Abstract
There has been a dramatic rise in the abuse of synthetic cathinones known as “bath salts,” including 3,4-methylenedioxypyrovalerone (MDPV), an analog linked to many adverse events. MDPV differs from other synthetic cathinones because it contains a pyrrolidine ring which gives the drug potent actions as an uptake blocker at dopamine and norepinephrine transporters. While MDPV is now illegal, a wave of “second generation” pyrrolidinophenones has appeared on the market, with α-pyrrolidinovalerophenone (α-PVP) being most popular. Here, we sought to compare the in vitro and in vivo pharmacological effects of MDPV and its congeners: α-PVP, α-pyrrolidinobutiophenone (α-PBP), and α-pyrrolidinopropiophenone (α-PPP). We examined effects of test drugs in transporter uptake and release assays using rat brain synaptosomes, then assessed behavioral stimulant effects in mice. We found that α-PVP is a potent uptake blocker at dopamine and norepinephrine transporters, similar to MDPV. α-PBP and α-PPP are also catecholamine transporter blockers but display reduced potency. All of the test drugs are locomotor stimulants, and the rank order of in vivo potency parallels dopamine transporter activity, with MDPV>α-PVP>α-PBP>α-PPP. Motor activation produced by all drugs is reversed by the dopamine receptor antagonist SCH23390. Furthermore, results of a functional observational battery show that all test drugs produce typical stimulant effects at lower doses and some drugs produce bizarre behaviors at higher doses. Taken together, our findings represent the first evidence that second generation analogs of MDPV are catecholamine-selective uptake blockers which may pose risk for addiction and adverse effects in human users.
Keywords: 3,4-methylenedioxypyrovalerone; α-PVP; functional observational battery; locomotor activity; monoamine transporter; synthetic cathinones
1.0 Introduction
In the past few years, products containing synthetic stimulants have flooded the recreational drug marketplace in the United States (U.S.) and elsewhere (Baumann et al., 2013a; Psychonaut, 2009; U.S. Drug Enforcement Administration, 2013a). These products, often sold under the guise of “bath salts,” “plant food,” or “research chemicals,” contain psychoactive cathinone derivatives and are purchased online, at gas stations, or at head shops as “legal” alternatives to illicit drugs (Karila and Reynaud, 2011; Schifano et al., 2011; Winstock and Ramsey, 2010; Winstock et al., 2011). From 2010 to 2011, the number of calls to U.S. poison control centers reporting exposure to synthetic cathinones increased from 303 to 6,138, and patients with acute toxicity began presenting to emergency departments (American Association of Poison Control Centers, 2012). The abuse of synthetic stimulants can result in severe side effects including tachycardia, hyperthermia, agitation, delusions, and violent behaviors leading to suicide or homicide (EMCDDA, 2010; Kelly, 2011; Ross et al., 2011; Spiller et al., 2011). In response to the heightened public health threat, federal legislation was enacted in 2012 and 2013 to permanently ban the three most common constituents in these products: 3,4-methylenedioxypyrovalerone (MDPV), 3,4-methylenedioxymethcathinone (methylone) and 4-methylmethcathinone (mephedrone).
Initial pharmacological investigations showed that synthetic cathinones exert their effects by interacting with monoamine transporters for dopamine (DAT), norepinephrine (NET) and serotonin (SERT) (Cozzi et al., 1999; Hadlock et al., 2011; López-Arnau et al., 2012; Martínez-Clemente et al., 2012). More recent data reveal that ring-substituted cathinones, like mephedrone and methylone are transporter substrates which cause the release of dopamine, norepinephrine and serotonin by reversing the normal direction of transporter flux (Baumann et al., 2012; Cameron et al., 2013; Simmler et al., 2013) in a manner similar to amphetamine (Baumann et al., 2013b; Fleckenstein et al., 2000). MDPV is structurally distinct from other synthetic cathinones due to the presence of a pyrrolidine ring, which gives the drug potent actions as a transporter blocker at DAT and NET (Baumann et al., 2013b; Eshleman et al., 2013; Simmler et al., 2013). Thus, MDPV displays a molecular mechanism of action that is similar to cocaine rather than amphetamine (Baumann et al., 2013b; Fleckenstein et al., 2000). Systemic administration of MDPV or mephedrone to rats increases extracellular concentrations of dopamine in mesolimbic reward circuits (Baumann et al., 2012; Baumann et al., 2013b; Kehr et al., 2011; Wright et al., 2012). Consistent with dopaminergic activation, synthetic cathinones increase locomotor activity in rodents, similar to the effects of classical stimulants like amphetamine and cocaine (Fantegrossi et al., 2013; Lisek et al., 2012; López-Arnau et al., 2012; Marusich et al., 2012). A functional observational battery (FOB) revealed that MDPV, mephedrone, and methylone produce typical stimulant effects including hyperactivity and stereotyped behavior, comparable to that found for cocaine, amphetamine and methamphetamine (Gauvin and Baird, 2008; Marusich et al., 2012). Perhaps more importantly, MDPV and mephedrone are readily self-administered by rats, indicating a propensity for abuse and addiction (Aarde et al., 2013; Hadlock et al., 2011; Motbey et al., 2013; Watterson et al., 2012a; Watterson et al., 2012b).
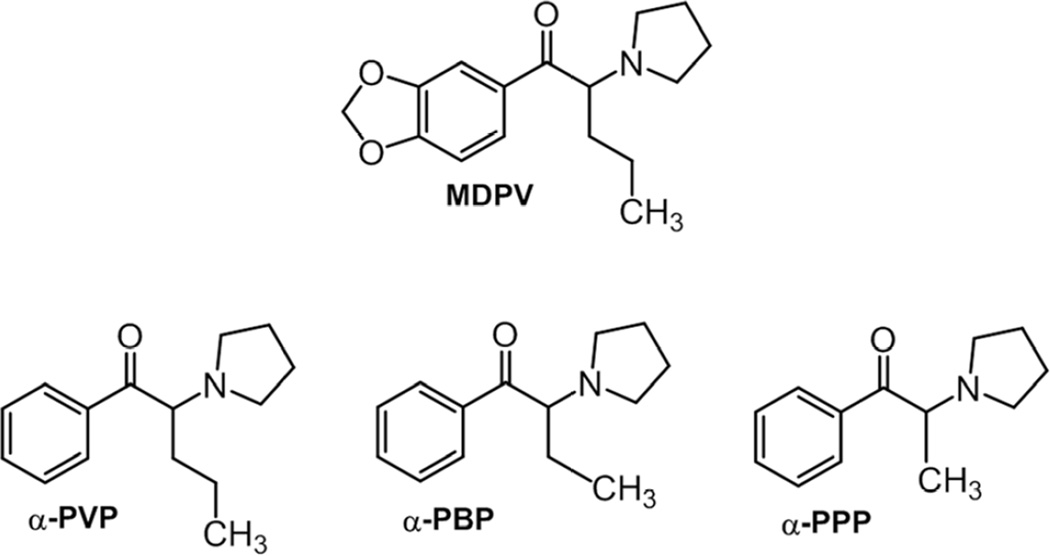
Since the three most common synthetic stimulants were banned in the U.S., manufacturers have introduced novel replacement cathinones as a means to skirt regulatory control, a trend which is expected to continue (Brandt et al., 2010; Shanks et al., 2012). For the purposes of the present paper, synthetic stimulants which were legal prior to legislation enacted in 2012 (U.S. Congress, 2012) are referred to as “first generation” drugs, while newer stimulants are referred to as “second generation” drugs. Despite the fact that a host of cathinone compounds may be present in synthetic stimulant products (Shanks et al., 2012; Spiller et al., 2011), MDPV is the chief compound found in blood and urine from patients admitted to emergency departments for treatment of acute toxicity due to synthetic stimulant exposure (Murray et al., 2012; Penders et al., 2012; Spiller et al., 2011; Wyman et al., 2013). Such data point to MDPV as a principal culprit in mediating medically-relevant adverse effects. Recently, a number of second generation MDPV analogs have appeared in the marketplace, with α-pyrrolidinovalerophenone (α-PVP) being the most popular and widespread (Marinetti and Antonides, 2013; Shanks et al., 2012; U.S. Drug Enforcement Administration, 2013b). As shown in Figure 1, pyrrolidinophenones like α-PVP, α-pyrrolidinobutiophenone (α-PBP) and α-pyrrolidinonpropiophenone (α-PPP) are structurally similar to MDPV (Meltzer et al., 2006), yet little is known about their mechanism of action or behavioral effects.
Figure 1.
Chemical structures of pyrrolidinophenone compounds in comparison to MDPV.
The purpose of the present study was to evaluate in vitro and in vivo effects of second generation stimulants that are structurally-related to MDPV. To this end, in vitro transporter activity at DAT, NET and SERT was assessed for MDPV, α-PVP, α-PBP, and α-PPP. In vivo pharmacology of these compounds was assessed by measuring locomotor activity and effects in an FOB. Our findings provide the first evidence that second generation pyrrolidinophenones like α-PVP are potent catecholamine-selective transporter blockers which can elicit psychomotor stimulant effects via a dopaminergic mechanism. As such, these agents would be expected to pose substantial risks for abuse and addiction.
2.0 Methods and Materials
2.1 Subjects
Adult male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) weighing 300–400g (total n=36) were housed three per cage. Adult male ICR mice (Harlan, Frederick, MD, USA) weighing 30–55 g (total n=112) were housed individually. Animals were housed in polycarbonate cages with hardwood bedding. All animals were drug and test naïve, and were housed in temperature-controlled conditions (20–24°C) with a 12 h standard light-dark cycle. Animals had ad libitum access to food and water in their home cages at all times. Rat experiments were approved by the Institutional Animal Care and Use Committee at NIDA IRP, while mouse experiments were approved by the Institutional Animal Care and Use Committee at RTI International. All research was conducted as humanely as possible, and followed the principles of laboratory animal care (National Research Council, 2011). The authors consulted the ARRIVE guidelines for reporting experiments involving animals, and all efforts were made to minimize animal suffering, reduce the number of animals used, and utilize alternatives to in vivo techniques, if available.
2.2 Drugs
α-PVP, α-PBP, and α-PPP were purchased from Cayman Chemical (Ann Arbor, MI, USA). SCH23390 was purchased from Tocris (Minneapolis, MN, USA). MDPV was synthesized in house at RTI using standard synthetic procedures. MDPV was formulated as a recrystallized HCl salt and was > 97% pure. The purity was assessed by several analytical techniques including carbon, hydrogen, nitrogen (CHN) combustion analysis and proton nuclear magnetic resonance spectroscopy. All drugs were dissolved in sterile saline (Butler Schein, Dublin, OH, USA). Doses are expressed as mg/kg of the salt, and were administered at a volume of 10 ml/kg in mice. Sterile saline was used as a comparison for all drugs for in vivo studies.
2.3 In Vitro Uptake and Release Assays
Rats were euthanized by CO2 narcosis, and brains were processed to yield synaptosomes as previously described (Baumann et al., 2013b; Rothman et al., 2003). Synaptosomes were prepared from rat striatum for the DAT assays, whereas synaptosomes were prepared from whole brain minus striatum and cerebellum for the NET and SERT assays. For uptake inhibition assays, 5 nM [3H]dopamine, 10 nM [3H]norepinephrine and 5 nM [3H]serotonin were used to assess transport activity at DAT, NET and SERT, respectively. The selectivity of uptake assays was optimized for a single transporter by including unlabeled blockers to prevent uptake of [3H]transmitter by competing transporters. Uptake inhibition assays were initiated by adding 100 µl of tissue suspension to 900 µl Krebs-phosphate buffer (126 mM NaCl, 2.4 mM KCl, 0.83 mM CaCl2, 0.8 mM MgCl2, 0.5 mM KH2PO4, 0.5 mM Na2SO4, 11.1 mM glucose, 0.05 mM pargyline, 1mg/mL bovine serum albumin, and 1 mg/mL ascorbic acid, pH 7.4) containing test drug and [3H]transmitter. Uptake inhibition assays were terminated by rapid vacuum filtration through Whatman GF/B filters, and retained radioactivity was quantified by liquid scintillation counting. For release assays, 9 nM [3H]1-methyl-4-phenylpyridinium ([3H]MPP+) was used as the radiolabeled substrate for DAT and NET, while 5 nM [3H]serotonin was used as a substrate for SERT. All buffers used in the release assay methods contained 1 µM reserpine to block vesicular uptake of substrates. The selectivity of release assays was optimized for a single transporter by including unlabeled blockers to prevent the uptake of [3H]MPP+ or [3H]serotonin by competing transporters. Synaptosomes were preloaded with radiolabeled substrate in Krebs phosphate buffer for 1 h (steady state). Release assays were initiated by adding 850 µl of preloaded synaptosomes to 150 µl of test drug. Release was terminated by vacuum filtration and retained radioactivity was quantified as described for uptake inhibition.
2.4 Apparatus for Behavioral Testing
Mouse locomotor activity was assessed in clear Plexiglass open field activity chambers measuring 47×25.5×22 cm. San Diego Instruments Photobeam Activity System software (model SDI: V-71215, San Diego, CA, USA) was used to calculate beam breaks. Each chamber contained two 4-beam infrared arrays that monitored horizontal movement. The FOB was conducted during handling, and in a clear Plexiglas open field measuring 47×25.5×22 cm.
2.5 Locomotor Activity and FOB
Mice were randomly assigned to receive a single dose of a particular drug (n=8 per group), and the same cohort of mice was used for all behavioral assessments. A single saline control group (n=16) was examined in the locomotor assessments and FOB. To facilitate visual comparison, data from this control group was included in all graphs and analyses. Locomotor activity was quantified by an automated system which provides a general measure of movement in the horizontal plane across time. Locomotor activity tests were conducted during week 1. The FOB consisted of observations by a trained technician and was conducted during weeks 2–3, and locomotor activity tests incorporating antagonist pretreatment were conducted during weeks 5 and 7. In total, each mouse was given 3 administrations of drug or vehicle, with a minimum of one week wash out between each administration. All pyrrolidinophenones were administered intraperitoneally (i.p.), and doses were chosen based on previous findings (Baumann et al., 2013b; Marusich et al., 2012) and in vitro research from the present study.
For initial locomotor assessments (week 1), mice received their assigned drug dose and immediately thereafter were placed individually into locomotor activity chambers for a 60-min test, conducted by a technician who was blind to treatment. Doses examined were saline, 0.3–3.0 mg/kg MDPV, 1.0–10.0 mg/kg α-PVP, 1.0–10.0 mg/kg α-PBP, and 3.0–30.0 mg/kg α-PPP. For antagonist tests (weeks 5 and 7), SCH23390, a potent D1 antagonist (0.03 mg/kg), or saline was administered s.c. 30 min prior to challenge injection with the assigned cathinone or saline. Mice were placed into the locomotor activity chamber immediately after injection for a 60-min test. For the antagonist testing phase, mice that received the highest dose of each cathinone during the FOB (weeks 2–3) were excluded. The remaining two groups of mice for each cathinone were counterbalanced across the groups pre-treated with saline or the antagonist, and the vehicle group was split in half for antagonist assessment.
An FOB (weeks 2–3), modified from a procedure commonly used by the Environmental Protection Agency (U.S. Environmental Protection Agency, 1998a; 1998b), was used to classify observable effects of the drugs, as determined 15 min post-injection. This assay allowed for assessment of a wide range of drug effects, and provided an overall behavioral profile for each compound, with an emphasis on detection of potential safety concerns. Methods were similar to those used in our previous study on first generation synthetic cathinones (Marusich et al., 2012). Doses examined were 1.0–10.0 mg/kg MDPV, 3.0–17.0 mg/kg α-PVP, 3.0–30.0 mg/kg α-PBP, 10.0–56.0 mg/kg α-PPP, and saline control. FOBs were scored by a trained technician who was blind to treatment. Dependent measures included ataxia, bizarre behavior (e.g. jumping, climbing, rearing while facing away from wall), circular ambulations, convulsions, ejaculation, exploration (e.g. excessive sniffing or reorienting of the head), flattened body posture, grooming, hyperactivity (increased ambulatory movements), hypoactivity, muscle relaxation, retropulsion, salivation, self-injury, stereotyped biting, stereotyped head circling, stereotyped head weaving, stereotyped licking, stimulation (e.g. increased heart rate, tense body), Straub tail, and tremor.
2.6 Data Analysis
For in vitro assays, statistical analyses were carried out using GraphPad Prism (v. 5.0; GraphPad Scientific, San Diego, CA, USA). IC50 values for inhibition of uptake and EC50 values for stimulation of release were calculated based on non-linear regression analysis. For in vivo assays, statistical analyses were conducted using NCSS (2004; Number Cruncher Statistical Systems, Kaysville, Utah, USA). Locomotor activity was expressed as total beam breaks per 10-min bin. Mixed model analysis of variance (ANOVA) was used to analyze dose effect and time course locomotor data, with time as the within-subject factor and dose as the between-subject factor. Results of antagonist tests were analyzed with an additional two-way (dose × pre-session injection) ANOVA for each drug. All tests were considered significant at p<0.05. FOB data were analyzed with Kruskal–Wallis one-way ANOVA by ranks for each dependent measure, corrected for ties. No dose of any drug produced convulsions, ejaculation, muscle relaxation, self-injury, Straub-tail, or tremor; therefore, these measures were not analyzed. Hence, 15 dependent variables remained for FOB and, the alpha level was controlled for this number of measures (α=0.0033). When ANOVAs revealed significant main effects or interactions, Tukey’s post hoc test was used to determine differences between group means. Data from all mice were included in all analyses.
3.0 Results
3.1 In Vitro Transporter Assays
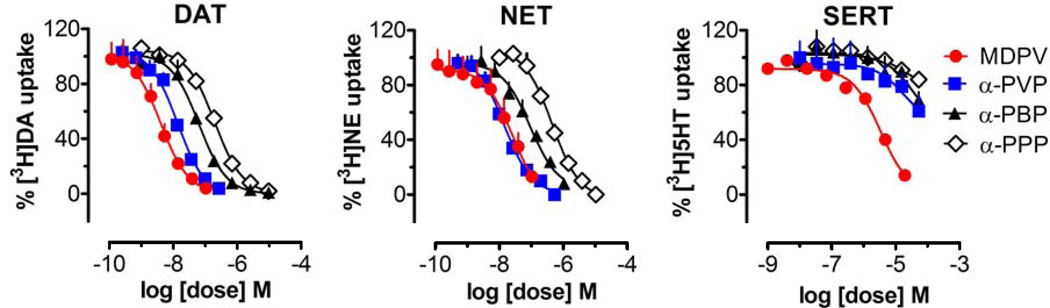
The IC50 values for inhibition of [3H]transmitter uptake at DAT, NET and SERT are summarized in Table 1; in vitro data for cocaine and amphetamine from a previously published study are included for comparison (Baumann et al., 2013b). Dose-response curves for uptake inhibition at DAT, NET, and SERT are depicted in Figure 2. MDPV was a potent catecholamine uptake blocker with IC50 values of 4.0±0.6 nM and 25.9±5.6 nM at DAT and NET, respectively. By contrast, MDPV was more than 100-fold weaker at blocking uptake of serotonin, with an IC50 of 3305±485 nM at SERT. Similar to MDPV, all of the other pyrrolidinophenones were catecholamine-selective uptake blockers. α-PVP exhibited IC50 values of 12.8±1.2 and 14.2±1.2 nM for DAT and NET, but had much weaker effects at blocking serotonin uptake with an IC50 of>10,000 nM. α-PBP and α-PPP were less potent at DAT and NET when compared to α-PVP, indicating shorter alkyl chain length produces progressively less potent effects on uptake. Nevertheless, α-PBP and α-PPP maintained selectivity for inhibition of catecholamine uptake versus SERT uptake. It should be noted that even the weakest pyrrolidinophenone compound tested here, α-PPP, is more potent than cocaine at inhibiting uptake at DAT. None of the pyrrolidinophenones displayed sufficient efficacy in the release assays to allow for the determination of EC50 values (data not shown), demonstrating that the drugs are not transporter substrates. We have previously shown that MDPV is not active in the release assay (Baumann et al., 2013b). Taken together, the in vitro data demonstrate that MDPV, α-PVP, α-PBP and α-PPP are catecholamine-selective transporter blockers, and decreasing alkyl chain length on the α-carbon reduces potency at uptake blockade.
Table 1.
Effects of pyrrolidinophenones, cocaine, and amphetamine on inhibition of [3H]transmitter uptake at DAT, NET and SERT in rat brain synaptosomes. Data are expressed as nM concentrations (mean±SD) for n=3 experiments performed in triplicate.
| Test Drug | [3H]Dopamine uptake, IC50 at DAT (nM) |
[3H]Norepinephrine uptake, IC50 at NET (nM) |
[3H]Serotonin uptake, IC50 at SERT (nM) |
DAT/SERT ratio |
|---|---|---|---|---|
| MDPV | 4.1±0.6 | 25.9±5.6 | 3305±485 | 806 |
| α-PVP | 12.8±1.2 | 14.2±1.2 | >10,000 | >781 |
| α-PBP | 63.3±5.7 | 91.5±12.8 | >10,000 | >159 |
| α-PPP | 196.7±9.9 | 444.7±39.2 | >10,000 | >51 |
| Cocainea | 211±19 | 292±34 | 313±17 | 1.5 |
| Amphetaminea | 93±17 | 67±16 | 3418±314 | 37 |
Figure 2.
Effects of test drugs on inhibition of [3H]transmitter uptake by DAT, NET, and SERT in rat brain tissue. Synaptosomes were incubated with different concentrations of test drug in the presence of 5 nM [3H]dopamine for DAT, 5 nM [3H]norepinephrine for NET, or 5 nM [3H]serotonin for SERT. Data are percentage of control uptake expressed as mean±SD for n=3 experiments performed in triplicate.
3.2 Locomotor Dose Response and Time Course
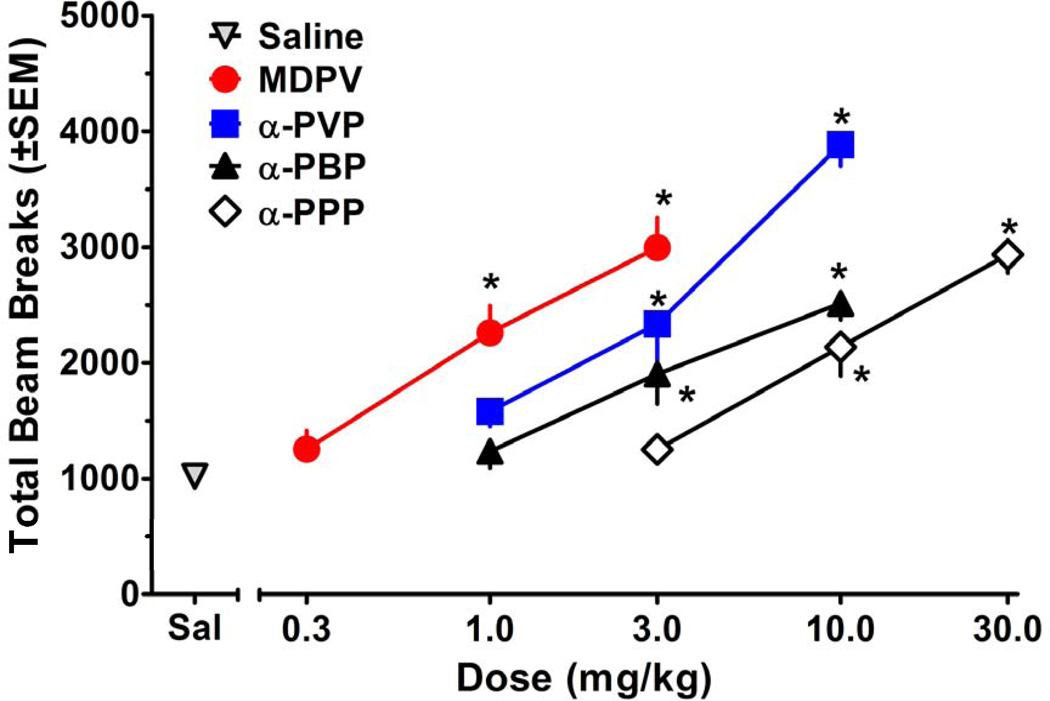
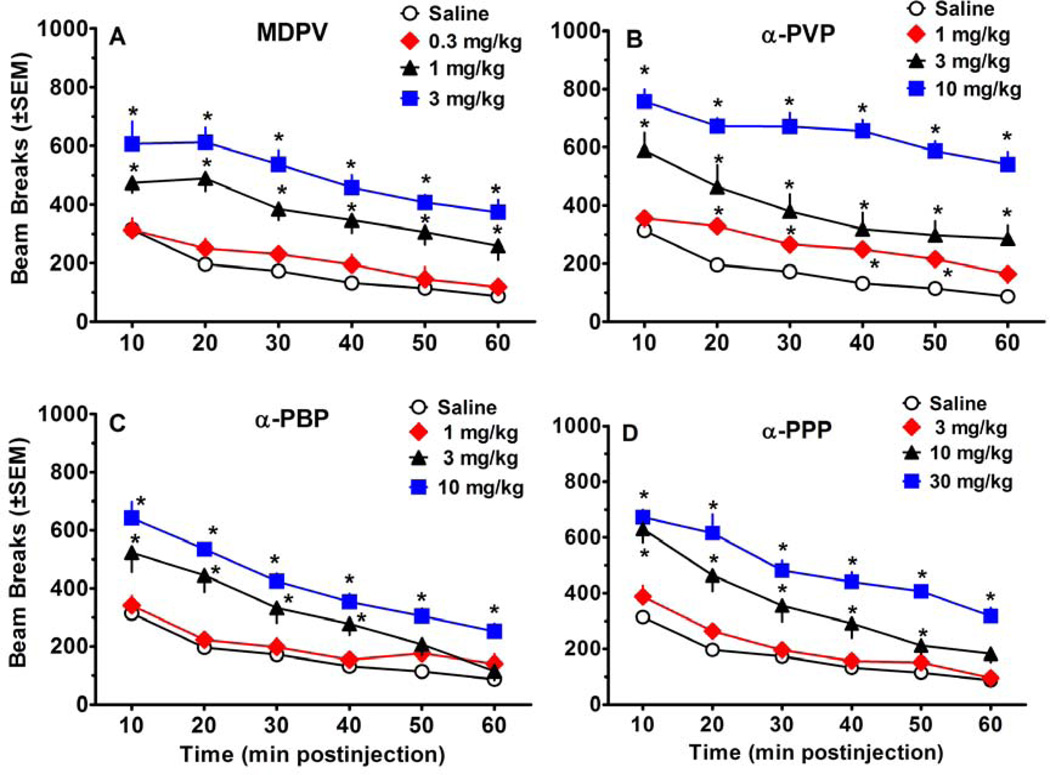
Figures 3 and 4 show the effects of test compounds on locomotor activity. As depicted in Figure 3, all compounds displayed a significant main effect of dose [MDPV:F(3, 36)=30.65, p<0.001; α-PVP:F(3, 36)=53.78, p<0.001; α-PBP:F(3, 36)=22.23, p<0.001; α-PPP:F(3, 36)=38.06, p<0.001]. Post-hoc tests revealed that 1.0–3.0 mg/kg MDPV, 3.0–10.0 mg/kg α-PVP, 3.0–10.0 mg/kg α-PBP, and 10.0–30.0 mg/kg α-PPP produced significant locomotor increases compared to saline. Significant main effects for time and significant interactions were also observed for all compounds, as illustrated in Figure 4. For the sake of comparison, the results for the saline group are shown in each panel. Over the course of the 60 min session, habituation occurred in the saline group, resulting in attenuation of activity in later bins. For MDPV, post hoc analysis of the significant interaction [F(15, 180)=2.31, p<0.01] revealed a session-long effect, with 1.0–3.0 mg/kg producing significant increases in beam breaks compared to saline for the duration of the session. α-PVP also produced a significant interaction [F(15, 180)=2.52, p<0.01]. While 3.0–10.0 mg/kg α-PVP significantly increased beam breaks for the duration of the session, the 1.0 mg/kg dose significantly increased beam breaks only during the 20–50 min postinjection interval. Post-hoc analysis of the significant interaction observed with α-PBP [F(15, 180)=6.56, p<0.001] revealed that 10.0 mg/kg produced significant increases in beam breaks compared to saline for all time points, whereas the 3.0 mg/kg dose significantly increased beam breaks only for the first 40 min. For α-PPP, post hoc analysis of the significant interaction [F(15, 180)=5.02, p<0.001] showed that 30.0 and 10.0 mg/kg produced significant increases in beam breaks for the duration of the session and for the first 50 min of the session, respectively.
Figure 3.
Effects of pyrrolidinophenone drugs on cumulative locomotor activity during 60 min sessions, plotted as a function of dose. Asterisks (*) represent doses that produced significant increases in beam breaks compared to saline.
Figure 4.
Time course effects of pyrrolidinophenone drugs on locomotor activity, plotted as a function of 10 min bins during a 60-min test session. Values represent mean±SEM expressed as number of beam breaks for each dose (n=8 per dose except n=16 for saline). Asterisks (*) within each panel indicate doses and time points that showed significant increases in beam breaks compared to saline at the same time point. Panel A shows data for MPDV, panel B shows data for α-PVP, panel C shows data for α-PBP, and panel D shows data for α-PPP.
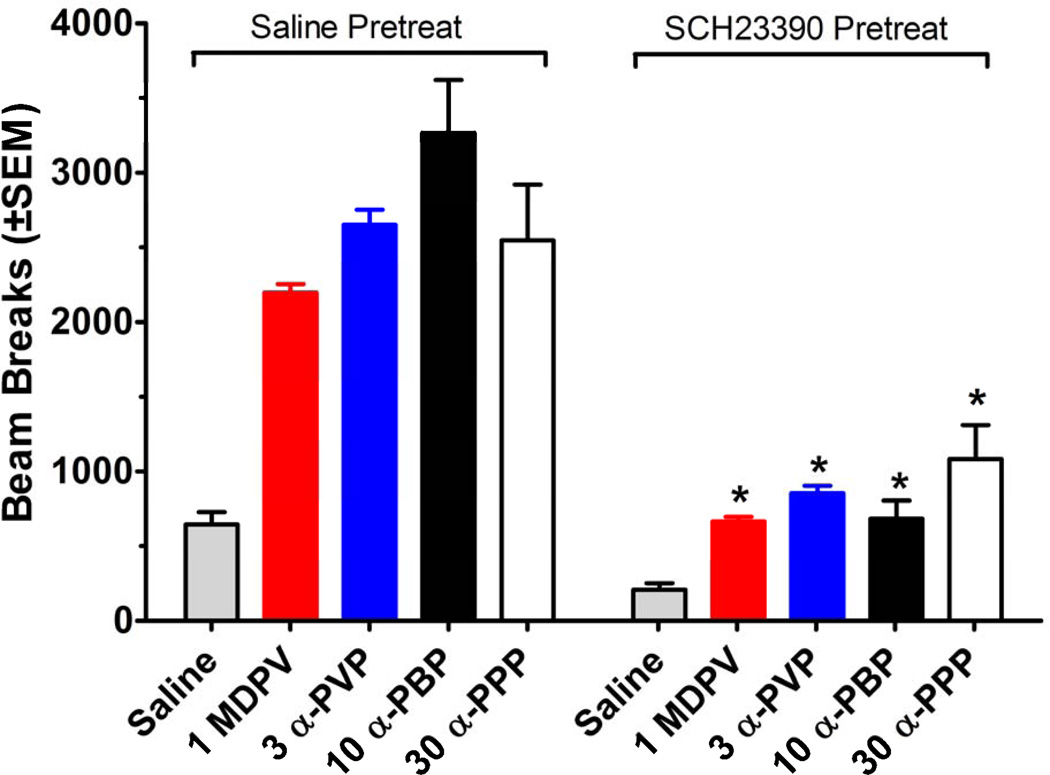
3.3 Locomotor Antagonist Testing
Combinations of the D1 receptor antagonist SCH23390 (0.03 mg/kg) and a single dose of each drug or saline were tested in the locomotor activity procedure. Figure 5 shows that antagonist pretreatment attenuated the hyperactivity produced by all pyrrolidinophenones at the doses administered. The data represent total beam breaks over 60 min motor activity sessions. When mice received 1.0 mg/kg MDPV, there was a main effect of pretreatment [F(1, 28)=88.28, p<0.001], drug [F(1, 28)=91.85, p<0.001], and a significant interaction [F(1, 28)=27.39, p<0.001], indicating that SCH23390 significantly decreased beam breaks for MDPV-treated mice. Administration of 3.0 mg/kg α-PVP produced a main effect of pretreatment [F(1, 28)=45.50, p<0.001], drug [F(1, 28)=64.05, p<0.001], and a significant interaction [F(1, 28)=16.92, p<0.001], with SCH23390 significantly decreasing beam breaks for the α-PVP group. When 10.0 mg/kg of α-PBP was administered there was a main effect of pretreatment [F(1, 28)=61.65, p<0.001], drug [F(1, 28)=64.85, p<0.001], and a significant interaction [F(1, 28)=31.18, p<0.001], indicating that SCH23390 significantly decreased beam breaks for α-PBP-treated mice. Mice that received 30.0 mg/kg α-PPP showed a main effect of pretreatment [F(1, 28)=18.12, p<0.001], drug [F(1, 28)=38.74, p<0.001], and a significant interaction [F(1, 28)=5.30, p< 0.05], indicating that SCH23390 significantly decreased beam breaks for the α-PPP group. Administration of SCH23390 plus saline did not significantly affect activity compared to saline/saline group.
Figure 5.
Effects of vehicle or SCH23390 (0.03 mg/kg) tested in combination with vehicle or a test drug on locomotor activity during 60 min sessions. Values represent mean±SEM expressed
3.4 Functional Observational Battery
Results of significant drug effects for the FOB are displayed in Table 2; behavioral data for cocaine and methamphetamine from a previously published study (Marusich et al., 2012) are included for comparison. Some drugs produced small non-significant effects on hypoactivity, salivation, stereotyped biting, and stereotyped licking; therefore, these measures are not included in Table 2. MDPV produced significant ataxia [H(3)=15.94, p<0.0033] at the 3.0 mg/kg dose, as compared to saline. α-PPP produced significant retropulsion compared to saline at 56.0 mg/kg [H(3)=18.92, p<0.0033]. All doses of all pyrrolidinophenones significantly increased exploration compared to saline [MDPV:H(3)=18.41, p<0.0033; α-PVP:H(3)=25.98, p<0.0033; α-PBP:H(3)=25.98, p<0.0033; α-PPP:H(3)=29.32, p<0.0033]. MDPV produced bizarre behavior such as rearing while facing away from the wall, climbing, and jumping [H(3)=27.92, p<0.0033], with 1.0–3.0 mg/kg producing significant differences from saline.
Table 2.
Effects of pyrrolidinophenones, cocaine (COC), and methamphetamine (METH) in the FOB. Arrows denote the direction of the difference from saline with corrected alpha level (p<0.0033). Doses (mg/kg) at which significant effects occurred are shown below arrows. For comparison purposes, doses at which locomotor activity was significantly increased during the first 10-min bin of the locomotor activity sessions are also shown.
| Dependent variable | MDPV | α-PVP | α-PBP | α-PPP | COCa | METHa |
|---|---|---|---|---|---|---|
| Locomotion (first 10 min) | ↑ 1 –3 |
↑ 3–10 |
↑ 3–10 |
↑ 10–30 |
↑ 10–42 |
↑ 1–5.6 |
| Ataxia | ↑ 3 |
|||||
| Retropulsion | ↑ 56 |
|||||
| Exploration | ↑ 1–10 |
↑ 3–17 |
↑ 3–30 |
↑ 10–56 |
||
| Bizzare Behavior | ↑ 1–3 |
|||||
| Circular Ambulations | ↑ 3–10 |
↑ 3–17 |
↑ 10–30 |
↑ 10–42 |
↑ 1–10 |
|
| Grooming | ↓ 30 |
|||||
| Flattened Body Posture | ↑ 3–17 |
|||||
| Hyperactivity | ↑ 3–10 |
↑ 3–17 |
↑ 10–30 |
↑ 10–30 |
↑ 10–42 |
↑ 1–10 |
| Stereotyped Head Weaving | ↑ 3–17 |
↑ 3 & 30 |
↑ 10–56 |
↑ 10 & 17 |
↑ 1–10 |
|
| Stereotyped Head Circling | ↑ 10 |
↑ 3–17 |
↑ 10 & 56 |
↑ 10–42 |
↑ 1–10 |
|
| Stimulation | ↑ 1–10 |
↑ 3–17 |
↑ 3–30 |
↑ 10–56 |
↑ 10–42 |
↑ 1–10 |
MDPV, α-PVP, and α-PBP significantly increased circular ambulations [MDPV:H(3)=24.37, p<0.0033; α-PVP:H(3)=26.01, p<0.0033; α-PBP:H(3)=19.60, p<0.0033]. The 3.0–10.0 mg/kg doses of MDPV, all doses of α-PVP, and 10.0–30.0 mg/kg α-PBP produced significant differences from saline. α-PPP produced a significant effect on grooming [H(3)=15.19, p<0.0033], with 30 mg/kg producing a significant decrease compared to saline. All doses of α-PVP produced significant flattened body posture compared to saline [H(3)=15.55, p<0.0033]. All drugs produced a significant increase in hyperactivity [MDPV:H(3)=19.46, p<0.0033; α-PVP:H(3)=27.24, p<0.0033; α-PBP:H(3)=18.07, p<0.0033; α-PPP:H(3)=17.88, p<0.0033], with 3.0–10.0 mg/kg MDPV, all doses of α-PVP, 10.0–30.0 mg/kg α-PBP, and 10.0–30.0 mg/kg α-PPP producing significant differences from saline.
α-PVP, α-PBP, and α-PPP produced significant stereotyped head weaving compared to saline [α-PVP:H(3)=22.13, p<0.0033; α-PBP:H(3)=18.07, p<0.0033; α-PPP:H (3)=23.82, p<0.0033] at all doses of α-PVP and α-PPP, and 3.0 and 30.0 mg/kg α-PBP. MDPV, α-PVP, and α-PPP significantly increased stereotyped circular head movements [MDPV:H(3)=21.14, p<0.0033; α-PVP:H(3)=23.85, p<0.0033; α-PPP:H(3)=23.78, p<0.0033], with 10.0 mg/kg MDPV, all doses of α-PVP, and 10.0 and 56.0 mg/kg α-PPP producing significant differences from saline. All doses of all drugs produced a significant increase in stimulation compared to saline [MDPV:H(3)=24.47, p<0.0033; α-PVP:H(3)=29.64, p<0.0033; α-PBP:H(3)=17.72, p<0.0033; α-PPP:H(3)=19.54, p<0.0033].
4.0 Discussion
Results of the present study demonstrate that MDPV and α-PVP are potent uptake blockers at DAT and NET, with much weaker effects at SERT. α-PBP and α-PPP are also catecholamine-selective uptake blockers but display reduced potency. Our in vitro results agree with those of Meltzer et al. (2006) who demonstrated that analogs of the pyrrolidinophenone compound, pyrovalerone, are potent catecholamine uptake blockers. Importantly, none of the compounds tested here are transporter substrates. In general, drugs interacting at monoamine transporters can be classified either as blockers which inhibit transmitter uptake, or as substrates (i.e., releasers) which cause transmitter release by reversing the normal direction of transmitter flux (Baumann et al., 2013a; 2013b). Previous studies using rat brain synaptosomes or cells expressing human DAT, NET and SERT have shown that MDPV is a transporter blocker and not a transporter substrate (Baumann et al., 2013b; Cameron et al., 2013; Eshleman et al., 2013; Simmler et al., 2013).
The present in vitro data extend previous findings to reveal that the presence of a pyrrolidine ring in any cathinone-like compound confers potent uptake blocking properties at DAT and NET. Thus, pyrrolidinophenones are mechanistically distinct from ring-substituted cathinones, such as mephedrone and methylone, which act as non-selective substrates for monoamine transporters and trigger transmitter release (Baumann et al., 2012; Cameron et al., 2013; Simmler et al., 2013). A comparison with our previous in vitro data reveals that all the pyrrolidinophenones tested in the present study are more potent than cocaine as DAT blockers, and all except α-PPP are more potent than amphetamine as DAT blockers. All pyrrolidinophenones except α-PPP are more potent NET blockers than cocaine, while MDPV and α-PVP are more potent NET blockers than amphetamine (Baumann et al., 2013b). The data included in Table 1 for cocaine and amphetamine were not collected at the same time as data for pyrrolidinophenones in the present study, but all of the in vitro experiments from both studies were conducted using similar assay conditions. Importantly, MDPV and α-PVP are similar in potency and transporter selectivity, indicating that the presence of the 3,4-methylenedioxy substituent in MDPV does not exert much influence on the profile of transporter activity. By contrast, alkyl chain length extending from the α-carbon is a critical structural feature, with shorter chain length (i.e., α-PPP, methyl) yielding less potent transporter-blocking properties when compared to longer chain length (i.e., α-PVP, propyl).
Consistent with their activity as catecholamine uptake blockers, all of the test drugs stimulate locomotion, and the rank order of in vivo potency parallels their potencies for blockade of DAT activity: MDPV>α-PVP>α-PBP>α-PPP. From a comparative perspective, all of the pyrrolidinophenones tested are more potent than cocaine at blocking DAT and at stimulating locomotion (Baumann et al., 2013b; Marusich et al., 2012).The time course of locomotor effects produced by the pyrrolidinophenones is comparable to what has been seen previously for various doses of cocaine and low doses of methamphetamine, with locomotor effects dissipating over the course of 60 min. In contrast, higher doses of methamphetamine can sustain locomotor increases throughout a 90 min session (Marusich et al., 2012). The present results confirm previous reports that MDPV is a powerful locomotor stimulant (Aarde et al., 2013; Fantegrossi et al., 2013; Gatch et al., 2013; Marusich et al., 2012), and extend this observation to other pyrrolidinophenones. Additionally, the vivo potency findings indicate that DAT blockade, inhibition of dopamine uptake, and the ensuing increase in extracellular dopamine seem essential for stimulant effects. Results of the FOB show that all test drugs produce typical psychomotor stimulant actions, similar to what we have found previously for cocaine and methamphetamine (Marusich et al., 2012), though each drug produces somewhat unique effects at higher doses. At the higher doses, hyperactivity was occasionally accompanied by bizarre behaviors such as jumping or rearing while facing away from the wall, or other atypical behavior such as ataxia, flattened body posture, or retropulsion. These behaviors are similar to those observed previously with other structural classes of synthetic stimulants found in bath salts (Marusich et al., 2012). It is noteworthy that our previous study comparing the in vivo effects of synthetic stimulants to cocaine and methamphetamine (Marusich et al., 2012) was conducted under identical conditions to the experiments described here, making the results from the two studies comparable.
The antagonist experiments with SCH23390 support the role of dopamine D1 receptors in mediating locomotor effects of pyrrolidinophenones. Activation of D1 receptors expressed on medium spiny output neurons in the striatum has been implicated in psychomotor stimulant effects of cocaine, amphetamine and other stimulants, suggesting that this mechanism may also be relevant to the acute actions of pyrrolidinophenones (Lobo and Nestler, 2011; Smith et al., 2013). While few studies have examined the effects of D1 antagonists on behaviors induced by newer synthetic stimulants, previous investigations have found that SCH23390 inhibits locomotor activation produced by mephedrone in rats (Lisek et al., 2012), and produces increased self-administration of cathinone in rats (Gosnell et al., 1996). Such results suggest that the D1 receptor subtype mediates the effects of cathinone-related stimulants on various types of rodent behavior, in addition to those behaviors examined in the present study. Research with ring-substituted amphetamine analogs has shown that DAT-selective compounds produce powerful stimulant and abuse-related effects in rodents (Bauer et al., 2013; Baumann et al., 2011). Compounds with mixed DAT and SERT activity have reduced stimulant qualities, apparently due to serotonergic dampening of dopamine-mediated effects (Baumann et al., 2011; Wee et al., 2005). Therefore, it might be predicted that the catecholamine selectivity of pyrrolidinophenones may render these agents especially addictive, due to lack of inhibitory serotonergic effects. This hypothesis warrants further investigation. Additionally, given the affinity of the pyrrolidinophenones for NET, future studies should test the ability of norepinephrine receptor antagonists to influence the behavioral effects of these stimulants. It will also be crucial to examine the pharmacology of any identified brain penetrant metabolites of α-PVP, α-PBP, and α-PPP, since bioactive metabolites may play a role in the in vivo effects of these drugs.
Human drug users prefer stimulants that possess a quick onset and short duration of action, similar to that of cocaine (Fischman, 1989), so humans are likely to prefer rapidly-acting pyrrolidinophenones over other types of stimulants. Due to the high potency of MDPV and α-PVP in comparison to prototypic stimulants such as cocaine, these synthetic stimulants may have higher abuse potential. Synthetic cathinone abuse can cause severe and life-threatening side effects in humans. MDPV has been implicated as a main culprit in causing toxic effects such as tachycardia, agitation, psychosis, and violent behaviors (Murray et al., 2012; Penders et al., 2012; Spiller et al., 2011; Wyman et al., 2013). One of the more severe side effects is excited delirium, a syndrome often accompanied by hyperthermia, rhabdomyolysis and kidney failure (Murray et al., 2012; Penders et al., 2012; Wyman et al., 2013). It is well established that excited delirium can be produced by cocaine and other psychomotor stimulants (Byard et al., 2011; Samuel et al., 2009), and a postmortem human study found that excited delirium was correlated with altered dopamine transporter levels (Mash et al., 2009), implicating central dopamine mechanisms. The potent dopaminergic actions of α-PVP and other pyrrolidinophenones suggest the possibility that abuse of these stimulants may increase the risk for excited delirium and other toxic effects. As second generation pyrrolidinophenones become more widespread (Marinetti and Antonides, 2013; Shanks et al., 2012; U.S. Drug Enforcement Administration, 2013b), it is increasingly important to understand their potential for producing toxic effects in preclinical models.
5.0 Conclusion
In conclusion, the present study found that MDPV, α-PVP, α-PBP, and α-PPP are uptake blockers at DAT and NET. All of the pyrrolidinophenones are efficacious locomotor stimulants, with motor activation reversed by the dopamine D1 receptor antagonist SCH23390. A functional observational battery showed that all test drugs produce typical psychomotor stimulant effects at low doses, along with bizarre behaviors at higher doses. Our findings represent the first evidence that second generation analogs of MDPV are catecholamine-selective uptake blockers which may pose substantial risk for addiction and adverse effects in human users. The present results imply that the more potent pyrrolidinophenones, MDPV and α-PVP, will continue to appear in bath salt formulations due to their high DAT potency as compared to less potent compounds such as α-PBP and α-PPP. Future studies should examine the reinforcing effects and toxic potential of these drugs in animal models, as a means to inform public health policy and aid medical professionals who may encounter patients under the influence of these substances.
Bath salts are emerging drugs of abuse that contain legal and illegal cathinones.
Structurally related cathinones were assessed in neurochemical and behavioral assays.
α-PVP, α-PBP, and α-PPP are catecholamine transporter blockers and locomotor stimulants.
Typical stimulant effects occurred at low doses, and bizarre behaviors occurred at higher doses.
Analogs of MDPV may pose substantial risk for addiction and adverse effects in human users.
Acknowledgements
The authors thank Tim Lefever and Tony Landavazo for technical assistance. Research was generously supported by the Intramural Research Program at NIDA, RTI International internal research and development funds, and NIH/NIDA Grant DA12970. These sources of funding did not play any role in study design, data collection, analysis, and interpretation, in writing the report, or in the decision to submit the article for publication.
Abbreviations
- methylone
3,4-methylenedioxymethcathinone
- MDPV
3,4-methylenedioxypyrovalerone
- mephedrone
4-methylmethcathinone
- α-PBP
α-pyrrolidinobutiophenone
- α-PPP
α-pyrrolidinonpropiophenone
- α-PVP
α-pyrrolidinovalerophenone
- DAT
dopamine transporter
- FOB
functional observational battery
- NET
norepinephrine transporter
- SERT
serotonin transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Association of Poison Control Centers. Bath Salts Data. 2012. Updated January 5, 2012. [Google Scholar]
- Bauer C, Banks M, Blough B, Negus S. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br. J. Pharmacol. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacol. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J. Pharmacol. Exp. Ther. 2011;337:218–25. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive "bath salts": not so soothing. Eur. J. Pharmacol. 2013a;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacol. 2013b;38:1–11. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Sumnall HR, Measham F, Cole J. Analyses of second-generation “legal highs” in the UK: initial findings. Drug Test Anal. 2010;2:377–382. doi: 10.1002/dta.155. [DOI] [PubMed] [Google Scholar]
- Byard RW, Summersides G, Thompson A. Confluent muscle pallor: a macroscopic marker of cocaine-induced rhabdomyolysis. Forensic. Sci. Med. Pathol. 2011;7:364–366. doi: 10.1007/s12024-011-9229-6. [DOI] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Verkariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of "bath salts," produce opposite effects at the human dopamine transporter. Psychopharmacology. 2013;227:493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P, Ruoho AE. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur. J. Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- EMCDDA (European Monitoring Centre for Drugs and Drug Addiction) EMCDDA and Europol step up information collection on mephedrone. 2010. Posted 03/11/13. Retrieved 8/26/13. [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: Drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacol. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW. Relationship between self-reported drug effects and their reinforcing effects: studies with stimulant drugs. NIDA Res. Monogr. 1989;92:211–230. [PubMed] [Google Scholar]
- Fleckenstein AE, Gibb JW, Hanson G. Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur. J. Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of 'bath salt' cathinones. Behav.Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Baird TJ. A functional observational battery in non-human primates for regulatory-required neurobehavioral assessments. J. Pharmacol. Toxicol. Methods. 2008;58:88–93. doi: 10.1016/j.vascn.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Yracheta JM, Bell SM, Lane KE. Intravenous self-administration of cathinone by rats. Behav. Pharmacol. 1996;7:526–531. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, et al. 4- Methylmethcathinone (mephedrone): Neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Reynaud M. GHB and synthetic cathinones: clinical effects and potential consequences. Drug Test Anal. 2011;3:552–559. doi: 10.1002/dta.210. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, et al. Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP. Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu Y-T, Reitz AB, Liu-Chen L-Y, et al. Mephedrone (’bath salt') elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front. Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br. J. Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti LJ, Antonides HM. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: Method development, drug distribution and interpretation of results. J. Anal. Toxicol. 2013;37:135–146. doi: 10.1093/jat/bks136. [DOI] [PubMed] [Google Scholar]
- Martínez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur. Neuropsychopharmacol. 2012;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Marusich J, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Duque L, Pablo J, Qin Y, Adi N, Hearn WL, et al. Brain biomarkers for identifying excited delirium as a cause of sudden death. Forensic Sci. Int. 2009;190:e13–e19. doi: 10.1016/j.forsciint.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J. Med. Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Clemens KJ, Apetz N, Winstock AR, Ramsey J, Li KM, et al. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: Neural consequences and comparison with methamphetamine. J. Psychopharmacol. 2013;27:823–836. doi: 10.1177/0269881113490325. [DOI] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug "bath salts" containing 3,4-Methylenedioxypyrovalerone (MDPV) J. Med. Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- Penders TM, Gestring RE, Vilensky DA. Intoxication delirium following use of synthetic cathinone derivatives. Am. J. Drug Alcohol Abuse. 2012;38:616–617. doi: 10.3109/00952990.2012.694535. [DOI] [PubMed] [Google Scholar]
- Psychonaut Research Web Mapping Project. MDPV report. London, UK: Institute of Psychiatry, King’s College London; 2009. [Google Scholar]
- Ross EA, Watson M, Goldberger B. "Bath salts" intoxication. N. Engl. J. Med. 2011;365:967–968. doi: 10.1056/NEJMc1107097. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, et al. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J. Pharmacol. Exp. Ther. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Samuel E, Williams RB, Ferrell RB. Excited delirium: Consideration of selected medical and psychiatric issues. Neuropsychiatr. Dis. Treat. 2009;5:61–66. doi: 10.2147/ndt.s2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, et al. Mephedrone (4-methylmethcathinone; 'meow meow'): Chemical, pharmacological and clinical issues. Psychopharmacology. 2011;214:593–602. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- Shanks KG, Dahn T, Behonick G, Terrell A. Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectrometry. J. Anal. Toxicol. 2012;36:360–371. doi: 10.1093/jat/bks047. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr. Opin. Neurobiol. 2013;23:546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of "bath salts" and "legal highs" (synthetic cathinones) in the United States. Clin. Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- U.S. Congress. Synthetic Drug Abuse Prevention Act. 2012. pp. 1–6. [Google Scholar]
- U.S. Drug Enforcement Administration. Synthetic Cannabinoids and Cathinones - DEA Request for Information. 2013a. Posted 01/22/13. Retrieved 8/26/13. [Google Scholar]
- U.S. Drug Enforcement Administration, Office of Diversion Control. National Forensic Laboratory Information System: Midyear Report 2012. Springfield, VA: U.S. Drug Enforcement Administration; 2013b. https://www.nflis.deadiversion.usdoj.gov. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Guidelines for neurotoxicity risk assessment. Risk assessment forum. Washington, DC: U.S. EPA; 1998a. 630/R-95/001F. Available from: http://www.epa.gov/raf/publications/guidelinesneurotoxicity-risk-assessment.htm. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Health effects guidelines: neurotoxicity screening battery. Washington, DC: U.S. EPA; 1998b. EPA 712-C-98-238. OPPTS 870.6200. Available from: http://www.epa.gov/ocspp/pubs/frs/publications/Test_Guidelines/series870.htm. [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, et al. The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts”. J. Addict. Res. Ther. 2012a;S9:002. doi: 10.4172/2155-6105.S9-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addicti. Biol. 2012b doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J. Pharmacol. Exp. Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Ramsey JD. Legal highs and the challenges for policy makers. Addiction. 2010;105:1685–1687. doi: 10.1111/j.1360-0443.2010.03163.x. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, et al. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS. One. 2012;7 (e):44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, et al. Postmortem tissue distribution of MDPV following lethal intoxication by "bath salts". J. Anal. Toxicol. 2013;37:182–185. doi: 10.1093/jat/bkt001. [DOI] [PubMed] [Google Scholar]