOLYTIC STREPTOCOCCUS. 22. ON THE INHIBITION BY GLUCOSE OF STREPTOLYSIN-S PRODUCTION IN RIBONUCLEIC ACID BROTH CULTURE. Jpn J Exp Med. 1964 Jun;34:109–118. [PubMed] [Google Scholar]
- Quinn R. W., Lowry P. N. Effect of Streptococcus pyogenes on tissue cells. J Bacteriol. 1967 Jun;93(6):1825–1831. doi: 10.1128/jb.93.6.1825-1831.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWAB J. H. An intracellular hemolysin of group A Streptococci. I. influence of sonic energy and pH on hemolytic potency. J Bacteriol. 1956 Jan;71(1):94–99. doi: 10.1128/jb.71.1.94-99.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWAB J. H. An intracellular hemolysin of group A Streptococci. II. Comparative properties of intracellular hemolysin, streptolysin S, and streptolysin O. J Bacteriol. 1956 Jan;71(1):100–107. doi: 10.1128/jb.71.1.100-107.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWAB J. H. An intracellular hemolysin of group A streptococci. III. Immunological studies. J Bacteriol. 1960 Apr;79:488–495. doi: 10.1128/jb.79.4.488-495.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOKAWA Y., EGAMI F. RELEASE OF THE HEMOLYTIC COMPONENT OF STREPTOLYSIN S BY ATP AND MAGNESIUM ION FROM A PROTOPLAST MEMBRANE FRACTION OF STREPTOCOCCI. J Biochem. 1965 Jan;57:64–74. doi: 10.1093/oxfordjournals.jbchem.a128058. [DOI] [PubMed] [Google Scholar]
- TAKETO A., TAKETO Y. BIOCHEMICAL STUDIES ON STREPTOLYSIN S FORMATION. I. STREPTOLYSIN S FORMATION IN CELL FREE SYSTEM. J Biochem. 1964 Dec;56:552–561. doi: 10.1093/oxfordjournals.jbchem.a128033. [DOI] [PubMed] [Google Scholar]
- TAKETO A., TAKETO Y. BIOCHEMICAL STUDIES ON STREPTOLYSIN S FORMATION. II. ON THE TRANSFER OF STREPTOLYSIN S. J Biochem. 1964 Dec;56:562–567. doi: 10.1093/oxfordjournals.jbchem.a128034. [DOI] [PubMed] [Google Scholar]
- Taketo A., Taketo Y. Biochemical studies on streptolysin S formation. 3. Intracellular streptolysins. J Biochem. 1965 Jun;57(6):787–792. doi: 10.1093/oxfordjournals.jbchem.a128145. [DOI] [PubMed] [Google Scholar]
- Taketo Y., Taketo A. Oncolytic activity in vitro of streptococci with special reference to "cell-bound" hemolysin. J Biochem. 1967 Apr;61(4):450–459. doi: 10.1093/oxfordjournals.jbchem.a128568. [DOI] [PubMed] [Google Scholar]



