Abstract
Chlamydia muridarum and C. trachomatis, mouse and human strains respectively, have been used to study immunity in a murine model of female genital tract infection. Despite evidence that unique genes of these otherwise genomically similar strains could play a role in innate immune evasion in their respective mouse and human hosts there have been no animal model findings to directly support this conclusion. Here, we infected C57BL/6 and adaptive immune deficient Rag1−/− female mice with these strains and evaluated their ability to spontaneously resolve genital infection. Predictably, C57BL/6 mice spontaneously cleared infection caused by both chlamydial strains. In contrast, Rag1−/− mice which lack mature T and B cell immunity but maintain functional innate immune effectors, were incapable of resolving C. muridarum infection but spontaneously cleared C. trachomatis infection. This distinct dichotomy in adaptive and innate immune-mediated clearance between mouse and human strains has important cautionary implications for the study of natural immunity and vaccine development in the mouse model.
Keywords: Chlamydiae, human and mouse strains, Rag−/− mice, female genital tract, innate immunity, adaptive immunity
Chlamydia trachomatis is an obligate intracellular bacterial pathogen that infects mucosal surfaces of the eye and urogenital tract. In the United States infections of the urogenital tract represent the most common cause of bacterial sexually transmitted infection (STI) (CDC Grand Rounds, 2011) with an estimated 92 million STI occurring annually worldwide (WHO, 2001). Complications of chlamydial STI in women can be severe resulting in pelvic inflammatory disease, ectopic pregnancy, and tubal factor infertility (Brunham & Rey-Ladino, 2005). Control of chlamydial STI is currently focused on national screening programs and antibiotic therapy (Johnson et al., 2002); however the effectiveness of this approach in interrupting chlamydial transmission has been questioned (Rekart & Brunham, 2008). Consequently, there has been a focus on vaccine development as the next step for controlling chlamydial STI (Brunham & Rappuoli, 2013).
Towards this end investigators have employed a female mouse urogenital infection model where they have interchangeably used a naturally occurring C. muridarum strain (Barron et al., 1981; Swenson et al., 1983) or human C. trachomatis urogenital isolates (Tuffrey et al., 1986) to study infection mediated immunity and vaccinology. The general paradigm that has collectively emerged for this work is that immunity against both mouse and human strains is largely the result of the adaptive immune response; specifically Th1 cells producing IFN-γ (Johansson et al., 1997; Morrison & Caldwell, 2002). However, there have been no reports that have directly examined the anti-chlamydial effects of the adaptive versus the innate arm of the host’s immune response against mouse and human chlamydial organisms in a head on comparison. Defining the roles of adaptive and innate immunity in this model is important as it directly affects conclusions about the relative roles of Th1 mediated immunity which have important consequences in the design and development of chlamydial vaccines.
C. trachomatis and C. muridarum are remarkably similar genetically sharing a high conservation in gene content and order (Read et al., 2003). Only a few open reading frames differ between the species and they are primarily located within the organism’s plasticity zone (PZ) (Read et al., 2003). It has been hypothesized that these pathogen-specific PZ genes play an important role in avoiding host specific IFN-γ induced immunity in mice and humans (Nelson et al., 2005). Thus, this host-pathogen interaction might influence both the susceptibility and infection dependent immunity observed by these strains in their natural hosts.
Here, we addressed the relative roles of murine innate and adaptive immunity in the spontaneous clearance of female urogenital tract infections caused by C. muridarum and C. trachomatis in recombination activation gene 1 deficient (Rag1−/−) mice. Rag deficient mice lack mature T and B cell adaptive immunity (Mombaerts et al., 1992), but retain normal innate immune functions including IFN-γ secreting NK cells (Shinkai et al., 1992). An advantage of using Rag1−/− mice instead of nude or severe combined immunodeficiency (SCID) mice for these experiments is that Rag1−/− mice are not “leaky” (Mombaerts et al., 1992) thereby providing an unambiguous interpretation for the roles of innate and adaptive immunity to chlamydial infection. We show that resolution of C. muridarum infection is dependent on adaptive immunity. Conversely, resolution of C. trachomatis infection is largely independent of adaptive immunity and is controlled by innate immunity.
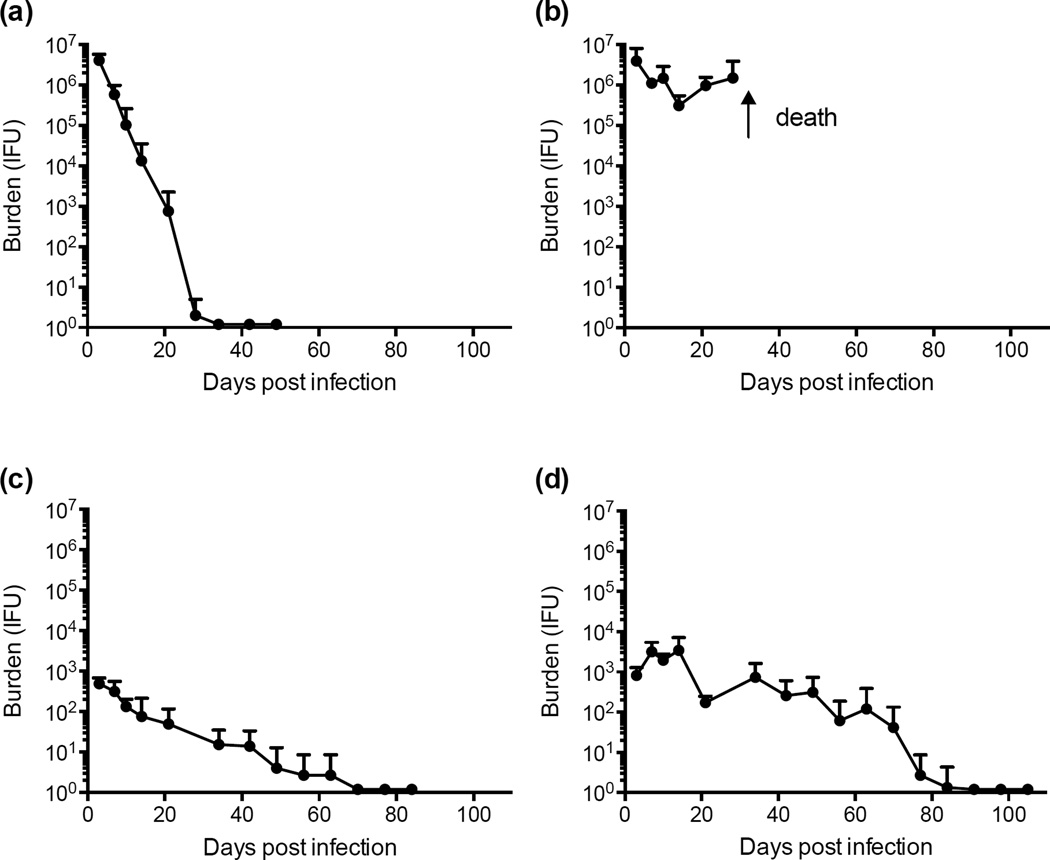
Progesterone treated female eight-week old C57BL/6 wild type and C57BL/6-derived Rag1−/− mice (Jackson Laboratory) were each infected cervico-vaginally with 1×105 inclusion forming units (IFU) of either C. muridarum (Weiss strain) or C. trachomatis serovar D, strain D-LC (Sturdevant et al., 2010). Five to ten mice were infected with each chlamydial strain. All animal procedures used throughout this study were conducted in accordance with Animal Care and Use Guidelines and were reviewed and approved by the Animal Care and Use Committee at RML. Chlamydial burdens and infection duration were monitored at weekly intervals by swabbing the vaginal vault and culturing recoverable organisms on monolayers of McCoy cells. Two-way ANOVA statistical analyses were calculated comparing strain infection course curves. The results are shown in Figure 1. C. muridarum genital tract infection of C57BL/6 female mice produced a self-limiting infection that cleared spontaneously by day 34 post-infection (PI, Fig. 1a). Infectious burdens were high (106 IFU) during the early time periods PI (days 3–7) and then rapidly decreased until infections resolved. In contrast, C. muridarum infected Rag1−/− mice yielded similarly high numbers of recoverable IFUs that were sustained over the first 28 days PI (P ≤ 0.02 at days 7, 21; Fig. 1b). C. muridarum infected Rag−/− mice developed a rampant lethal systemic infection by 28 days post-vaginal infection as determined by the isolation of C. muridarum from the spleen, lung, and liver of infected animals; findings similar to those reported by Cotter (Cotter et al., 1997) using IFN-γ knockout (KO) mice. These results demonstrate that spontaneous clearance of C. muridarum infection from the female genital tract and prevention of disseminating genital tract infection is dependent on an adaptive immune response.
Figure 1. Infectivity of C. muridarum and C. trachomatis in C57BL/6 and Rag1−/− female mice.
(a) C. muridarum infected C57BL/6 mice. (b) C. muridarum infected Rag1 −/− mice. (c) C. trachomatis infected C57BL/6 mice. (d) C. trachomatis infected Rag1−/− mice. At days 3, 7, 10, 14, and weekly intervals thereafter cervico-vaginal specimens were collected and recoverable IFU enumerated by titration on monolayers of McCoy cells. The results show the five-mouse mean chlamydial burden and duration of infection for each group over the entire period with standard deviations for each time point indicated. C. muridarum infected Rag1−/− mice developed disseminating infection resulting in death at approximately five weeks post infection.
In contrast, C57BL/6 and Rag1−/− female mice infected with C. trachomatis spontaneously cleared infection. Rag1−/− mice (Fig. 1d) produced higher infectious burdens (P ≤ 0.05 at days 7, 10, 21) than C57BL/6 animals (Fig. 1c) over the entire culture positive period with a similar time required to completely resolve infection. These results show that the mouse innate immune response is capable of eradicating C. trachomatis genital tract infection. The reduction in infectious burdens between C57BL/6 and Rag1−/− over the entire infection period implicates a dual role for adaptive and innate immunity in spontaneous clearance; nevertheless, it is patently clear that the primary immune component that controls C. trachomatis infection in the mouse genital tract is innate, not adaptive, immunity. These results are the first to definitively show in a side-by-side study that adaptive and innate immunity play distinct roles in control of C. muridarum and C. trachomatis infection of the female mouse genital tract.
Tuffrey (Tuffrey et al., 1982) and Rank (Rank et al., 1985) previously reported on C. trachomatis and C. muridarum infection of female genital tract in nude mice, respectively. Their findings were similar to those described herein; C. trachomatis infection of the female mouse genital tract resolved spontaneously in the absence of T cells (Tuffrey et al, 1982) whereas T cells were required for the resolution of C. muridarum infection (Rank et al, 1985). An important difference between those studies and ours is that these investigators used nude mice which have greatly reduced, but not complete deficiency, in T cell immunity (Belizario, 2009). Consequently, a definitive role, or lack of a role, for T cells in immunity cannot be concluded from their work. In contrast, Rag−/− mice are entirely deficient for both T and B cell immunity. Therefore our findings provide a conclusive answer with respect to the roles of innate and adaptive immunity against the human and mouse strains. We do not know what innate immune mechanism(s) are responsible for the resolution of C. trachomatis infection. We speculate, because of the strong inhibitory function of IFN-γ in both in vivo (Johansson et al., 1997; Perry et al, 1999) and in vitro (Nelson et al., 2005; Roshick et al., 2006) murine infection models, that IFN-γ secreting local NK cells could be important. However, additional studies using NK KO and double NK-IFN-γ KO mice will be required to answer this question. Lastly, our findings and those of Williams (Williams et al., 1988) and Rank (Rank et al., 1992) are not in complete agreement. They showed that C. muridarum infection of both the lung and genital tract can be protracted by IFN-γ treatment. In contrast, we (Perry et al., 1999) and others (Cotter et al., 1997) have shown that IFN-γ is not essential for the clearance of C. muridarum from the genital tract but does prevent disseminating infection and death. A possible explanation for these discrepancies is that different C. muridarum strains were used in these reports. The Perry, Cotter, and Williams studies used the C. muridarum Weiss strain whereas Rank used the Nigg strain. Interestingly, Ramsey (Ramsey et al., 2009) recently showed that the Weiss strain is more virulent than the Nigg strain in the mouse model. We believe that the use of strains differing in virulence could at least in part explain these conflicting findings. Nevertheless, collectively the results warrant further studies in this model using additional strains and isolates.
In summary, because the mouse innate immune system is capable of independently controlling urogenital infections of the female genital tract caused by human C. trachomatis strains, studies designed to ascertain natural adaptive immune control or vaccine mediated protective immunity using human isolates should be interpreted with cautionary implications.
ACKNOWLEDGEMENTS
We thank Kelly Matteson for editorial assistance and Dan Sturdevant for performing statistical analyses. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Barron AL, White HJ, Rank RG, Soloff BL, Moses EB. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981;143:63–66. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- 2.Belizario JE. Immunodeficient mouse models: An overview. The Open Immunology Journal. 2009;2:79–85. [Google Scholar]
- 3.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 4.Brunham RC, Rappuoli R. Chlamydia trachomatis control requires a vaccine. Vaccine. 2013;31:1892–1897. doi: 10.1016/j.vaccine.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC Grand Rounds. Chlamydia Prevention: Challenges and strategies for reducing disease burden and sequelae. MMWR. 2011;60:370–373. [PubMed] [Google Scholar]
- 6.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson M, Schön K, Ward M, Lycke N. Studies in knockout mice reveal that anti-chlamydial protection requires TH1 cells producing IFN-gamma: is this true for humans? Scand J Immunol. 1997;46:546–552. doi: 10.1046/j.1365-3083.1997.d01-167.x. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RE, Newhall WJ, Papp JR, et al. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections. MMWR Recomm Rep. 2002;51:1–38. [PubMed] [Google Scholar]
- 9.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 10.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson DE, Virok DP, Wood H, et al. Chlamydial IFN-gamma immune evasion is linked to host infection tropism. Proc Natl Acad Sci. 2005;102:10658–10663. doi: 10.1073/pnas.0504198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry LL, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell HD. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- 13.Ramsey KH, Sigar IM, Schripsema JH, Denman CJ, Bowlin AK, Myers GA, Rank RG. Strain and virulence diversity in the mouse pathogen Chlamydia muridarum. Infect Immun. 2009;77:3284–3293. doi: 10.1128/IAI.00147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rank RG, Soderberg LS, Barron AL. Chronic chlamydial genital infection in congenitally athymicnude mice. Infect Immun. 1985;48:847–849. doi: 10.1128/iai.48.3.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rank RG, Ramsey KH, Pack EA, Williams DM. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Read TD, Myers GS, Brunham RC, et al. Genome sequence of Chlamydophilacaviae (Chlamydiapsittaci GPIC): examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 2003;31:2134–2147. doi: 10.1093/nar/gkg321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rekart ML, Brunham RC. Epidemiology of chlamydial infection: are we losing ground? Sex Transm Infect. 2008;84:87–91. doi: 10.1136/sti.2007.027938. [DOI] [PubMed] [Google Scholar]
- 18.Roshick C, Wood H, Caldwell HD, McClarty G. Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells. Infect Immun. 2006;74:225–238. doi: 10.1128/IAI.74.1.225-238.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinkai Y, Rathbun G, Lam KP, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 20.Sturdevant GL, Kari L, Gardner DJ, et al. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infect Immun. 2010;78:3660–3668. doi: 10.1128/IAI.00386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swenson CE, Donegan E, Schachter J. Chlamydia trachomatis-induced salpingitis in mice. J Infect Dis. 1983;148:1101–1107. doi: 10.1093/infdis/148.6.1101. [DOI] [PubMed] [Google Scholar]
- 22.Tuffrey M, Falder P, Taylor-Robinson D. Genital-tract infection and disease in nude and immunologically competent mice after inoculation of a human strain of Chlamydia trachomatis. Br J Exp Pathol. 1982;63:539–546. [PMC free article] [PubMed] [Google Scholar]
- 23.Tuffrey M, Falder P, Gale J, Taylor-Robinson D. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br J Exp Pathol. 1986;67:605–616. [PMC free article] [PubMed] [Google Scholar]
- 24.Williams DM, Byrne GI, Grubbs B, Marshal TJ, Schachter J. Role in vivo for gamma interferon in control of pneumonia caused by Chlamydia trachomatis in mice. Infect Immun. 1988;56:3004–3006. doi: 10.1128/iai.56.11.3004-3006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. World Health Organization. Geneva, Switzerland: 2001. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. [Google Scholar]