Abstract
Sleep is widely believed to play an essential role in synaptic plasticity. However, the precise mechanisms governing this presumptive function are largely unknown. There is also evidence for independent circadian oscillations in synaptic strength and morphology. Therefore, synaptic changes observed after sleep reflect interactions between state-dependent (e.g. wake vs. sleep) and state-independent (circadian) processes. In this article we review how sleep and biological clocks influence synaptic plasticity. We discuss these findings in the context of current plasticity-based theories of sleep function and propose a new model that integrates circadian and brain state influences on synaptic plasticity.
Keywords: synapse, function, circadian, cortex, invertebrate, vertebrate, brain
Investigating the role of sleep in synaptic plasticity
Sleep is widely found in the animal kingdom yet its core functions are unknown. A popular and perennial idea is that sleep plays an essential role in synaptic plasticity. Synaptic plasticity is defined as changes in the strength of existing synapses (synaptic efficacy), changes in synapse number or size, or changes in morphological structures that contain synapses (e.g. dendritic spines). These latter two processes are often referred to as ‘structural plasticity’ and involve the formation and growth of synapses, pre-synaptic boutons and dendritic spines or the elimination of pre-existing ones [1, 2] (Box 1).
Box 1.
Synaptic efficacy and structural plasticity. The precise relationship between changes in synaptic efficacy and structural plasticity is not entirely understood. It has been proposed that a continuum exists between the two, such that changes in synaptic efficacy precede and may instruct structural changes [104]. However, in other cases, structural changes may occur without stimulation that first produces a change in synaptic efficacy. An example is the Drosophila flight motor neuron. A circadian mechanism increases the size of synaptic boutons independent of synaptic activity in the circuit [85].
The idea that sleep promotes synaptic plasticity is supported by many findings. For example, sleep promotes learning and memory and is accompanied by electrophysiological and molecular changes consistent with synaptic remodeling [3-5]. These findings have spawned numerous theories regarding sleep function (Box 2). There remain, however, a number of unresolved issues. Sleep does not appear to have a single effect on synapses (Table 1). Rather, as is true for the waking brain, diverse forms of plasticity accompany the sleeping brain. There is also evidence that circadian processes that coincide with sleep, but are not themselves sleep-dependent, influence the strength or number of synapses (Table 2) [6].
Box 2.
Theories of sleep and plasticity. Sleep has been variously hypothesized to stabilize [88], strengthen [105], or remove synapses [106, 107]. The Synaptic Homeostasis Hypothesis (SHY) is a more recent version of the latter idea. SHY proposes that sleep promotes global (or ‘net’) synaptic weakening (called ‘downscaling’ or ‘renormalization’) which offsets global synaptic strengthening during wakefulness [7]. This preserves the relative strength between synapses, allows for further synaptic changes and prevents maladaptive metabolic costs associated with excessive synaptogenesis. Therefore, SHY predicts that synapses should be weaker, not stronger after sleep. Global measures of plasticity (e.g. from tissue homogenates) are difficult to interpret, because different neurons (or glial cells) may contribute differently to a particular measurement of protein or mRNA. In addition, ‘net’ or ‘global’ plastic changes are difficult to define in vivo (e.g. what constitutes a ‘global’ measure? measurements from a thousand, ten thousand, or a million neurons?). Nevertheless, some electrophysiological and molecular findings are consistent with SHY [7]. Other studies, however, show that synapses may be stronger or more numerous after sleep (Table 1). Therefore, SHY does not appear to fully explain the role of sleep in brain plasticity (for further discussion see [6]).
These observations have led to new, more integrative theories. According to the ‘Boom and Bust’ theory [108], the first few hours of sleep promote synaptic potentiation which is predicted to be greatest in synapses stimulated according to Hebbian rules. This is followed by a slower, non-Hebbian scaling that proportionately reduces synaptic strength across the network. This model is compatible with the ‘State-Clock’ model (Figure 2) and shares features in common with other recent theories which also predict that sleep has dual effects on synaptic strength [109, 110]. These theories differ from SHY in that they do not require that sleep only have a net or global downscaling effect on synapses.
Table 1.
The effects of sleep on mammalian synapses vary by circuit and prior waking experience.
| Species (developmental stage) |
Circuit examined | Learning or plasticity inducing stimuli prior to sleep? |
Results after sleep |
|---|---|---|---|
| Mouse (juvenile & adult) |
Motor cortex, apical dendrites, layer (L) 5 neurons |
No | Juvenile mice: ratio of dendritic spines eliminated vs. formed higher in sleep than wake. Adult mice: no change [50, 51]. |
| Mouse (adult) | Frontal cortex (not specified) |
No | Electrically evoked EPSPs smaller after sleep relative to wakefulness [40]. |
| Mouse (adult) | Frontal cortex (L2,3) | No | Mini EPSPs in situ at lower frequency or amplitude after sleep relative to wakefulness[112]. |
| Mouse (adult) | Motor cortex, apical dendrites, L5 neurons |
Yes (rotarod motor learning) |
Dendritic spines increase during sleep. No evidence of spine elimination [52]. |
| Mouse (adult) | Visual cortex (L2-5) | Yes (exposure to single oriented grating) |
Single cortical neuron activity enhanced in response to experienced visual stimuli after sleep [9]. |
| Cat (juvenile) | Visual cortex (L2,3&5,6) |
Yes (monocular deprivation) |
Single cortical neuron firing rates enhanced in response to non- deprived eye stimulation after sleep [13]. |
| Cat (adult) | Somatosensory cortex (not specified) |
No | Evoked extracellular and intracellular electrophysiological potentials larger after sleep relative to wakefulness [20]. |
Table 2.
Circadian rhythms in synaptic efficacy and structural plasticity.
| Animal species | Circuit and measurements |
Rhythm properties | Driven by endogenous oscillator? |
|---|---|---|---|
|
Drosophila Melanogaster and Leucophea maderae |
Olfactory electroantennograms |
Peak during subjective day(light phase); trough in subjective night (dark phase) |
Abolished in clock gene mutants, rhythm persists in situ. [68] [69] [70] |
|
Drosophila
Melanogaster |
Flight motor neuron synaptic bouton number |
Peak during subjective night, trough in subjective day |
Persists in constant conditions and following decapitation, abolished in clock gene mutants [84] |
| Zebrafish (Danio rerio) |
Hypocretin>pineal gland, synaptic markers |
Peak during subjective day, trough in subjective night |
Persists under constant conditions, abolished in mutants arrhythmic for nptxb (gene involved in excitatory synapse plasticity)[53] |
| Mouse (Mus musculus) and Hamster (Mesocricetus auratus) |
Hippocampus CA1 LTP |
Easier to induce (or of greater magnitude) in subjective night |
Persists ex vivo [64, 65] |
In this article we review how sleep and circadian rhythms influence synaptic plasticity. We begin by summarizing key findings that demonstrate that the effects of sleep vary depending on several factors. These include developmental age, the type of waking experience (or stimulation protocols) that precede sleep, the type of synapse under examination, and the presence or absence of strong circadian rhythms (Figure 1). We then present evidence that circadian rhythms also influence synaptic strength independently from sleep. We conclude by discussing the implications of these findings in light of recent theories of sleep function and present a theoretical model that integrates the effects of sleep and circadian rhythms on synaptic plasticity. According to this model (‘State-Clock’) many synaptic changes ascribed to sleep and wakefulness are in fact driven by circadian oscillators (Figure 2).
Figure 1. Sleep does not have a single effect on synaptic efficacy or morphology.
Direct and indirect measures of synaptic plasticity after sleep show changes consistent with increases or decreases in synaptic strength depending on several factors. These include the animal species, the type of circuit under examination, the presence of absence of strong circadian rhythms, and the developmental age of the organism. See main text for citations.
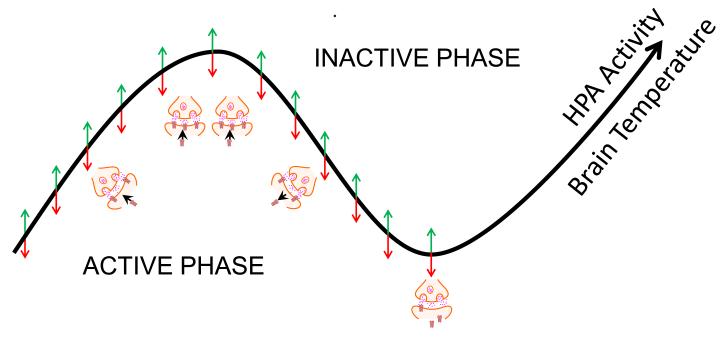
Figure 2. A State-Clock model of synaptic plasticity.
According to the State-Clock model, biological clocks drive 24-hour rhythms in synaptic plasticity. These form a canvas against which experience-dependent changes can be induced in wakefulness and further consolidated during sleep (small green and red arrows). The direction of the latter changes, however, is not fixed and dependent upon the type of experience that precedes sleep and the type of circuit. In mammals with strong circadian rhythms, oscillations in brain temperature and hypothalamic-pituitary-axis (HPA) activity globally increase cortical synaptic efficacy (i.e. more glutamate receptor signaling, trafficking to existing synapses) and morphology (i.e. more synapses forming) during the active (wake) phase. During the inactive phase, brain temperature and HPA activity decrease, resulting in global reductions in these processes.
Changes in synaptic efficacy across the sleep-wake cycle
Sleep-wake (i.e. vigilance state) differences in synaptic efficacy have been measured directly via electrophysiological potentials and indirectly via measurements of plasticity-related mRNAs or proteins that are concentrated at pre or post-synaptic sites. The latter may change in concentration or phosphorylation state which can be indicative (but not proof positive) of synaptic potentiation or depression.
Direct measures
Direct measures of synaptic efficacy demonstrate no single effect of sleep on synaptic strength. Some studies in nocturnal adult rodents have shown that excitatory electrophysiological potentials (EPSPs, see Glossary) in frontal/parietal cortices are larger when measured after long periods of wake (e.g. 6-12 hours) compared to sleep. There is also a reduction in cortical neuron firing rate during non-rapid eye movement (nonREM) stages of sleep after long periods of sleep. Conversely, long periods of waking are associated with a general increase in cortical excitability. These changes in rodents may reflect changes in intracortical inhibition or transient neuromodulator effects and/or they may also reflect changes in synaptic efficacy [7, 8]. Different results, however, are obtained from other parts of the rodent brain, at different developmental periods and in different animal species.
Direct evidence that sleep promotes cortical potentiation was recently reported in the adult mouse visual cortex [9]. Stimulus specific-response plasticity (SRP) is a form of in vivo synaptic long-term potentiation (LTP) that manifests as a potentiated response to an experienced visual stimulus [10]. SRP is only observed after a period of sleep, and suppressed by sleep deprivation [9]. These results are consistent with an earlier report in adult rats that thalamic-evoked EPSPs in the visual cortex do not decrease across the sleeping phase as might be expected if synaptic efficacy is reduced during sleep [11]. Moreover, in juvenile mice manipulations that induce non-Hebbian synaptic upscaling in vivo produce equivalent changes in cortical activity irrespective of sleep [12]. This is consistent with a recent suggestion that synaptic scaling may not require sleep [6].
Sleep also increases synaptic efficacy in cats, which have very weak circadian rhythms. Studies of ocular dominance plasticity in developing cats show that sleep promotes cortical potentiation [13]. In these experiments, blocking patterned vision in one eye (monocular deprivation (MD)) in the awake animal is sufficient to weaken cortical circuits serving the deprived eye. After sleep, cortical circuits serving the non-deprived eye become stronger [13-15]. Moreover, visual cortical firing rates (in animals with MD or normal vision) do not show the overall decline in neuronal firing rates during sleep that has been reported in adult rodents [15]. These results are also not caused by ‘non-physiological’ processes resulting from MD as recently asserted [7]. For example, it was claimed that short periods of MD lead to ‘massive’ synaptic weakening and a 40% reduction in nonREM slow wave activity (SWA) [7]. In fact, 6 hours of MD has only small effects on the visual cortex [13]. It takes days (between 2 and 7 days) of continuous, 24-hour MD to produce ‘massive’ synaptic weakening in cats [16]. There are also no decreases in visual cortical nonREM SWA after 6 hours of MD [14]. Raising animals in complete darkness does reduce nonREM SWA by 40% [17], but this is a completely different paradigm than MD that produces very different effects on the visual cortex [18, 19].
Sleep has similar effects in the adult cat. Intracellular and extracellular recordings in somatosensory adult cat cortex show that electrophysiological potentials in wakefulness are increased when interleaved with short episodes of nonREM sleep, but unaffected by similar periods of wakefulness [20]. It has been suggested that this is due to sleep inertia [7]. Sleep inertia refers to a transient reduction in cognitive and neural function immediately following awakening. Sleep inertia, however is an unlikely explanation for several reasons. First, no significant differences in membrane potential in wake before or after nonREM sleep were reported, as might be expected if sleep inertia were present. Second, the initial enhancement of the electrophysiological response persisted over several sleep-wake cycles, which would be unlikely if this was due to a transient inertial effect. Moreover, sleep inertia generally requires much longer periods of sleep and is more common in animals with strong circadian rhythms [21]. Third, experiments in vitro which simulated nonREM SWA specifically led to synaptic potentiation, while simulations of waking activity did not [20].
The results from studies of human cortical excitability are equivocal with respect to sleep-dependent changes in synaptic efficacy. Some studies report heightened cortical excitability when assessed during wakefulness or after REM sleep deprivation (relative to undisturbed sleep) [22, 23] whereas other studies reported no state-dependent changes in cortical excitability. In two separate studies [24, 25], cortical excitability and intracortical inhibition were measured at different times of day, after periods of sleep or wake, or after sleep deprivation. There was no evidence of a general increase in cortical excitability in wakefulness relative to sleep. The only significant changes were in intracortical inhibition, which declined across the day independently of brain state [25]. An additional prediction of an increase in cortical synaptic efficacy is a corresponding increase in brain connectivity. This was recently tested by Shannon et al., using sophisticated functional imaging and analytical techniques. Measurements before and after the normal waking or sleep periods showed no net increase in global circuit connectivity in wakefulness relative to sleep [26].
There are very few studies that have examined electrophysiological changes in invertebrates across the sleep-wake cycle. The principle findings are that while there are state-specific changes in neuronal activity, these vary widely across species. In Drosophila melanogaster, sleep is accompanied by a general reduction of ongoing electrophysiological activity relative to wakefulness in the medial protocerebrum relative to wakefulness [27, 28]. In aquatic invertebrates, sleep-like states are instead accompanied by periodic bursts or oscillations of activity in central ganglia [29, 30]. In none of these studies, however, is there any evidence that electrical excitability or synaptic potentials are significantly reduced or enhanced after a period of sleep. Instead, they appear to occur when there is a change in brain state (or at different circadian times, see below)—but themselves do not reflect overall changes in synaptic efficacy.
Indirect measures
Indirect measures of synaptic efficacy present an equally complex picture. Some studies in adult rodents show that plasticity-related mRNAs, such as the immediate early gene arc are expressed at lower levels in the cortex or hippocampus when measured after long periods of sleep vs. long periods of wake (and reviewed in [3]). It has been suggested that these findings indicate that synaptic efficacy is reduced during sleep [31, 32]. However, this interpretation is complicated by several factors. First, genes like arc can have pleiotropic effects on synaptic strength. Therefore increases or decreases in mRNAs like arc do not necessarily equate to similar changes in synaptic efficacy (discussed in [6]). Second, sleep is associated with a general increase in levels of mRNAs (and proteins) involved in protein synthesis [14, 33]; a key process in the formation of new synapses or increases in synaptic efficacy. Third, the expression of other plasticity-related immediate early genes, such as zif-268, is not only modulated by vigilance state, but by the types of stimuli that precede sleep. For example, zif-268 levels increase after sleep when sleep is preceded by novel experience or stimulus-protocols that induce LTP [34, 35].
No simple picture has emerged from measurements of proteins across the sleep/wake cycle. Although sleep promotes protein synthesis, very little is known about the identity of these proteins [36]. Nor can one simply infer protein concentration based on the expression of corresponding mRNAs. This is because mRNA transcription and translation do not always occur in parallel [14, 37, 38]. Additionally, while some rodent studies report changes in protein levels consistent with decreases in synaptic efficacy after sleep, others do not. For example, in one study cortical brain-derived neurotrophin factor (BDNF) protein was reduced after long periods of sleep, relative to long periods of wake (or sleep deprivation) [39]. However, in a different study, cortical BDNF was maximal when animals were sacrificed in the middle of the sleep phase [37]. In other studies, many forebrain proteins implicated in LTP were reduced after long periods of wakefulness while others were elevated after sleep (reviewed in [3, 6]).
As is true for electrophysiological recordings, developing animals with weak circadian rhythms show different patterns of mRNA and protein expression than adult rodents. Sleep/wake changes in cortical glur1 and camkii mRNA reported in adult rodents [40, 41] (and reviewed in [3]) were not found in developing cats [13, 14]. In instances where similar changes in mRNA were reported in both species, in cats this tracked visual input and not wakefulness per se [14]. An additional important difference is that while cortical Arc and BDNF mRNA and proteins are co-expressed at higher levels in the awake rat, this is not true for the developing cat. More specifically, sleep in the developing cat visual cortex is characterized by a decrease in arc and bdnf mRNA and a transient increase in the corresponding proteins [14].
Interestingly, these molecular changes in kitten cortex are accentuated following MD during the critical period of visual development [14]. This suggests that sleep in early life may specifically consolidate experience-dependent forms of plasticity that involve synaptic potentiation. This is supported by findings in developing birds. Visual imprinting in the chick (Gallus gallus domesticus) involves a neuronal gain of response to the imprinted stimulus which is not paralleled with reductions in response to the non-imprinted stimulus. It also appears to involve synaptic potentiation in forebrain neurons. Imprinting is also associated with increases in both postsynaptic density size and glutamate receptor numbers [42-44]. As is true for the developing visual cortex, sleep immediately after imprinting enhances and consolidates this form of developmental plasticity [43].
Changes in structural plasticity across the sleep-wake cycle
The effect of sleep on structural plasticity has been minimally explored. An interesting finding in mammals is that sleep deprivation, even when carried to lethal extremes, has negligible effects on gross features of the brain. In the classic studies carried out in the Rechtschaffen laboratory, rats that are totally sleep-deprived or selectively REM sleep-deprived die after 2-4 weeks. Surprisingly, total or selective REM sleep deprivation for this period of time has no effect on morphological or cytoarchitectural features of the brain [45]. For example, no changes in axons, dendrites, synaptic density or organelles were noted using electron microscopy (EM) or cresyl violet staining in cortical and subcortical regions (Box 3). These latter results should be cautiously interpreted since they were principally reported in abstract form. Subsequent studies using weeks of sleep deprivation also failed to show changes in markers of neuronal degeneration, stress or apoptosis [45-47] (but see [48]). There are also no significant differences in (whole-brain) synaptic proteins (e.g. synapsin, synaptobrevin II, synaptotagmin) in adult mice sacrificed either in the sleeping or active phases [49]. These findings are difficult to reconcile with arguments that sleep prevents or offsets unchecked synaptogenesis in wakefulness [31, 32]. It remains possible, however, that more fine-grained analyses of synaptic morphology might have revealed subtle changes.
Box 3.
Counting synapses. The quantification of number and size of synapses (active sites) in the brain requires the use of electron microscopy (EM) or similar quantitative techniques. This is labor-intensive and instead synapses are often estimated indirectly by confocal microscopy of preparations in which synapse-enriched structures (dendritic spines, synaptic boutons) are stained with specific markers. The measurement of synaptic proteins or synaptic branches (axon terminals and dendrites) provides in the best case, approximate data that should be interpreted with caution. This is because a single bouton or dendrite might contain one or more (or no) functional synapses.
This possibility was investigated in developing and adult mice. In developing mice there was a slight increase in the rate of cortical spine elimination vs. formation when spines were measured after sleep vs. wakefulness or sleep deprivation [50, 51]. However, these results were only obtained at ages when there is an overall pruning of cortical synapses. No sleep/wake differences in spine elimination were observed in adult mice. Interestingly, in the latter studies sleep was not preceded by any specific learning tasks or other stimuli known to cause synaptic remodeling. Very different results were obtained when mice were engaged in learning tasks before they went to sleep. Motor learning in adult mice is accompanied by the formation of new dendritic spines in motor cortex. This process was recently shown to be sleep-dependent [52]. Therefore, as is true for electrophysiological measures of plasticity, the effects of sleep are highly dependent on the kinds of experience that precede sleep.
The zebrafish is the only other vertebrate examined with respect to sleep and structural plasticity. In zebrafish larva, changes in synapse number in hypocretin (HCRT) neurons were measured at different times of day, and after sleep deprivation, by EGFP-labeled synaptophysin; a marker of presynaptic boutons and synapses [53]. The principle findings were that changes in HCRT synapses were highly dependent on circadian rhythms and the targets of innervation. Sleep had relatively modest effects on synaptic markers compared to circadian effects. It is also unknown if sleep-dependent changes in these synapses in this model are restricted to early stages of development as appears to be the case in mice.
State-dependent structural changes in synapses have also been found in Drosophila. Gilestro et al., [54] reported that the levels of pre-synaptic and post-synaptic proteins are higher in whole brain homogenates after sleep deprivation compared to periods of undisturbed sleep [54]. In a different study, synaptic markers were examined in ventral lateral neurons (LNvs) after social enrichment and sleep. The number of synaptic boutons expressing GFP-tagged synaptic markers were elevated in large LNv terminals after social experience and reduced after sleep (relative to 48 hours of sleep deprivation) [55]. Similar results were obtained in the same type of LNv neurons when sleep was experimentally induced after social enrichment [56]. Complementary results were reported in small LNv neurons using different synaptic markers which localize to pre-synaptic boutons [57]. An examination of axonal processes in mushroom body gamma neurons further showed that periods of prolonged wakefulness were, relative to sleep, accompanied by an increase in axonal tip diameter and labeled puncta. Morphological changes were also found in dendrites in a different sensory circuit. Interestingly, these state-dependent changes in dendrites were not observed when compared across baseline periods of wake or sleep, but were only revealed after sleep deprivation [57].
Summary: Sleep and plasticity
Sleep has been shown to increase, decrease or have no effect on electrophysiological and morphological measurements of synaptic plasticity. What precisely determines the effect of sleep on a given synapse is unclear. However, a consistent finding in mammals is that this is strongly determined by the kinds of waking experience or stimulation that precede sleep. When animals are trained in learning paradigms or exposed to sensory input that trigger synaptic plasticity, sleep increases the strength and possibly the number of synapses. When animals are instead merely sacrificed after long periods of sleep not preceded by such experience or stimulation, synapses appear to be weaker (Table 1). Results from invertebrate models indicate that sleep has a more uniform, synaptic weakening effect in insects. However, there is also evidence that when insects are engaged in learning tasks, sleep may also lead to synaptic strengthening [56]. These results raise the possibility that uniform, or ‘net’ changes in synapses observed under basal conditions may be driven by the biological clock.
Circadian influences on synaptic plasticity
Circadian rhythms refer to oscillations in biological processes with a period of approximately 24 hours. In addition to the sleep/wake cycle, in many animal species there are strong circadian rhythms in metabolism, body temperature, hormone output, organ function and gene expression [58, 59]. There is also evidence of circadian rhythms in synaptic plasticity, in some cases driven by a master clock (in mammals, the suprachiasmatic nucleus [SCN]) and in other cases, by peripheral clocks. In the following sections, we review the evidence for circadian influences on synaptic plasticity outside the SCN (see [60] for discussion of plastic changes within the SCN). One consideration in assessing these studies is that it can be difficult to disentangle the effects of circadian time vs. brain state in a given measurement. That is, changes in tissues obtained at different circadian times may reflect a pure clock-driven process or the brain state at the time of sacrifice. Circadian influences, however, are more likely if the changes persist ex vivo or disappear when clock mechanisms are removed (e.g. following SCN lesions).
Direct measures
Circadian rhythms have been reported in evoked electrophysiological potentials and classic Hebbian LTP. In these studies, evoked responses or LTP was assayed at different times in the circadian day. Some of the earliest evidence of a circadian rhythm in evoked responses was reported by Barnes et al., who showed diurnal/nocturnal rhythms in hippocampal EPSPs in rat and monkey [61]. Hippocampal LTP is also easier to induce (or is of greater magnitude) in hippocampal slices obtained from rodents sacrificed in the dark (active) phase (relative to the light phase). However, this appears to vary in different hippocampal circuits (CA1 vs. dentate gyrus) [62, 63]. In two studies, time-of-day effects persisted for several hours ex vivo, which strongly suggests that the hippocampus itself generates rhythms in synaptic efficacy [64, 65]. This view is consistent with recent demonstrations of circadian rhythms in hippocampal kinase activity and hippocampal based learning [66].
The evidence of circadian rhythms in vertebrate neuronal excitability or evoked potentials outside of the hippocampus is less clear. Hanada and Kawamura reported circadian rhythms in rat visual circuits in vivo that were independent of vigilance state and abolished by SCN lesions [67]. In this study, lateral geniculate nucleus and optic tract responses to stimulation were consistently higher in the dark phase. This was true irrespective of the vigilance state in which stimuli were presented. In addition there did not appear to be differences in the evoked responses across vigilance states.
Circadian rhythms in neuronal excitability and activity have been observed in a wide range of invertebrates. Electroantennograms recorded in situ in Drosophila [68] and the cockroach Leucophea maderae [69] show a circadian rhythm in the response to specific odorants with significantly smaller amplitude at times of wake, relative to sleep. This rhythm is not present in flies with mutations in clock genes and is driven from a peripheral clock mechanism resident in olfactory neurons [70].
Circadian changes in neuronal excitability may also be present in the Drosophila clock neurons of the LNv group. Electrophysiological recordings done in freshly dissected brains of adult flies kept under Light-Dark (LD) cycles show rhythms in membrane excitability. The resting membrane potential of large LNv neurons is more depolarized at the end of the night and more hyperpolarized at the end of the day [71, 72]. Only one of these two groups was able to confirm that the large LNvs continue to be more depolarized in the day compared with the night when the brain explants were obtained from flies kept in constant conditions (continuous darkness: DD) [72]. In both cases, these results are consistent with a circadian influence, but the brain state at time of sacrifice cannot be excluded as a factor.
Circadian rhythms in structural plasticity
A large number of studies of synapses in mammals, bony fishes, flies and other animals have firmly established the existence of circadian changes in synaptic structure. The largest body of data is represented by the study of structural plasticity among vertebrate ribbon synapses (RS) [73]. RS are found in the retina, the pineal, the vestibular organ and other sensory organs. Their structural plasticity comprises changes in number, size, shape and location and has been studied as a function of light conditions, developmental stage and aging and circadian rhythms. RS were first described by Sjöstrand (1953) at the same time as the pioneering studies that established our current understanding of the basic structural organization of the synapse [74]. This type of synapse is characterized by graded potentials based on their capacity for sustained exocytosis which is conveyed by specific structural and molecular traits of which an electron-dense ‘ribbon’ with tethered vesicles is the most studied component [75]. Among pineal RS the number of ribbons, and sometimes also their size, is larger in the night compared with the day regardless of whether the animal is nocturnal (rat), diurnal (baboon) or has no distinct 24-hour sleep/wake cycle (guinea pig). Retinal RS cells generally exhibit a reverse pattern [76] with an extreme example noted in zebrafish larvae which disassemble all their ribbons during the night [77].
EM has also been used to examine two well-defined classes of synapses in the visual centers of the (diurnal) housefly Musca domestica and Drosophila [78, 79]. The houseflies were kept in LD cycles and thereafter sacrificed for EM at time points either under the same light conditions or after being kept for different periods in DD. During LD cycles the synapses made by photoreceptors on a specific type of interneuron (L2) are more abundant during the day but the ‘feedback synapses’ made by the same interneurons onto photoreceptors are more abundant in the night. In flies kept in DD, on the other hand, only the L2 synapses onto photoreceptors continue to change in number across the subjective day and night [78]. In Drosophila maintained in LD the photoreceptor synapses on both interneurons L1 and L2 are more abundant and synaptic terminals are larger during the day than night [80]. However, terminal size and synapse numbers do not simply increase in the day and decline during the night. Instead they start to decrease several hours before the end of the day and begin to increase again during the night (sleep phase).
Fluorescence microscopy and EM demonstrate similar changes in axonal and dendrite morphology in tissue obtained at different circadian time points. The axons of Drosophila L1 and L2 interneurons swell at the onsets of the light and dark periods, with a maximum observed at the latter time point [79].The dendrites of L2 are larger at the beginning of the day with a unimodal circadian rhythm [81]. Small LNvs also show a rhythmic change in branching complexity along the day in LD and DD, with more complex branching early in the day in LD (relative to early night) and the same relationship when the flies are kept in DD [82].
The only motor neuron for which circadian changes in synaptic structure have been investigated is a flight motor neuron in Drosophila. This neuron (MN5) is very large and has axonal terminals innervating two large flight muscles with about 3000 synapses [83]. The synaptic boutons (measured with confocal microscopy after immunostaining of the neuronal membrane) of this neuron grow in size during the morning and decrease in the night (84, 85), whereas their numbers show a minimum at midday and a maximum at midnight (83). These changes reflect the influence of the biological clock as they persist in DD and are prevented by mutations in clock genes or normal aging [84]. Moreover, they are unaffected by sleep-deprivation during the early night, synaptic silencing during the morning peak of activity, or complete lack of activity over two LD cycles resulting from decapitation [85]. Interestingly, contrary to synapses in the brain, the synapses (active sites) in Drosophila motor neurons can be counted not only with EM but also with the aid of confocal microscopy in preparations stained with specific synaptic markers. Counting boutons and their synapses with both methods in the MN5 shows that both types of structures are more numerous at midnight compared with midday under LD cycles [83]. In the same synapses, the size and distribution of synaptic vesicles change with a bimodal cycle under LD cycles, with smaller vesicles at the beginning of the day and the night, coincident with moments of more intense locomotion activity [86].
A very intriguing demonstration of circadian influences on mammalian structural plasticity was recently reported by Liston et al., (2013) [87]. In mammals the secretion of glucocorticoids is strongly regulated by the SCN. In this study, the normal peaks in glucocorticoid concentrations (during the rodent active phase) were shown to directly promote the cortical dendritic spine formation that accompanies motor learning. Interestingly, the normal troughs (which correspond to the sleep phase) had dual effects; they promote the stabilization of newly formed spines associated with learning and the pruning of pre-existing spines not associated with learning.
Summary: Circadian rhythms and plasticity
Considerable evidence indicates that biological clocks influence synaptic plasticity independently of vigilance state. In insects and vertebrates biological clocks produce rhythms in synaptic efficacy and number that appear timed to match or anticipate changes in activity. For example, a peripheral clock in the hippocampus produces peaks in the ability to generate LTP that coincide with the subjective night—when rodents are awake and exploring their environments. Similarly, a peripheral clock in the zebrafish retina produces rhythms in RS, leading to a disassembly during periods of darkness, and a reassembly during the day. A second general observation is that different circuits express different circadian rhythms in plasticity. The zebrafish provides a good example, as circadian rhythms in HCRT synapses have different 24-hour phases, depending on their post-synaptic targets [53].
Mechanisms governing synaptic plasticity: Clock-dependent or sleep-dependent?
Numerous theories have been proposed to explain how sleep influences synaptic plasticity (Box 2). Some propose that sleep stabilizes underused synapses [88], while others propose that sleep instead globally weakens synapses [7]. A number of recent findings are consistent with the latter view [7], but many are not (for additional discussion, see [6, 89]). For example, evidence of global synaptic weakening after sleep is observed in some parts of the rodent brain but not others, not when learning tasks precede sleep, and not at all in cats (Table 1). Nor can these latter findings be dismissed as indirect or non-physiological effects of a given experimental procedure (as some have argued [7]). Moreover, a number of changes in invertebrate synaptic efficacy and morphology appear to be driven by circadian rhythms and do not occur in one direction during the sleep phase. Instead, depending on the circuit, synapses may form during the sleeping or waking phase.
Disentangling the dual roles of brain state and the biological clock in synaptic plasticity is difficult. One important clue is that ‘global’ measures of synaptic weakening after sleep are found in species with strong circadian rhythms. Indeed, many findings ascribed to changes in brain state may instead be driven by outputs of the biological clock. For example, in rodents and humans, glucocorticoid concentrations are tightly regulated by the SCN and rise and fall in parallel with wakefulness and sleep [90]. Changes in circulating glucocorticoid concentrations can induce changes in cortical excitability, the amplitude of evoked potentials and AMPAR trafficking (for review see [6]). Small, transient increases in corticosterone can lead to rapid spinogenesis in vivo, which slowly declines over 5 hours [91] and, as discussed above, circadian cycles of glucocorticoid secretion strongly influence learning-induced structural plasticity [87]. These latter findings are consistent with previously reported biphasic effects of glucocorticoids which are comprised of rapid increases in synaptic efficacy (and/or spine formation) followed by a slower, time-dependent normalization of synapses to baseline levels (for discussion, see [92, 93]). These biphasic synaptic changes are strikingly similar to those ascribed to wakefulness and sleep in some theories [7]. They are, however, ultimately driven by the biological clock and are thus not state-dependent.
Temperature is another variable that may also explain changes in synaptic efficacy and morphology reported in vertebrate and invertebrate species. The biological clock produces 24 hour rhythms in core and brain temperature [94]. In endotherms, this involves direct mechanisms of thermogenesis, and in ectotherms, temperature is instead behaviorally regulated, either by selecting warmer environments or through muscle activity [95]. In both endotherms and ectotherms temperature can have significant effects on synaptic plasticity ([6]). In rodents, studies in vitro show that dendritic spines rapidly remodel as a function of temperature, and studies in vivo show that hippocampal EPSPs increase when animals explore novel environments. However, the latter changes are due to accompanying changes in brain temperature and not learning or experience per se [96]. Similar temperature gradients across the subjective day and night have been reported in rodent cortex [97, 98].
The effects of temperature appear to be even more extreme in ectotherms commonly used in sleep studies. Temperature gradients as small as ≈ 8° C are sufficient to alter synaptic structures in Drosophila [99, 100]. These include increased axonal arborization in mushroom body neurons [100] and motor nerve terminals in vivo [99] and neurite extension in vitro [100]. Intriguingly these temperature effects are mediated by signaling pathways shared by activity-dependent synaptic plasticity (e.g. cAMP) [100]. Whether similar temperature gradients exist across insect wake and sleep is unknown as this has yet to be measured. However, similar gradients in ambient temperature are encountered under natural conditions [101] and may even occur in insects housed under constant ambient temperatures. This is because core temperature tracks motor/muscle activity in small terrestrial insects [95]; processes which are strongly influenced by the biological clock.
An integrative model of clock and state-driven changes in synaptic plasticity
The above considerations suggest a new model of how sleep and the biological clock work in tandem to influence synaptic plasticity. According to the ‘State-Clock’ model, outputs of the biological clock produce circuit-specific, 24 hour rhythms in synaptic efficacy and synapse number (Figure 2). For example, in rodents the active phase is accompanied by a net increase in these two parameters in the cortex, while the inactive phase is accompanied by general declines. However, in contrast to other theories [7, 53], it proposes that global synaptic downscaling observed after sleep in these species is driven by the clock and not brain state. The normal controls for circadian influences (i.e. sleep deprivation in the sleep phase) are inadequate, because sleep deprivation can independently increase hypothalamic-pituitary-axis (HPA) activity and brain temperature relative to sleep [6]. In other species, (e.g. Drosophila, zebrafish), circadian influences appear more complex but are also not unidirectional. In all animals, in those circuits that are modifiable by experience, these clock-driven cycles in synapses provide a background for additional state-driven plastic changes.
The State-Clock model thus may account for the variability in synaptic changes reported in different species and in different circuits. This model is also consistent with how outputs of the biological clock (e.g. brain temperature, hormone release) are likely to influence synapses. Circadian rhythms in hormone concentration and temperature are not restricted to specific synapses, but occur throughout the brain. Global changes in brain temperature and hormone concentration in turn provide a simple way of globally adjusting synapse number or efficacy up or down, as described in models of non-Hebbian synaptic scaling. This model is also easily falsifiable by empirical investigation. For example, if circadian rhythms in glucocorticoid secretion in mammals play an essential role, then eliminating such rhythms (via adrenalectomy [102]) should likewise eliminate reported sleep-wake differences in EPSPs and AMPAR phosphorylation [40]. There may also be ways to ‘clamp’ core temperature in mammals [94], which would also eliminate the influence of circadian temperature cycles (or activity) on synaptic efficacy and morphology. The State-Clock model further predicts that forced-desynchrony protocols (which decouple the sleep-wake cycle from the biological clock [103]) will show that biochemical or electrophysiological indices of global cortical potentiation [8] will prove to be clock-driven, rather than state-driven.
Summary and Future Directions
In summary, an inclusive review of the literature indicates that sleep does not appear to have a simple, unidirectional effect on synaptic strength. Instead, the effects of sleep vary considerably depending on several factors. These include the circuit under examination, the age of the animal, the types of stimuli that precede sleep, and the presence or absence of strong circadian rhythms. Circadian rhythms, either as an output of a master clock or due to peripheral oscillators, also influence synaptic efficacy and morphology. An important future challenge will be to identify the relative roles of state-driven vs. clock-driven changes in synaptic strength (Box 4). As discussed above the State-Clock model provides a theoretical framework for such investigations.
Box 4. Outstanding questions.
What are the cellular and molecular mechanisms governing sleep and clock dependent synaptic plasticity?
Are they distinct or do they share common signaling pathways?
When do these mechanisms appear in early life and how do they align?
Are all synapses influenced by brain state or clocks?
Do misalignments in sleep and clock dependent plasticity cause neuropathology?
What are the respective roles of central and peripheral clocks in synaptic plasticity?
Highlights.
Sleep does not have a single effect on synapses
The effects of sleep vary across brain regions and species
Circadian factors independently influence synaptic efficacy and morphology
Sleep and circadian rhythms interact to strengthen and weaken synapses
Acknowledgements
This research was supported by NIH EY019022 and HL114161 to MGF. R.C. is supported by Agencia Nacional de Investigacion e Innovacion.
Glossary
- Constant conditions
A means of identifying endogenous biological rhythms. Diurnal/nocturnal rhythms can occur under normal Light-Dark (“LD”) cycles, but this may be due to masking effects of light rather than an endogenous pacemaker. If the rhythms persist in the absence of external light cues (i.e. when animals are maintained in complete darkness “DD”) then they likely reflect circadian influences. Subjective day and night: In animals maintained under constant conditions, the terms ‘subjective day’ and ‘subjective night’ refer to those segments of the free-running circadian cycle that correspond to the illuminated and non-illuminated portions of a LD cycle often composed of 12:12 hours. These terms are also used for tissue explants studied in isolation from the whole organism.
- Excitatory post-synaptic potentials (EPSPs)
EPSPs refer to extracellular, electrophysiological recordings of synaptic potentials, typically performed as field recordings (fEPSPs) either in brain slices or in vivo. Miniature EPSPs (‘minis’ or mEPSPs) are intracellular recordings of synaptic potentials under conditions where action potentials are blocked. The response to spontaneous release of neurotransmitter is then used to assess the strength of the synapse (e.g. an increase in the frequency or amplitude of mEPSPs).
- Hebbian and non-Hebbian plasticity
Synaptic plasticity is considered either Hebbian or non-Hebbian. Hebbian plasticity is input-specific and requires correlated activity in the pre-synaptic and post-synaptic neurons. In contrast, non-Hebbian forms of synaptic plasticity involve upward or downward changes in all synapses in a neuron, or network of neurons, and do not require input-specificity. One of the best described forms of non-Hebbian plasticity is synaptic scaling, which produces proportionate increases or decreases in all synapses in response to overall decreases or increases in neuronal activity, respectively [111].
- Rapid eye-movement (REM) and nonREM sleep
Birds and mammals have two distinct types of sleep. REM (or ‘paradoxical’) sleep is characterized by neuronal activity in many brain regions that resembles activity during wakefulness. It is also accompanied by phasic activity in brainstem and visual circuits, atonia of skeletal muscle, and a steep reduction in monoamine neurotransmission. NonREM sleep is characterized by rhythmic, oscillatory firing in the thalamus, cortex and hippocampus and an overall decrease in excitatory neuromodulator release. Both sleep states are thought to influence synaptic plasticity [3].
- Monocular deprivation (MD)
An experimental means of probing an endogenous, physiological form of cortical plasticity in vivo. MD is achieved by closing the eyelids of one eye, or patching one eye. MD during a critical period of mammalian development triggers synaptic changes in the visual cortex that favor the seeing eye. This involves two changes in visual cortical circuits, synaptic weakening in the deprived-eye pathways and synaptic potentiation in the non-deprived eye pathways. The latter plastic change occurs during sleep [13].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sala C, Segal M. Dendritic spines: The locus of structural and functional Plasticity. Physiological Reviews. 2014;94:141–188. doi: 10.1152/physrev.00012.2013. [DOI] [PubMed] [Google Scholar]
- 2.De Roo M, et al. Progress in Brain Research. Elsevier; 2008. Chapter 11 Spine dynamics and synapse remodeling during LTP and memory processes; pp. 199–207. [DOI] [PubMed] [Google Scholar]
- 3.Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Progress in Neurobiology. 2003;69:77–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 4.Rasch B.r., Born J. About sleep’s role in memory. Physiological Reviews. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, et al. Synaptic plasticity in sleep: learning, homeostasis and disease. Trends in Neurosciences. 2011;34:452–463. doi: 10.1016/j.tins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank MG. Erasing synapses in sleep: is it time to be SHY? Neural plasticity. 2012 doi: 10.1155/2012/264378. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tononi G, Cirelli C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanlon EC, et al. Synaptic potentiation and sleep need: Clues from molecular and electrophysiological studies. Current topics in medicinal chemistry. 2011;11:2472–2482. doi: 10.2174/156802611797470312. [DOI] [PubMed] [Google Scholar]
- 9.Aton S, et al. Sleep promotes cortical potentiation following visual experience. Sleep. 2014 doi: 10.5665/sleep.3830. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. The Journal of Neuroscience. 2010;30:16304–16313. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsanov M, Manahan-Vaughan D. The adult visual cortex expresses dynamic synaptic plasticity that is driven by the light/dark cycle. The Journal of Neuroscience. 2007;27:8414–8421. doi: 10.1523/JNEUROSCI.1101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hengen KB, et al. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron. 2013;80:335–342. doi: 10.1016/j.neuron.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aton SJ, et al. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seibt J, et al. Protein synthesis during sleep consolidates cortical plasticity in vivo. Current Biology. 2012;22:676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aton SJ, et al. Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proceedings of the National Academy of Sciences. 2013;110:3101–3106. doi: 10.1073/pnas.1208093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson C, Freeman RD. Progressive changes in kitten striate cortex during monocular deprivation. Journal of Neurophysiology. 1975;38:26–32. doi: 10.1152/jn.1975.38.1.26. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto H, et al. Experience-dependent slow-wave sleep development. Nat Neurosci. 2003;6:553–554. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- 18.Fagiolini M, et al. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Research. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 19.Buisseret P, Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. The Journal of Physiology. 1976;255:511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chauvette S, et al. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75:1105–1113. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matchock RL, et al. International Review of Neurobiology. Academic Press; 2010. Circadian and sleep episode duration influences on cognitive performance following the process of awakening; pp. 129–151. [DOI] [PubMed] [Google Scholar]
- 22.Huber R, et al. Human cortical excitability increases with time awake. Cerebral cortex. 2013;23:1–7. doi: 10.1093/cercor/bhs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Placidi F, et al. Increased cortical excitability after selective REM sleep deprivation in healthy humans: A transcranial magnetic stimulation study. Sleep Medicine. 2013;14:288–292. doi: 10.1016/j.sleep.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Doeltgen SH, Ridding MC. Behavioural exposure and sleep do not modify corticospinal and intracortical excitability in the human motor system. Clinical Neurophysiology. 2010;121:448–452. doi: 10.1016/j.clinph.2009.11.085. [DOI] [PubMed] [Google Scholar]
- 25.Lang N, et al. Circadian modulation of GABA-mediated cortical inhibition. Cerebral Cortex. 2011;21:2299–2306. doi: 10.1093/cercor/bhr003. [DOI] [PubMed] [Google Scholar]
- 26.Shannon BJ, et al. Morning-evening variation in human brain metabolism and memory circuits. Journal of Neurophysiology. 2012 doi: 10.1152/jn.00651.2012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nitz DA, et al. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Current Biology : CB. 2002;19:1934–1940. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- 28.van Alphen B, et al. A dynamic deep sleep stage in drosophila. The Journal of Neuroscience. 2013;33:6917–6927. doi: 10.1523/JNEUROSCI.0061-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown ER, et al. Brain and behavioural evidence for rest-activity cycles in Octopus vulgaris. Behavioural Brain Research. 2006;172:355–359. doi: 10.1016/j.bbr.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Ramon F, et al. Slow wave sleep in crayfish. PNAS. 2004;101:11857–11861. doi: 10.1073/pnas.0402015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Medicine Reviews. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Vecsey CG, et al. Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiological Genomics. 2012;44:981–991. doi: 10.1152/physiolgenomics.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribeiro S. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J. Neurosci. 2002;22:10914–10923. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro S, et al. Brain gene expression during REM sleep depends on prior waking experience. Learn Mem. 1999;6:500–508. doi: 10.1101/lm.6.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seibt J, Frank MG. Translation regulation in sleep: making experience last. Communicative and Integrative Biology. 2012;5:491–495. doi: 10.4161/cib.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollock GS, et al. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. The Journal of Neuroscience. 2001;21:3923–3931. doi: 10.1523/JNEUROSCI.21-11-03923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neuner-Jehle M, et al. Sleep deprivation differentially affects the mRNA and protein levels of neurogranin in rat brain. Brain Res. 1995;685:143–153. doi: 10.1016/0006-8993(95)00416-n. [DOI] [PubMed] [Google Scholar]
- 39.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. The Journal of Neuroscience. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vyazovskiy VV, et al. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nature Neuroscience. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 41.Cirelli C, et al. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 42.Horn G. Pathways of the past: the imprint of memory. Nat Rev Neurosci. 2004;5:108–120. doi: 10.1038/nrn1324. [DOI] [PubMed] [Google Scholar]
- 43.Jackson C, et al. Dynamics of a memory trace: effects of sleep on consolidation. Current Biology. 2008;18:393–400. doi: 10.1016/j.cub.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 44.Horn G. Visual imprinting and the neural mechanisms of recognition memory. Trends in Neurosciences. 1998;21:300–305. doi: 10.1016/s0166-2236(97)01219-8. [DOI] [PubMed] [Google Scholar]
- 45.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25:18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- 46.Cirelli C, et al. No evidence of brain cell degeneration after long-term sleep deprivation in rats. Brain Research. 1999;840:184–193. doi: 10.1016/s0006-8993(99)01768-0. [DOI] [PubMed] [Google Scholar]
- 47.Hipolide DC, et al. Sleep deprivation does not affect indices of necrosis or apoptosis in rat brain. Int J Neurosci. 2002;112:155–166. doi: 10.1080/00207450212022. [DOI] [PubMed] [Google Scholar]
- 48.Biswas S, et al. Increased apoptosis in rat brain after rapid eye movement sleep loss. Neuroscience. 2006;142:315–331. doi: 10.1016/j.neuroscience.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 49.Yelamanchili SV, et al. Differential sorting of the vesicular glutamate transporter 1 into a defined vesicular pool is regulated by light signaling involving the clock gene period2. Journal of Biological Chemistry. 2006;281:15671–15679. doi: 10.1074/jbc.M600378200. [DOI] [PubMed] [Google Scholar]
- 50.Maret S, et al. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci. 2011;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang G, Gan W-B. Sleep contributes to dendritic spine formation and elimination in the developing mouse somatosensory cortex. Developmental Neurobiology. 2012;72:1391–1398. doi: 10.1002/dneu.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang G, et al. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Appelbaum L, et al. Circadian and homeostatic regulation of structural synaptic plasticity in hypocretin neurons. Neuron. 2010;68:87–98. doi: 10.1016/j.neuron.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilestro GF, et al. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donlea JM, et al. Use-dependent plasticity in clock neurons regulates sleep need in drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donlea JM, et al. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bushey D, et al. Sleep and synaptic homeostasis: structural evidence in drosophila. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore RY, Martha UG. Progress in Molecular Biology and Translational Science. Academic Press; 2013. Chapter One - The Suprachiasmatic Nucleus and the Circadian Timing System; pp. 1–28. [DOI] [PubMed] [Google Scholar]
- 59.Pegoraro M, Tauber E. Animal clocks: a multitude of molecular mechanisms for circadian timekeeping. Wiley Interdisciplinary Reviews: RNA. 2011;2:312–320. doi: 10.1002/wrna.58. [DOI] [PubMed] [Google Scholar]
- 60.Girardet C, et al. Neuroglial and synaptic rearrangements associated with photic entrainment of the circadian clock in the suprachiasmatic nucleus. The European Journal of Neuroscience. 2010;32:2133–2142. doi: 10.1111/j.1460-9568.2010.07520.x. [DOI] [PubMed] [Google Scholar]
- 61.Barnes CA, et al. Circadian Rhythm of Synaptic Excitability in Rat and Monkey Central Nervous System. Science. 1977;197:91–92. doi: 10.1126/science.194313. [DOI] [PubMed] [Google Scholar]
- 62.Harris KM, Teyler TJ. Age differences in a circadian influence on hippocamapl LTP. Brain Research. 1983;261:69–73. doi: 10.1016/0006-8993(83)91284-2. [DOI] [PubMed] [Google Scholar]
- 63.Bowden JB, et al. Differential effects of strain, circadian cycle, and stimulation pattern on LTP and concurrent LTD in the dentate gyrus of freely moving rats. Hippocampus. 2012;22:1363–1370. doi: 10.1002/hipo.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaudhury D, et al. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2005;20:225–236. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raghavan AV, et al. Diurnal modulation of long-term potentiation in the hamster hippocampal slice. Brain Research. 1999;833:311–314. doi: 10.1016/s0006-8993(99)01523-1. [DOI] [PubMed] [Google Scholar]
- 66.Eckel-Mahan KL. Circadian oscillations within the hippocampus support hippocampus-dependent memory processing. Frontiers in Molecular Neuroscience. 2012;5 doi: 10.3389/fnmol.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanada Y, Kawamura H. Circadian rhythms in synaptic excitability of the dorsal lateral geniculate nucleus in the rat. Int J Neurosci. 1984;22:253–261. doi: 10.3109/00207458408990682. [DOI] [PubMed] [Google Scholar]
- 68.Krishnan B, et al. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature. 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- 69.Page TL, Koelling E. Circadian rhythm in olfactory response in the antennae controlled by the optic lobe in the cockroach. Journal of insect physiology. 2003;49:697–707. doi: 10.1016/s0022-1910(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 70.Tanoue S, et al. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Current biology : CB. 2004;14:638–649. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 71.Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. The Journal of Neuroscience. 2008;28:6493–6501. doi: 10.1523/JNEUROSCI.1503-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheeba V, et al. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. Journal of Neurophysiology. 2008;99:976–988. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vollrath L, Spiwoks-Becker I. Plasticity of retinal ribbon synapses. Microscopy Research and Technique. 1996;35:472–487. doi: 10.1002/(SICI)1097-0029(19961215)35:6<472::AID-JEMT6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 74.Sjostrand FS. The ultrastructure of the retinal rod synapses of the guinea pig eye. J Appl Physics. 1953;24:1422. doi: 10.1016/s0022-5320(58)90050-9. [DOI] [PubMed] [Google Scholar]
- 75.Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 76.McNulty JA. Synaptic ribbons in the pineal organ of the goldfish: circadian rhythmicity and the effects of constant light and constant darkness. Cell Tissue Res. 1981;215:491–497. doi: 10.1007/BF00233525. [DOI] [PubMed] [Google Scholar]
- 77.Emran F, et al. Zebrafish larvae lose vision at night. Proceedings of the National Academy of Sciences. 2010;107:6034–6039. doi: 10.1073/pnas.0914718107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pyza E, Meinertzhagen IA. Daily and circadian rhythms of synaptic frequency in the first visual neuropile of the housefly’s (Musca domestica L.) optic lobe. Proceedings. Biological sciences / The Royal Society. 1993;254:97–105. doi: 10.1098/rspb.1993.0133. [DOI] [PubMed] [Google Scholar]
- 79.Pyza E, Meinertzhagen IA. Daily rhythmic changes of cell size and shape in the first optic neuropil in Drosophila melanogaster. J. Neurobiol. 1999;40:77–88. doi: 10.1002/(sici)1097-4695(199907)40:1<77::aid-neu7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 80.Barth M, et al. Circadian plasticity in photoreceptor cells controls visual coding efficiency in Drosophila melanogaster. PLoS ONE. 2010;5:e9217. doi: 10.1371/journal.pone.0009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weber P, et al. Circadian control of dendrite morphology in the visual system of Drosophila melanogaster. PLoS ONE. 2009;4:e4290. doi: 10.1371/journal.pone.0004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernandez MP, et al. Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol. 2008;6:e69. doi: 10.1371/journal.pbio.0060069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruiz S, et al. Rhythmic changes in synapse numbers in Drosophila melanogaster motor terminals. PLoS ONE. 2013;8:e67161. doi: 10.1371/journal.pone.0067161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mehnert KI, et al. Circadian changes in Drosophila motor terminals. Dev Neurobiol. 2007;67:415–421. doi: 10.1002/dneu.20332. [DOI] [PubMed] [Google Scholar]
- 85.Mehnert KI, Cantera R. A peripheral pacemaker drives the circadian rhythm of synaptic boutons in Drosophila independently of synaptic activity. Cell Tissue Res. 2008;334:103–109. doi: 10.1007/s00441-008-0670-0. [DOI] [PubMed] [Google Scholar]
- 86.Ruiz S, et al. Synaptic vesicles in motor synapses change size and distribution during the day. Synapse. 2010;64:14–19. doi: 10.1002/syn.20699. [DOI] [PubMed] [Google Scholar]
- 87.Liston C, et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kavanau JL. Memory, sleep, and dynamic stabilization of neural circuitry: evolutionary perspectives. Neurosci Biobehav Rev. 1996;20:289–311. doi: 10.1016/0149-7634(95)00019-4. [DOI] [PubMed] [Google Scholar]
- 89.Frank MG. Why I’m not SHY: A reply to Tononi and Cirelli. Neural Plasticity. 2013 doi: 10.1155/2013/394946. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Cauter E. Endocrine physiology. In: Kryger M, et al., editors. Principles and Practice of Sleep Medicine. 5th edn Elsevier; 2005. pp. 266–282. [Google Scholar]
- 91.Komatsuzaki Y, et al. Corticosterone induces rapid spinogenesis via synaptic glucocorticoid receptors and kinase networks in hippocampus. PLoS ONE. 2012;7:e34124. doi: 10.1371/journal.pone.0034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tse YC, et al. Dynamic regulation of NMDAR function in the adult brain by the stress hormone corticosterone. Frontiers in Cellular Neuroscience. 2012;6 doi: 10.3389/fncel.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joels M, et al. Stress-induced changes in hippocampal function. Progress in brain research. 2008;167:3–15. doi: 10.1016/S0079-6123(07)67001-0. [DOI] [PubMed] [Google Scholar]
- 94.Heller HC. Temperature, Thermoregulation and Sleep. In: Kryger MH, et al., editors. Principles and Practice of Sleep Medcine. Fourth edn Elsevier; 2005. pp. 292–304. [Google Scholar]
- 95.Stevenson RD. Body size and limits to the daily range of body temperature in terrestrial ectotherms. The American Naturalist. 1985;125:102–117. [Google Scholar]
- 96.Moser E, et al. Association between brain temperature and dentate field potentials in exploring and swimming rats. Science. 1993;259:1324–1326. doi: 10.1126/science.8446900. [DOI] [PubMed] [Google Scholar]
- 97.Franken P, et al. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. The American journal of physiology. 1991;261:R198–208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 98.Franken P, et al. Effects of 12-h sleep deprivation and of 12-h cold exposure on sleep regulation and cortical temperature in the rat. Physiolo. Behav. 1993;54:885–894. doi: 10.1016/0031-9384(93)90297-s. [DOI] [PubMed] [Google Scholar]
- 99.Zhong Y, Wu C-F. Neuronal activity and adenylyl cyclase in environment-dependent plasticity of axonal outgrowth in Drosophila. The Journal of Neuroscience. 2004;24:1439–1445. doi: 10.1523/JNEUROSCI.0740-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng IF, et al. Temperature-dependent developmental plasticity of drosophila neurons: cell-autonomous roles of membrane excitability, Ca2+ influx, and cAMP signaling. The Journal of Neuroscience. 2007;27:12611–12622. doi: 10.1523/JNEUROSCI.2179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vanin S, et al. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature. 2012;484:371–375. doi: 10.1038/nature10991. [DOI] [PubMed] [Google Scholar]
- 102.Mongrain V, et al. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep. 2010;33:1147–1157. doi: 10.1093/sleep/33.9.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. The Journal of Neuroscience. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dahlhaus M, Levelt CN. Structure and function relationships during ocular dominance plasticity in the visual cortex. Rev Neurosci. 2010;21:223–237. doi: 10.1515/revneuro.2010.21.3.223. [DOI] [PubMed] [Google Scholar]
- 105.Datta S, Patteron EH. Activation of phasic pontine wave (P-wave): A mechanism of learning and memory processing. In: Maquet P, et al., editors. Sleep and Brain Plasticity. Oxford University Press; 2003. pp. 135–156. [Google Scholar]
- 106.Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–114. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 107.Giuditta A, et al. The sequential hypothesis of the function of sleep. Behavioral Brain Research. 1995;69:157–166. doi: 10.1016/0166-4328(95)00012-i. [DOI] [PubMed] [Google Scholar]
- 108.Frank MG. Sleep and synaptic plasticity in the developing and adult brain. Current topics in behavioral neurosciences. 2014 doi: 10.1007/7854_2014_305. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ribeiro S. Sleep and plasticity. Pflugers Archiv European Journal of Physiology. 2011:1–10. doi: 10.1007/s00424-011-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Genzel L, et al. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends in Neurosciences. 2014;37:10–19. doi: 10.1016/j.tins.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 111.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Z-W, et al. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. Journal of Neuroscience. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]