Abstract
Background
Individuals with schizophrenia demonstrate deficits in context processing. These deficits can be characterized by examining the influence of auditory context on ERP responses to rare target tones. Previous studies demonstrate that target ERP deficits in schizophrenia depend on the number of non-targets that precede the target ERP. Our goal was to extend these findings by examining whether patients with schizophrenia demonstrate a reduced sensitivity to subtle differences in the auditory context preceding rare target stimuli, as quantified by Itti and Baldi’s Bayesian prediction error model.
Methods
Cortical responses to auditory oddball tones were measured within 59 individuals with schizophrenia (SZ) and 59 controls (HC). Individual trial amplitudes were estimated by conducting group ICA on the EEG time series and analyzing the reconstructed individual temporal sources. We quantified the auditory context of target tones using the Bayesian prediction error model and determined whether ERP amplitudes to tones were sensitive to this measure of context, or the number of preceding non-targets directly, within HC and SZ.
Results
Individuals with schizophrenia show a significant reduction in ERP response amplitudes to targets approximately 244 – 412 ms following target onsets. Individual amplitudes within this window showed significantly greater sensitivity to modeled prediction error within the controls than in individuals with schizophrenia. These differences approached significance when examining differences in amplitudes as a function of the number of preceding non-targets.
Conclusions
These findings further clarify differences in HC and SZ with regards to their attentional and perceptual sensitivity to subtle environmental regularities.
Keywords: perceptual organization, context processing, acoustic regularities, Bayesian prediction error, auditory oddball, schizophrenia
1. Introduction
A prominent characteristic of schizophrenia is a perceived loss of environmental continuity or a reduced ability to integrate multiple stimuli coherently (Cox & Leventhal, 1978). These deficits in perceptual organization appear to underlie a number of symptoms of schizophrenia, including a reduced ability to integrate visual elements into a coherent whole (Butler et al., 2001; Place & Gilmore, 1980) and a reduced ability to utilize auditory context when detecting auditory targets (Silverstein, et. al. 1996).
Perceptual organization deficits can be examined in greater detail by examining event-related potential (ERP) responses to a series of frequent and infrequent auditory stimuli, as in auditory oddball tasks. It has long been known that individuals diagnosed with schizophrenia demonstrate reduced ERP amplitudes ~300ms following rare auditory targets (Levit, Sutton, & Zubin, 1973; Roth & Cannon, 1972). These responses, termed the P3, appear sensitive to the sequence of auditory tones that precede the behaviorally relevant target. For example, Gonsalvez et al., 1995 demonstrate P3 amplitude differences between controls and schizophrenia patients when targets appear after 3–7 non-targets, but not after a shorter (1) or longer (9) sequence of non-targets. Javitt, et. al., 1998 demonstrate greater ERP amplitude differences in schizophrenia in blocks with a reduced probability of rare stimuli (i.e. in situations where rare stimuli generally appear following a greater number of non-targets).
These oddball ERP deficits in schizophrenia patients suggest sensitivity differences between patients and controls with respect to the acoustic context (Gilmore, et. al., 2005; Gonsalvez et al., 1995; Javitt et al., 1998; Shelley, et. al., 1999). In addition to manipulating rare target probability or the number of preceding non-targets, the acoustic context can be further characterized by modeling the level of Bayesian surprise or prediction error of rare stimuli (Baldi and Itti, 2010; Itti and Baldi, 2005, 2009). According to this model, individuals have a prior expectation of the probability in which each stimulus occurs. This prior expectation is updated when a new stimulus is encountered, and the prediction error is reflected by the degree in which the probability is updated. Stimuli that are more unexpected correspond to greater differences between the two probabilities, which corresponds to greater prediction error (Baldi and Itti, 2010; Itti and Baldi, 2005, 2009), and a greater ERP modulation (Lieder, et. al., 2013).
Our goal was to examine whether patients with schizophrenia demonstrate a reduced sensitivity to differences in the context of rare target stimuli, as quantified by the Bayesian prediction error model. This model allows estimates of ERP amplitudes for individual targets even when the number of preceding non-targets is fixed, and when a target follows another target. Thus, the model allows additional flexibility in the complexity of the auditory environment (i.e. with non-target intervals between 0 and 20), in contrast to previous studies which focus on a comparatively reduced range of non-target intervals (Gilmore et al., 2005; Gonsalvez et al., 1995), or broad measures of regularity such as target probability (Javitt et al., 1998; Shelley et al., 1999). In order explore differences in auditory regularity sensitivity between the two groups, individual target ERP responses were linearly fit to the modeled prediction error, or the number of preceding non-targets, and differences in the linear fit between the HC and SZ groups was demonstrated. These results help further clarify differences between schizophrenia patients and healthy controls with respect to their sensitivity to auditory regularities.
2. Methods
2.1 Participants and Procedures
One hundred and eighteen individuals participated at the Institute of Living at Hartford Hospital. The study was conducted in accordance with an experimental protocol approved by the Institutional Review Board (IRB). Trained clinicians acquired medical history, conducted Structured Clinical Interviews for DSM-IV diagnosis (First, et. al., 1997), and acquired symptom information (Positive and Negative Syndrome Scale (Lancon, et al., 2000)). Further details on recruitment, diagnosis, and exclusion criteria can be found in Ethridge et al., 2012 and Supplementary Methods.
Among the 118 participants, 59 were healthy controls, 45 were diagnosed with schizophrenia, and 14 were diagnosed with schizoaffective disorder, depressed type using DSM-IV criteria based on a SCID interview. The two diagnostic groups were combined into a single group with schizophrenia spectrum psychotic disorders (SZ group), since the schizoaffective depressed type patients demonstrate similar overall cognitive impairment as patients diagnosed with schizophrenia (Hill et al., 2013). Medication information and mean symptom scores are provided in Supplementary Methods and Supplementary Table 1. The healthy control group consisted of 27 males (mean age = 35 ± 11 years) and 32 females (mean age = 36 ± 11 years). The SZ group consisted of 42 males (mean age = 30 ±10 years) and 17 females (mean age = 36 ± 13 years). The groups did not significantly differ in age (T(116) = 1.656; p = 0.100), but showed significant differences in sex (χ2 = 7.853; p = 0.005). Sex was included as a covariate in all statistical tests examining differences between the two groups.
2.2 Oddball Task
Individuals pressed a button when an infrequent target tone (1000 Hz) appeared within a stream of frequent standard tones (1500 Hz). The tones were delivered by two 8-ohm speakers 50 cm away from the individual. The tones were presented every 1.3 seconds over the course of the 14 min 50 sec experimental session. The tones were presented in a pseudorandom order with target tones appearing 15% of the time (n = 98).
2.3 EEG acquisition and preprocessing
EEG was recorded with a 66-channel Neuroscan system (Compumedics, Charlotte, NM). Silver/silver chloride electrodes were placed according to the International 10-10 system with a mid-forehead ground and nose reference (sampling rate = 1000 Hz; impedance ≤ 5 kΩ). An additional four electrodes were used to record horizontal and vertical eye movements. EEG preprocessing was conducted in Matlab (http://www.mathworks.com) using custom functions, built-in functions, and the EEGLAB toolbox (http://sccn.ucsd.edu/eeglab). The EEG data was linearly detrended, forward and backward filtered with a Butterworth filter (bandpass: 0.01 to 50 Hz), downsampled to 250 Hz, and average referenced. Eye blink and muscle artifacts were attenuated by conducting a temporal ICA decomposition on the individual recordings (see Supplementary Methods).
2.4 Group temporal ICA of EEG
The average difference between the target and standard responses is indicated in Supplementary Fig. 1 for the HC (N = 59) (in red) and SZ groups (N = 59) (in blue). The results are consistent with previous studies indicating an increased ERP modulation to the rare relevant tone within healthy controls. Group temporal ICA was used to decompose the multiplexed ERP response into distinct components which potentially reflect the distinct ERP peaks (Eichele, et. al., 2011; Supplementary Fig. 2 and Supplementary Methods).
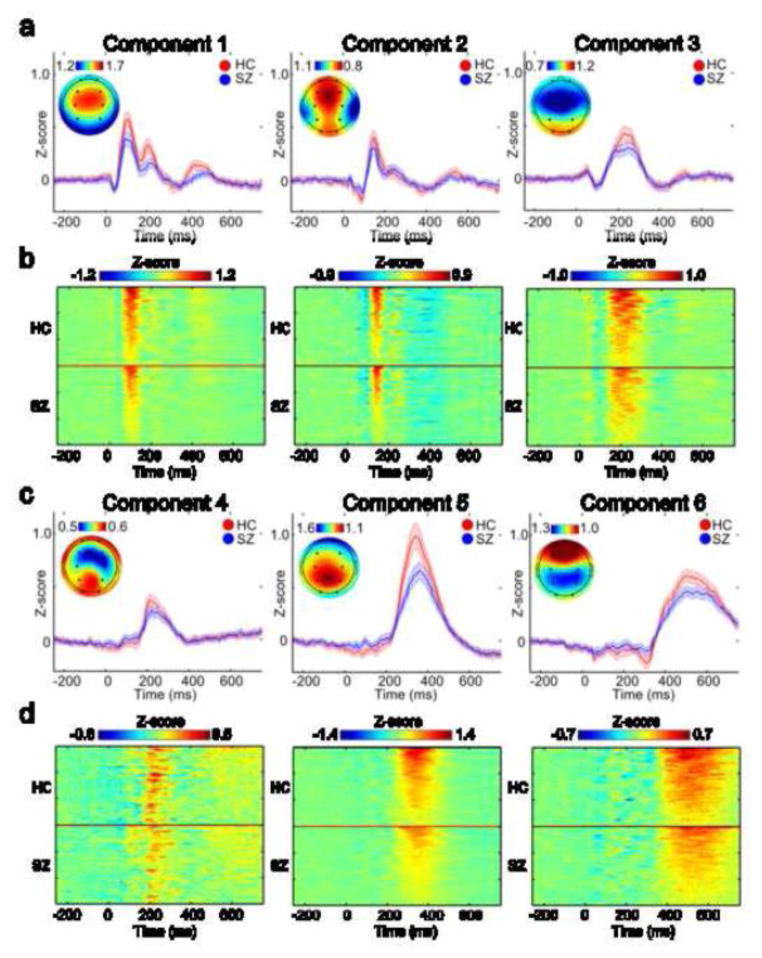
Single trial group responses were subtracted by the average response within −250 ms to −4 ms to correct for baseline fluctuations. Six components were selected for further analysis since they demonstrated a peak following either the standard or target tone. Group component amplitudes were calculated for each tone by averaging between the interval that corresponds to the full width half maximum of the component peak response across targets and standards (see Table 1). Component topographies were determined by averaging the individual mixing matrices across subjects. The average individual amplitudes were calculated from the back-reconstructed single subject data, as implemented in EEGIFT software (Eichele et al., 2011), using the same temporal window as the group component.
Table 1.
Peak time, window (full width half maximum, fwhm), and peak location of the overall average ERP response for the 6 selected components. O = occipital, T = temporal, P = parietal, C = central, F = frontal.
| Component | Peak time (ms) | Peak window (fwhm) (ms) | Spatial peak Min (z-score) | Spatial peak Max |
|---|---|---|---|---|
| 1 | 96 | 68 – 136 | O/T (−1.7) | F/C (1.2) |
| 2 | 144 | 120 – 168 | T (−1.2) | F/C (0.7) |
| 3 | 196 | 148 – 264 | O (−0.7) | F/C (1.3) |
| 4 | 208 | 172 – 240 | F/C (−0.5) | F/O (0.6) |
| 5 | 340 | 244 – 412 | O/P (−1.1) | F (1.6) |
| 6 | 484 | 372 – 656 | O/P (−1.0) | F (1.3) |
2.5 Auditory statistical regularities
The prediction error of each tone was modeled using the model of Baldi and Itti (Baldi and Itti, 2010; Einhäuser, et al., 2007; Itti and Baldi, 2009; Itti and Baldi, 2005), implemented in Nathan Mundhenk’s Bayesian Surprise Matlab Toolkit (http://sourceforge.net/projects/surprise-mltk/). In order to calculate Bayesian surprise or prediction error, we assume that individuals probabilistically model the frequency in which a particular tone will appear based upon the sequence of previous tones. This probability, or model M, is updated with a new observation, D = λ, generating a posterior distribution of the expected stimulus frequency. The prior and posterior densities are characterized by Gamma densities γ(λ;α,β) and (λ;α′,β′), respectively. The posterior density is adjusted given a new observation λ by
where the “working memory” parameter ζ adjusts α and β to reduce the influence of less recent stimuli. Zeta (ζ was set to 0.7 in the current study, which is consistent with Itti and Baldi (2009) and estimates of neural adaptation (Muller, et. al., 1999).
The difference between each density is given by the Kullback-Leibler (KL) divergence (Kullback, 1959) between the posterior and prior gamma (γ) densities as given by:
where Γ and Ψ are the Euler gamma and digamma functions, respectively (see Itti and Baldi, 2009 for additional details). Frequent (i.e. standard) tones were coded as λ̄ = 1 and infrequent target tones were coded as λ̄ = 2. The greater the stimulus deviates from expectations, the greater difference between the posterior probability density and the prior probability density, and the larger the prediction error S. The prediction error for individual stimuli in a sequence is demonstrated in Fig. 1. Supplementary Fig. 3 demonstrates the stimulus sequence for a subset of the targets, organized by their level of prediction error.
Figure 1.
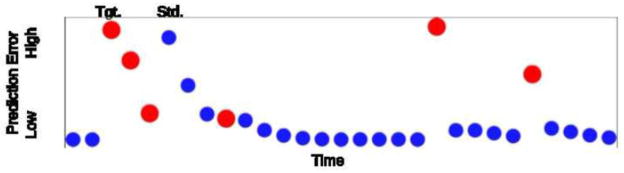
A series of frequent (standard; small blue circles) and infrequent (target; large red circles) tones are depicted. The position along the x axis indicates the position of the tone in time, and the position along the y-axis indicates the modeled prediction error of each tone given the recent history of tones presented, as described in the Methods 2.5.
2.6 Statistical analysis
Statistical tests are reported as “significant” if they pass Holm-Bonferroni correction for the 24 comparisons conducted (alpha = .05) (Holm, 1979). Further details are provided in Supplementary Methods.
3. Results
3.1 Behavior
The SZ group responded to fewer target’s than the HC group, with average hit rates of 0.910 ± 0.028 and 0.956 ± 0.017, respectively (T(116) = 2.82, p = .005). The differences in hit rate appear to result due to differences in perceptual sensitivity between the two groups (see Supplementary Results). The SZ group was slower at responding to targets than the HC group, with average target reaction times of 447 ± 22 ms and 401 ± 18 ms, respectively (T(116) = 3.13, p = .002). The false alarm rates were 0.002 ± 0.001 and 0.007 ± 0.006 within HC and SZ, respectively.
3.2 Group temporal ICA of EEG
The average difference between the target and standard responses is indicated in Supplementary Fig 1 for HC (N = 59) (in red) and SZ (N = 59) (in blue). The results are consistent with previous studies indicating a reduced ERP modulation to the rare relevant tones within SZ, and with previous studies analyzing a larger dataset which included individuals within the current group (Ethridge et al., 2014; Ethridge et al., 2012). The predominance of noise within unaveraged ERP responses motivated further processing steps for single trial analysis. Group ICA was used to decompose the multiplexed ERP response into distinct components which potentially reflect the distinct ERP peaks. The components were averaged across epochs for target and standard tones and 6 out of 15 components were selected for further analysis since they demonstrate an average peak to either the standard or target tones.
The average event-related component response is indicated in Fig. 2a and 2c for target (red) and standard tones (blue) along with the average topography across all subjects. The peak time, window, and topographic peak locations are indicated in Table 1. The individual time courses were determined by back reconstructing the group components on the individual data. Fig. 2b and 2d demonstrate the reconstructed response averaged across targets. Each row indicates the response for a particular subject, with HC above the solid line and SZ below.
Figure 2.
Average and individual Component ERP responses to tones. The average ERP response to targets is shown in (a) for HC (N = 59) (red) and SZ (N = 59) (blue). The error bars represent the 95% confidence interval. The topographic plots indicate the average spatial loadings (118 subjects) for each group temporal component. The large black circles within each topographic plot denote electrodes Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, and O2 (from top left to bottom right). The average back reconstructed response to tones is indicated for each subject in (b). Each row represents the response for an individual subject. The HC group is displayed above the solid line and the SZ group is displayed below the solid line. The individual responses are organized for each group with the largest differences on top. The graphs are repeated for components 4, 5, and 6 in c and d.
3.3 Behavioral relevance and regularities
We found that the component amplitude to behaviorally relevant infrequent tones were significantly larger than component amplitudes to the irrelevant frequent standard tones for all 6 selected components (Table 2, rows 2 and 3). Differences in amplitudes between HC and SZ were examined with an ANOVA analysis with the difference in reconstructed amplitudes between targets and standards as the independent variable, and diagnosis and sex as dependent variables. Amplitudes significantly differed between the HC and SZ groups for components 5 and 6 (F(1,116) = 18.6; p = 0.00003, F(1,116) = 10.0; p = 0.002), with target responses significantly larger (i.e. more positive) than standard responses for HC compared to SC (table 2, rows 6 and 7). These results indicate that HC and SZ show differences in auditory processing ~340 and ~484 ms following an auditory stimulus.
Table 2.
Statistical tests for differences in the components and reconstructed responses. The Component Difference section displays the results for component differences between target and standard amplitudes (relevance) and correlations between component amplitudes and the prediction error (regularity) for targets. The Reconstructed Difference section displays the results for relevance and regularity differences between HC and SZ. The results are displayed for 6 selected components.
| Component Difference | Reconstructed Difference | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Group Source | Relevance | Regularity | Relevance | Regularity | ||||
| T(654) | p | r(96) | p | F(1,116) | p | F(1,116) | p | |
| 1 | 7.9 | ≪.001 | .19 | .060 | 3.8 | .055 | 3.6 | .060 |
| 2 | 9.6 | ≪.001 | −.01 | .912 | 2.3 | .129 | 0.0 | .947 |
| 3 | 3.56 | ≪.001 | .09 | .374 | 0.1 | .760 | 0.0 | .867 |
| 4 | 4.8 | ≪.001 | .11 | .280 | 1.0 | .314 | 0.4 | .545 |
| 5 | 71.3 | ≪.001 | .54 | ≪.001 | 18.6 | .00003 | 10.6 | .002 |
| 6 | 65.7 | ≪.001 | .45 | ≪.001 | 10.0 | .002 | 0.4 | .545 |
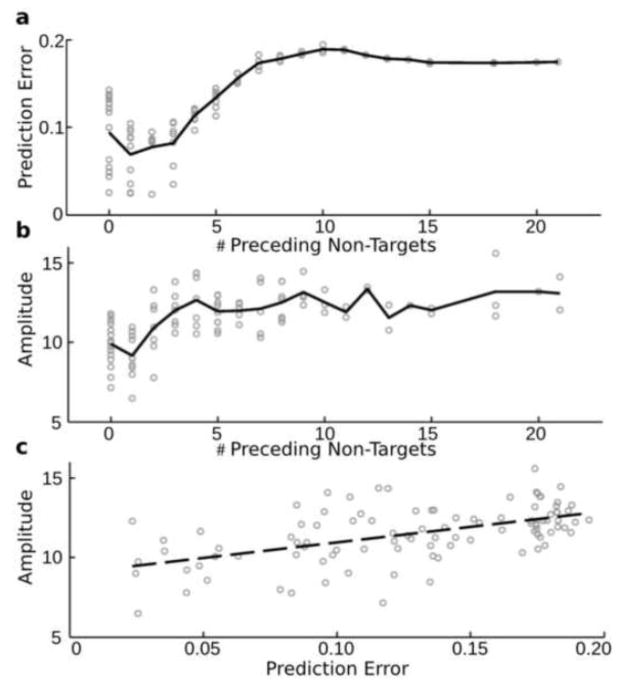
In addition to differences in the response to targets and standards, we also examined whether the response to individual standard tones was modulated by the subtle auditory context, as indicated by the tones prediction error. The modeled prediction error was plotted for each target as a function of the number of preceding non-targets averaged across both groups (Fig 3a). Similar trends are observed when comparing the prediction error (Fig 3a) and the average amplitude for component 5 (Fig. 3b) and component 6 (Supplementary Figure 4b) against the number of preceding non-targets. In general, these figures suggest a non-linear relationship between amplitude and the number of preceding non-targets. This relationship appears linear when amplitudes are plotted against the modeled prediction error (Fig 3c and Supplementary Figure 4c), suggesting that prediction error may be a more appropriate dependent variable in linear statistical tests.
Figure 3.
Estimated prediction error and observed target amplitudes as a function of preceding non-targets. The prediction error (y-axis) is indicated for all stimuli (excluding the first 9) as a function of the number of previous non-targets (x-axis) in a. The amplitude (y-axis) is plotted in b, and c, for component 5 against the number of preceding non-targets (in b) or against the prediction error (in c). The solid lines indicate the average level of prediction error (in a) or the average amplitude (in b) for the particular x axis value. The dotted line in c indicates the linear fit between amplitude and prediction error.
We found that the two group components with the greatest delay (e.g. components 5 and 6) each demonstrate a significant correlation with prediction error (r(96) = 0.54; p < 0.001; r(96) = 0.45; p < 0.001), with increases in prediction error associated with increased ERP amplitudes (table 2, rows 4 and 5). Differences in the sensitivity to prediction error between HC and SZ were examined with an ANOVA analysis. The individual subject amplitudes to target tones were linearly fit to the prediction error for each tone, and the slope of the fit served as the independent variable, with diagnosis and sex as dependent variables. Slopes significantly differed between the HC and SZ groups for component 5 (F(1,116) = 10.6; p = 0.002) (which overlaps with the P3 ERP component, see Supplementary Figure 5 and Supplementary Results). This difference is characterized by a greater slope in the linear fit between amplitude and prediction error within the HC group compared to the SZ group. The relationship between the component amplitude and the target prediction error is indicated in Fig 4, along with the average amplitude for each target separately for HC and SZ. This relationship approached uncorrected significance when the amplitudes were linearly fit to the number of preceding non-targets instead of prediction error (F(1,116) = 3.8; p = 0.053).
Figure 4.
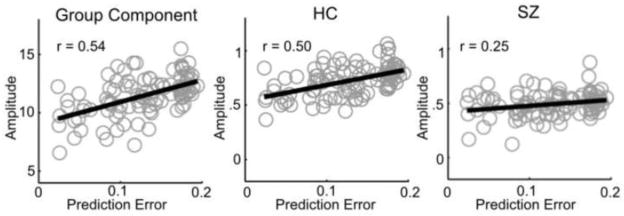
Sensitivity to prediction error. The amplitude to each target tone is plotted (y-axis) against the prediction error of the tone (x-axis) for component 5 (left) and for the component 5 reconstructed data in HC (middle) and SZ (right).
4. Discussion
This study demonstrates differences between controls and individuals with schizophrenia in their attentional and perceptual sensitivities to subtle environmental regularities. Single trial modulations in target amplitudes were related to the modeled prediction error of the tones. The ERP components which peaked at 340 and 484 ms each showed sensitivity to prediction error at the group level. However, healthy controls showed a greater sensitivity to prediction error within component 5, which peaked at 340 ms, while the two groups did not significantly differ within component 6, which peaked at 484 ms. It is interesting to note that these components appear to correspond to the P3 (see Supplementary Results) and P3b ERP response, respectively, and that these responses previously demonstrated differences with regards to attentional modulation and their relationship to gray matter volumes. For example, the P3 response appears when rare stimuli are either attended or ignored, and is associated with frontal gray matter volume, while the P3b appears primarily when stimuli are attended, and is associated with parietal gray matter volume (Ford et al., 1994; Squires, Squires, & Hillyard, 1975). The present study further clarifies differences between these responses with respect to the sensitivity to environmental regularities in healthy controls and individuals diagnosed with schizophrenia.
4.1. Comparing Bayesian prediction error and the number of preceding non-targets
Within auditory oddball tasks, context processing deficits have primarily been explored by considering the number of non-targets that precede rare stimuli. For example, ERP amplitudes have been compared separately for rare stimuli that follow between 1 and 9 non-targets (Gilmore et al., 2005; Gonsalvez et al., 1995), or as a function of rare stimulus probability (Javitt et al., 1998; Shelley et al., 1999). In some cases, the number of preceding non-targets may be an incomplete summary of acoustic regularities. According to the Bayesian prediction error model, this may apply in particular for targets that precede 1–3 non-targets (see Supplementary Figure 3). For example, all rare stimuli that follow 1 non-target are assumed to have the same ERP amplitude, disregarding the sequence of stimuli that appeared prior to the non-target. The prediction error model includes a weighted contribution of past events (e.g. incorporating biologically plausible assumptions about working memory), which improves predictions of fluctuations in rare stimulus amplitudes in instances with a fixed number of preceding non-targets, or in instances where a target precedes another target (which occurred within 17% of targets within the current study).
Individual’s sensitivity to auditory context was summarized in the current study by calculating the slope of the linear fit between single trial rare target amplitudes and the prediction error, or the number of preceding non-targets. It is interesting to note the close correspondence between the modeled prediction error and the observed amplitude, when plotted against the number of preceding non-targets (Fig 3a and 3b). This result cautions against assuming a linear relationship between ERP amplitudes and the number of preceding non-targets, further supports the utility of the prediction error model for predicting ERP amplitudes (Lieder, et. al., 2013), and is consistent with the observation of significant differences between HC and SZ when linearly fitting amplitudes with prediction error, compared to with the number of preceding non-targets.
4.2. Context processing deficits and symptoms
The reduced sensitivity to environmental regularities in schizophrenia could potentially be related to the frequency of hallucinations (e.g. internal distractions), be related to the influence of medication, or result due to a general cognitive deficit. In an exploratory analysis, the component which differed in prediction error between the groups was not significantly associated with positive (r = −0.07) or negative (r = 0.13) PANSS scores, or with total current antipsychotic dosage (r = −0.16). The absence of a significant relationship may result due to the relatively modest sample size. Thus, a larger heterogeneous sample could disentangle the underlying factors that contribute to these results.
4.3. Conclusion and implications
The present findings further clarify differences in HC and SZ with regards to their sensitivity to subtle environmental regularities. Rare target ERP amplitudes in schizophrenia were less influenced by the context of acoustic events before each target, indicating a relative ‘uncoupling’ of the response to a particular target from its acoustic context. This is consistent with impairments in working memory (Park & Holzman, 1992) and a more fragmented perceptual experience (Cox & Leventhal, 1978; Silverstein et al., 1996) in schizophrenia.
Supplementary Material
Acknowledgments
Role of the Funding Source
This work was supported by NIMH grant MH 5R3743775 and MH 1R01077945 (Dr. Pearlson), and NIH grant NIBIB 1R01EB006841 (Dr. Calhoun).
The authors would like to thank the participants and their families.
Footnotes
Conflict of Interest
Dr. Pearlson reports serving as a consultant for Bristol-Myers Squibb in 2012. Dr. Bridwell, Dr. Kiehl, and Dr. Calhoun have no interest to declare.
Contributors
GP contributed to experimental design. DB conducted analysis and wrote the manuscript. GP, KK, and VC edited the manuscript and assisted with interpretation of the results. All authors have contributed to and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldi P, Itti L. Of bits and wows: a Bayesian theory of surprise with applications to attention. Neural Networks. 2010;23 (5):649–666. doi: 10.1016/j.neunet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiat. 2001;158 (7):1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Cox MD, Leventhal DN. A multivariate analysis and modification of a preattentive perceptual dysfunction in schizophrenia. J Nerv Ment Dis. 1978;166:709–718. doi: 10.1097/00005053-197810000-00004. [DOI] [PubMed] [Google Scholar]
- Eichele T, Rachakonda S, Brakedal B, Eikeland R, Calhoun VD. EEGIFT: group independent component analysis for event-related EEG data. Comput Intell Neurosci. 2011:1–9. doi: 10.1155/2011/129365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhäuser W, Mundhenk TN, Baldi P, Koch C, Itti L. A bottom–up model of spatial attention predicts human error patterns in rapid scene recognition. J Vis. 2007;7 (10):1–13. doi: 10.1167/7.10.6. [DOI] [PubMed] [Google Scholar]
- Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, Clementz BA. Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.032. http://dx.doi.org/10.1016/j.biopsych.2014.03.032. [DOI] [PMC free article] [PubMed]
- Ethridge LE, Hamm JP, Shapiro JR, Summerfelt AT, Keedy SK, Stevens MC, Sweeney JA. Neural activations during auditory oddball processing discriminating schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2012;72 (9):766–774. doi: 10.1016/j.biopsych.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Arlington, Va: American Psychiatric Publishing; 1997. [Google Scholar]
- Ford JM, Sullivan EV, Marsh L, White PM, Lim KO, Pfefferbaum A. The relationship between P300 amplitude and regional gray matter volumes depends upon the attentional system engaged. Electroencephalogr Clin Neurophysiol. 1994;90 (3):214–228. doi: 10.1016/0013-4694(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Clementz BA, Buckley PF. Stimulus sequence affects schizophrenia–normal differences in event processing during an auditory oddball task. Cognitive Brain Res. 2005;24 (2):215–227. doi: 10.1016/j.cogbrainres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Gonsalvez CJ, Gordon E, Anderson J, Pettigrew G, Barry RJ, Rennie C, Meares R. Numbers of preceding nontargets differentially affect responses to targets in normal volunteers and patients with schizophrenia: A study of event-related potentials. Psychiat Res. 1995;58:69–75. doi: 10.1016/0165-1781(95)02315-n. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the bipolar-schizophrenia network on intermediate phenotypes (B-SNIP) study. Am J Psychiat. 2013;170 (11):1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Itti L, Baldi P. A principled approach to detecting surprising events in video. CVPR. 2005;1:631–637. [Google Scholar]
- Itti L, Baldi P. Bayesian surprise attracts human attention. Vision Res. 2009;49:1295–1306. doi: 10.1016/j.visres.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Grochowski S, Shelley AM, Ritter W. Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroen Clin Neuro. 1998;108 (2):143–153. doi: 10.1016/s0168-5597(97)00073-7. [DOI] [PubMed] [Google Scholar]
- Kullback S. Information theory and statistics. New York: Wiley; 1959. [Google Scholar]
- Lancon C, Auquier P, Nayt G, Reine G. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS) Schizophr Res. 2000;42 (3):231–239. doi: 10.1016/s0920-9964(99)00129-2. [DOI] [PubMed] [Google Scholar]
- Levit RA, Sutton S, Zubin J. Evoked potential correlates of information processing in psychiatric patients. Psychol Med. 1973;3 (4):487–494. doi: 10.1017/s0033291700054295. [DOI] [PubMed] [Google Scholar]
- Lieder F, Daunizeau J, Garrido MI, Friston KJ, Stephan KE. Modelling Trial-by-Trial Changes in the Mismatch Negativity. PLoS Comput Biol. 2013;9 (2):e1002911. doi: 10.1371/journal.pcbi.1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JR, Metha AB, Krauskopf J, Lennie P. Rapid adaptation in visual cortex to the structure of images. Science. 1999;285 (5432):1405–1408. doi: 10.1126/science.285.5432.1405. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49 (12):975. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Place EJ, Gilmore GC. Perceptual organization in schizophrenia. J Abnorm Psychol. 1980;89:409–418. doi: 10.1037//0021-843x.89.3.409. [DOI] [PubMed] [Google Scholar]
- Roth WT, Cannon EH. Some features of the auditory evoked response in schizophrenics. Arch Gen Psychiatry. 1972;27 (4):466–471. doi: 10.1001/archpsyc.1972.01750280034007. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37 (1):65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Matteson S, Knight RA. Reduced top-down influence in auditory perceptual organization in schizophrenia. J Abnorm Psychol. 1996;105 (4):663–667. doi: 10.1037//0021-843x.105.4.663. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroen Clin Neuro. 1975;38 (4):387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.