Abstract
Cells of the innate immune system are important mediators of multiple sclerosis (MS). We have previously identified Kruppel-like Factor 2 (KLF2) as a critical negative regulator of myeloid activation in the setting of bacterial infection and sepsis, but the role of myeloid KLF2 in MS has not been investigated. In this study, myeloid KLF2 deficient mice exhibited more severe neurological dysfunction and increased spinal cord demyelination and neuroinflammation in experimental autoimmune encephalomyelitis. This study represents the first description of a significant role of myeloid KLF2 in neuroinflammation, identifying KLF2 as a potential target for further investigation in patients with MS.
Keywords: KLF2, Multiple sclerosis, Experimental autoimmune encephalomyelitis, Macrophage, Neuroinflammation, Demyelination
1. Introduction
Multiple Sclerosis (MS) is a chronic and often debilitating immune mediated inflammatory disease that affects the central nervous system (CNS) causing significant neurological disability(Noseworthy et al., 2000). In MS the normally immunologically privileged brain and spinal cord are invaded by multiple leukocyte cell types that initiate an inflammatory process resulting in demyelination and axonal degeneration(Shechter and Schwartz, 2013). In addition to T lymphocytes, cells of the innate immune system, in particular peripheral blood macrophages and microglial cells, have also been demonstrated to play important roles in mediating MS. Multiple loss of function studies using the experimental autoimmune encephalomyelitis (EAE) murine model have been conducted that demonstrate the detrimental role of these cells in MS(Rawji and Yong, 2013).
Kruppel-like factor 2 (KLF2) is a member of the Kruppel-like family of zinc-finger transcription factors that are critically involved in regulating cellular development and function(McConnell and Yang, 2010). Recent studies from our laboratory identified KLF2 as a critical negative regulator of myeloid pro-inflammatory activation and alterations in KLF2 levels serve as a key determinant of the innate immune response in vivo (Das et al., 2006, Mahabeleshwar et al., 2011). Further, a reduction in myeloid KLF2 levels occurs in patients with acute and chronic inflammatory disease states such as sepsis(Mahabeleshwar et al., 2011) and atherosclerosis(Das et al., 2006). While these seminal studies were the first to identify an in vivo role for myeloid KLF2, the importance of this regulatory pathway in CNS disease has not been investigated.
The aim of the current study is to evaluate the role of myeloid KLF2 in MS. Our study demonstrates that myeloid KLF2 is an important mediator of murine EAE via regulation of neuroinflammation.
2. Materials and methods
2.1. Animals and EAE murine model
All animal experiments were performed in accordance with guidelines of and approved by the Institutional Animal Care and Use Committee, Case Western Reserve University. Myeloid specific KLF2 deficient mice (LysMCre/Cre:KLF2fl/fl, designated MY-K2-KO) were generated as previously described(Mahabeleshwar et al., 2011). In MY-K2-KO mice greater than 95% deletion of KLF2 is observed in myeloid cells(Mahabeleshwar et al., 2011) with no significant change in KLF4 and KLF6 expression(two KLF family members demonstrated to have important roles in myeloid cells(Date et al., 2014, Liao et al., 2011)(Supplemental Figure 1). In addition, no substantial effect was seen on other hematopoietic lineages, tissues, or monocytic subsets(Mahabeleshwar et al., 2011). EAE was induced in 8 week old female MY-K2-KO mice and age matched control mice (LysMCre/Cre) (n=20–25 mice per group) by subcutaneous immunization with myelin oligodendrocyte glycoprotein (MOG)35–55 peptide (300 μg/mouse, Cleveland Clinic Molecular Biotechnology Core Laboratory) emulsified in Freund’s complete adjuvant (Sigma). On day 0 and day 2 after immunization mice received an intraperitoneal injection of pertussis toxin (200 ng/mouse, Sigma). Mice were weighed and observed daily for 28 days after immunization for neurological deficits. Mean daily clinical neurological scores were determined based on the observed neurological deficit of each group: 0=healthy, 1=limp tail, 2=limp tail and hind leg weakness or impaired righting reflex or paresis of one limb, 3=limp tail and complete paralysis of hind legs or limp tail with paralysis of one front and one hind leg, 4=limp tail, complete hind leg and partial front leg paralysis, 5=complete hind and complete front leg paralysis, no movement around cage.
2.2. Histology and immunofluorescence
Lumbar spinal cord cryosections (10 μm) from three evenly spaced levels between T12 and L2 of each spinal cord (n=5–7 spinal cords per group) were stained with Luxol fast blue (LFB) and hematoxylin and eosin (H&E), or fixed in 4% paraformaldehyde for 10 minutes, washed and blocked for 30 minutes with 5% BSA in PBS-T (0.1 M PBS containing 0.2% Tween 20) and incubated overnight at 4 °C with antibodies to myelin basic protein (MBP; Millipore), CD3 (Dako), chemokine C-C motif ligand 2 (CCL2; Santa Cruz), inducible nitric oxide synthase (iNOS), CD45, CD68 (BD Biosciences) as indicated, followed by Alexa-488 or 594 conjugated secondary antibodies (Invitrogen). All imaging was performed using a Leica video imaging system. To quantify immunostaining results identical light intensity and exposure times were applied to all photographs from each experimental set. Images were acquired separately from the bilateral dorsal, ventral, and lateral white matter columns from three levels of the spinal cord for each mouse. All images were converted to grayscale and then analyzed by density measurement with ImageJ software. A fixed threshold range of 0–160 was chosen to highlight the staining signals in normal spinal cord sections, and the total area within this range was measured, averaged, and compared.
2.3. Real time quantitative PCR analysis
Lumbar spinal cords from MY-K2-KO and Control mice at peak stage (i.e., day 16–19 after immunization, n=3–4 mice per group) were harvested and total RNA was extracted from spinal cord tissue with TRIzol Reagent (Invitrogen) according to the manufacturers instructions. 2 μg of total RNA was reverse transcribed with M-MuLV reverse transcriptase (New England BioLabs Inc.) and oligo-dT primers. Real-time PCR was performed with Universal SYBR Green PCR Master Mix (Roche) on a LightCycler 480 System (Roche) with gene specific primers for iNOS, CCL2, TNFα, IL1β, CD16, CD86, MMP-2, and MMP-9.
2.4. Statistics
Data are expressed as mean±SEM. Differences between statistical groups were evaluated for statistical significance using the Student’s t-test for unpaired data. P<0.05 was considered statistically significant.
3. Results
3.1. Myeloid KLF2 deficiency exacerbates neurological dysfunction and spinal cord demyelination in EAE
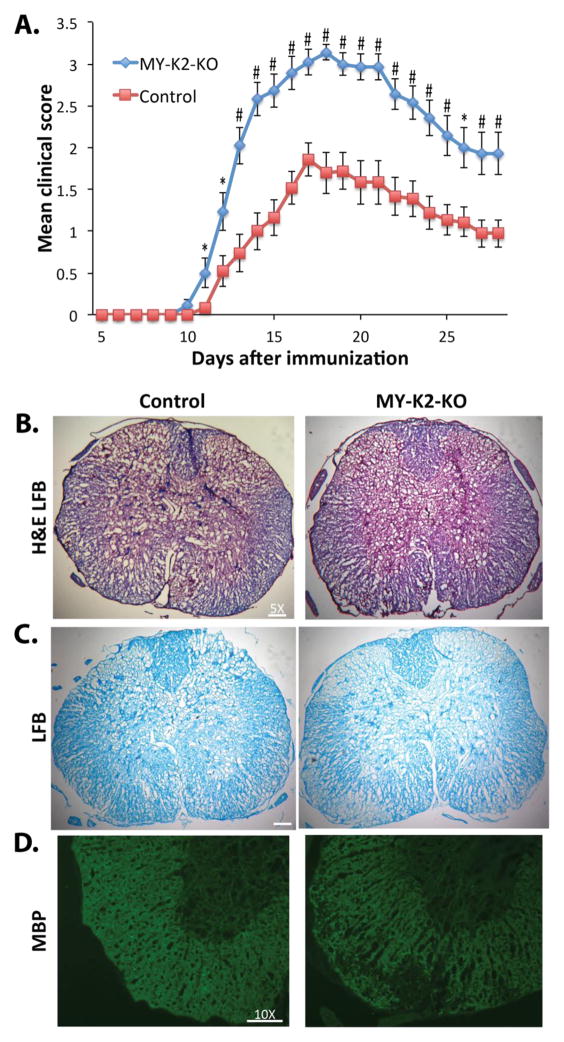
To assess the role of myeloid KLF2 in MS, EAE was induced in MY-K2-KO and control mice. MY-K2-KO mice exhibited a more rapid onset of neurological dysfunction and higher clinical neurological scores when compared to control mice (Figure 1A). Myelin staining of lumbar spinal cords using LFB (Figures 1B and 1C) or immunostaining for MBP (Figure 1D) reveals less staining in MY-K2-KO mice compared to control mice, indicating greater demyelination of the CNS. These data demonstrating that myeloid KLF2 inhibits myelin degradation and neurological dysfunction in EAE strongly implicate myeloid KLF2 is a protective factor in EAE.
Fig 1. Myeloid KLF2 deficient mice have more severe neurological deficits and spinal cord demyelination in EAE.
(A) Clinical neurological scores from 8 week old female MY-K2-KO and Control mice after immunization with MOG35–55 peptide (n=20–25 per group). *P<0.05, #P<0.005. (B–D) Lumbar spinal cords from MY-K2-KO and Control mice at peak stage (i.e., day 16–19 after immunization) stained with: (B) hematoxylin, eosin, and Luxol fast blue (H&E LFB), (C) Luxol fast blue alone (LFB), and (D) myelin basic protein (MBP) antibody. Representative results are shown (n=5–7 per group).
3.2. Myeloid KLF2 deficiency increases leukocyte infiltration and neuroinflammation of the spinal cord in EAE
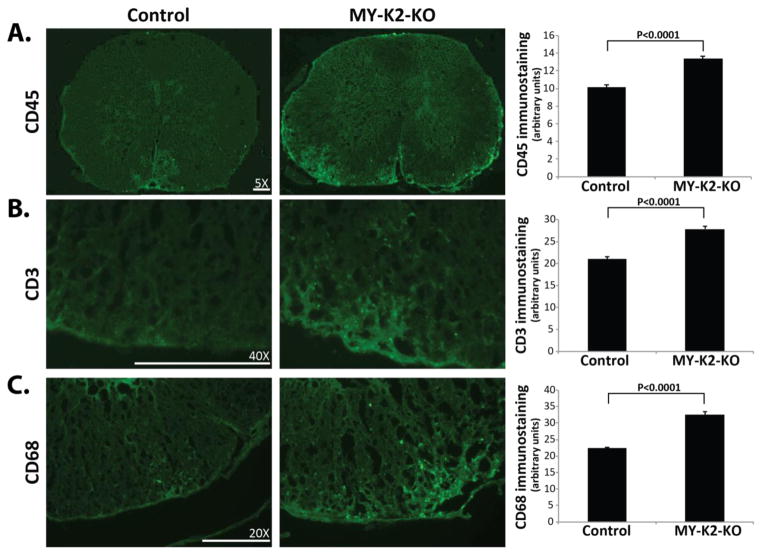
In order to investigate the cellular mechanisms by which myeloid KLF2 exerts its protective functions in EAE, we next conducted immunostaining of lumbar spinal cords to assess the levels of neuroinflammation at peak stage (i.e., 16–19 days after immunization) of immunized mice. CD45 immunostaining (Figure 2A) reveals greater leukocyte infiltration in the spinal cords of MY-K2-KO mice compared to controls (13.40±0.23 vs 10.12±0.26; P<0.0001). Staining for the T lymphocyte specific marker CD3 (Figure 2B) and the macrophage/microglia specific marker CD68 (Figure 2C) demonstrates the presence of more T lymphocytes (27.87±0.69 vs 21.03±0.60; P<0.0001) and macrophages/microglia (32.50±0.99 vs 22.38±0.37; P<0.0001) in the spinal cords of MY-K2-KO mice.
Fig 2. Myeloid KLF2 deficient mice have increased spinal cord invasion by leukocytes in EAE.
Lumbar spinal cords from MY-K2-KO and Control mice at peak stage (i.e., day 16–19 after immunization) immunostained for (A) CD45 (leukocyte marker), (B) CD3 (T lymphocyte marker), and (C) CD68 (macrophage marker). Representative results are shown. (n=5–7 per group). Quantification of immunostaining was performed as described in Methods.
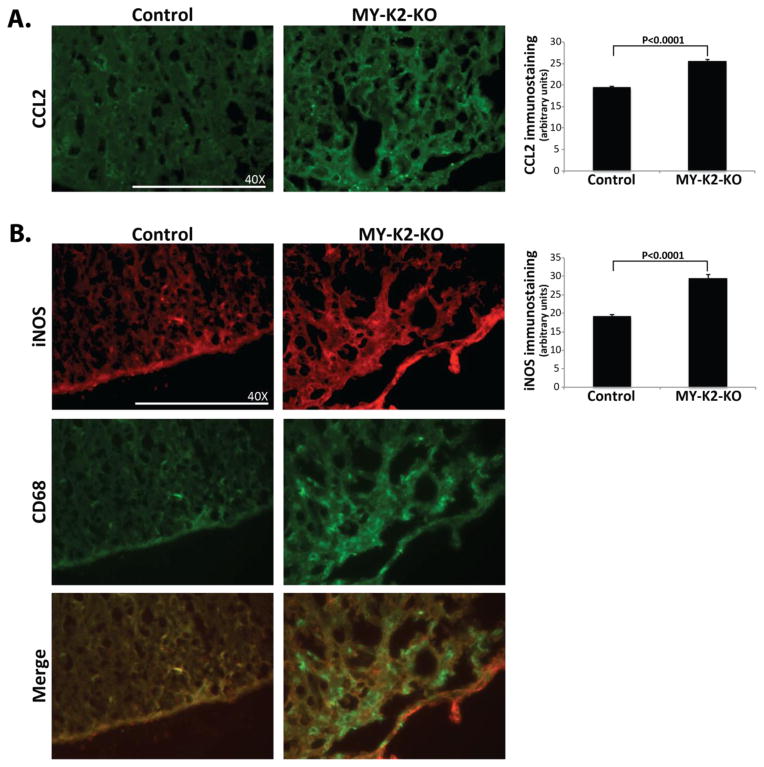
Recent work suggests that a spectrum of distinct macrophage/microglial phenotypes are observed in the inflammatory lesions of EAE(Mantovani et al., 2004, Martinez et al., 2009). Classically activated macrophages are considered pro-inflammatory and are identified by their expression of cell surface markers such as CD16/32 and CD86 as well as the expression of iNOS, and the expression and secretion of pro-inflammatory cytokines (e.g., tumor necrosis factor α; TNFα and interleukin 1β; IL1β), chemotactic factors (e.g., CCL2), and matrix metalloproteinases (e.g., MMP-2 and MMP-9)(David and Kroner, 2011, Mantovani et al., 2013, Aguzzi et al., 2013). Staining of spinal cords from immunized mice reveals an enhanced pro-inflammatory phenotype in MY-K2-KO mice as shown by the presence of increased immunostaining for CCL2 (25.65±0.30 vs 19.47±0.31; P<0.0001; Figure 3A) and iNOS (29.46±0.95 vs 19.19±0.53; P<0.0001; Figure 3B). Notably, co-staining with CD68 reveals co-localization of enhanced iNOS staining within the regions of increased macrophage invasion (Figure 3B), suggesting that the predominant source of iNOS is the macrophage/microglial cell.
Fig 3. Myeloid KLF2 deficient mice have increased iNOS and CCL2 immunostaining in their spinal cords in EAE.
Lumbar spinal cords from MY-K2-KO and Control mice at peak stage (i.e., day 16–19 after immunization) (A) immunostained for CCL2 and (B) co-stained for CD68 and iNOS. Representative results are shown. (n=5–7 per group). Quantification of immunostaining was performed as described in Methods.
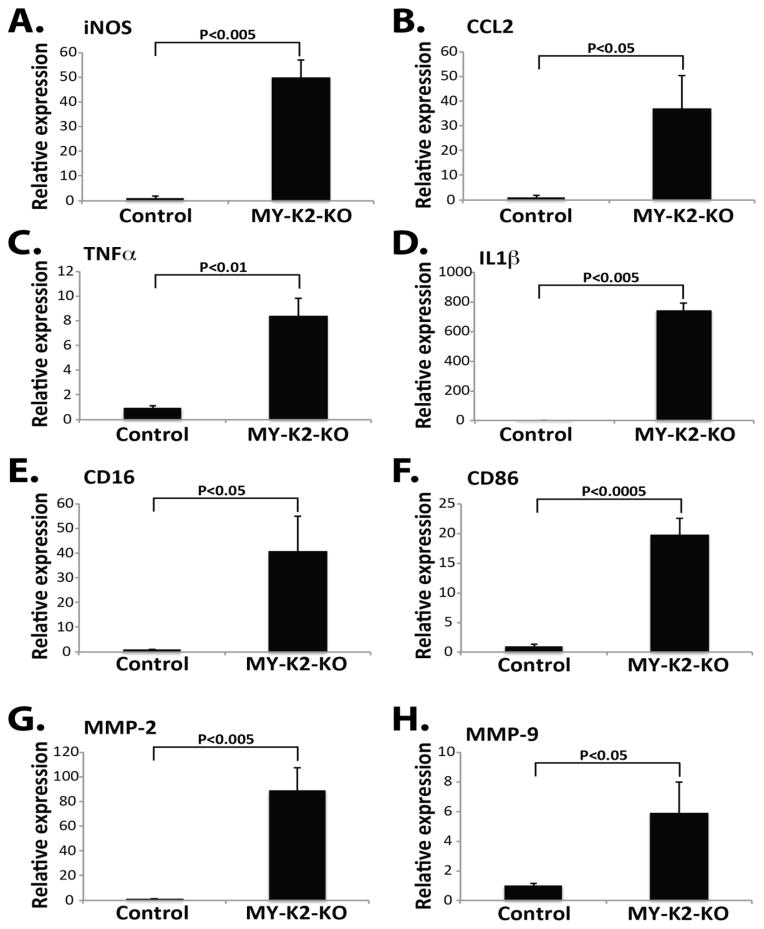
Finally, total RNA was harvested from spinal cords at peak stage and qPCR analysis performed. MY-K2-KO mice demonstrated markedly increased mRNA expression of several pro-inflammatory factors (i.e., iNOS, CCL2, TNFα, IL1β, CD16, CD86, MMP-2, and MMP-9; Figures 4A-H). Taken together these findings strongly suggest that myeloid KLF2 is a protective factor in EAE via its inhibition of neuroinflammation.
Fig 4. Myeloid KLF2 deficient mice have increased spinal cord expression of pro-inflammatory factors in EAE.
Lumbar spinal cords from MY-K2-KO and Control mice at peak stage (i.e., day 16–19 after immunization) were harvested for total RNA and qPCR analysis performed for (A) iNOS, (B) CCL2, (C) TNFα, (D) IL1β, (E) CD16, (F) CD86, (G) MMP-2, and (H) MMP-9. (n=3–4 per group).
4. Discussion
There are several mechanisms by which activated macrophages/microglia promote demyelination of neurons(Rawji and Yong, 2013). They have been implicated in facilitating the entry of pathogenic T cells into the CNS by releasing harmful proteases (e.g., MMPs) that breakdown the blood brain barrier(Agrawal et al., 2006, Yong et al., 2001, Toft-Hansen et al., 2004, Agrawal et al., 2011). In addition activated macrophages/microglia produce pro-inflammatory cytokines (e.g., IL1β and TNFα) that stimulate the release of the excitotoxin glutamate, resulting in neuronal and oligodendrocyte death(Takeuchi et al., 2005, Shijie et al., 2009). Secreted pro-inflammatory cytokines and chemokines promote further inflammation as well as the recruitment of inflammatory cells and reactivation of T lymphocytes via antigen presentation(Raivich and Banati, 2004, Murphy et al., 2010). Activated macrophages/microglia also release damaging free radicals, such as nitric oxide (NO) through the induction of iNOS, resulting in oxidative and nitrosative stress(Reynolds et al., 2007).
The central finding of our current study is that myeloid KLF2 is a negative regulator of EAE induced neuroinflammation via the inhibition of several pro-inflammatory factors. These results are consistent with our prior studies using MY-K2-KO mice where we demonstrated that myeloid KLF2 deficiency enhances lipopolysaccharide (LPS) mediated induction of iNOS, IL1β, TNFα, and CCL2, thereby determining the outcome in models of polymicrobial infection and endotoxemia(Mahabeleshwar et al., 2011). Our studies implicate KLF2 as playing a key role in inhibiting the pro-inflammatory M1 macrophage phenotype during the peak stage of EAE. Interestingly, M2 macrophages have been shown to be anti-inflammatory and reparative in the recovery stage of mouse models of MS(Rawji and Yong, 2013). In the future it would be interesting to investigate if KLF2 has the ability to regulate the polarization of macrophages to the M2 phenotype and if this might play a role in the recovery stages of EAE.
As mentioned earlier we have previously identified KLF2 as playing an important role in regulating the macrophage response to bacterial infection and sepsis. Specifically, myeloid KLF2 deficiency results in increased bactericidal activity and bacterial clearance by macrophages but increased susceptibility to endotoxic shock(Mahabeleshwar et al., 2011). One potential confounding issue with the currently used mouse model is the use of pertussis toxin during EAE induction. Notably, we observe no ill effects on either MY-K2-KO or Control mice immediately after pertussis toxin injection. Bacterial exotoxins have been previously described to increase the expression of KLF’s, specifically KLF2 and KLF6, thereby exploiting the KLF regulatory cascade to modulate pro-inflammatory cytokine expression(O'Grady et al., 2007). To investigate the possibility that this mechanism is playing a role in our studies we assessed the levels of KLF2 and KLF6 expression in the spinal cords of Control mice before and after EAE induction/pertussis toxin injection and we observed no statistically significant change in either KLF2 or KLF6 expression (Supplemental Figure 3). In fact there was a statistically insignificant trend towards a decrease in KLF2 and KLF6 expression after EAE induction, presumably due to the enhanced inflammation after EAE induction.
Given the central role of nuclear factor kappa B (NFκB) in general inflammation and studies specifically outlining its role in EAE induced neuroinflammation(Pahan and Schmid, 2000, van Loo et al., 2006, Ellrichmann et al., 2012), we suspect that KLF2 may regulate pro-inflammatory gene expression through modulation of NFκB activity. Members of the KLF family have been shown in multiple cell types to interact with the NFκB pathway(Atkins and Simon, 2013). Specifically KLF2 has previously been shown to inhibit NFκB in endothelial(SenBanerjee et al., 2004) and myeloid cells(Das et al., 2006, Mahabeleshwar et al., 2011). Therefore, in order to elucidate a more detailed molecular mechanism of KLF2’s action, additional studies are necessary to investigate the NFκB-KLF2 axis in myeloid cells in the context of neuroinflammation.
In conclusion, our studies are the first to recognize myeloid KLF2 as a critical mediator of neuroinflammation and neurological dysfunction in EAE. Our findings identify KLF2 as a potential target for further investigation in patients with MS and other CNS disease states involving neuroinflammation.
Supplementary Material
Fig S1. Myeloid KLF2 deficient mice have no significant alteration in expression of KLF4 and KLF6. Peritoneal macrophages from MY-K2-KO and Control mice were harvested for total RNA and qPCR analysis performed for (A) KLF4 and (B) KLF6. (n=5 per group).
Fig S2. EAE induction/pertussis toxin injection does not result in significant change in expression of KLF2 and KLF6. Lumbar spinal cords from Control mice before EAE induction and after EAE induction/pertussis toxin injection were harvested for total RNA and qPCR analysis performed for (A) KLF2 and (B) KLF6. (n=3–5 per group).
Acknowledgments
This work was supported by the Hemostasis and Thrombosis Research Society Mentored Research Award (L.N.), National Institutes of Health Grants HL087595, HL117759 (Z.L.); HL110630, HL097593, HL112486, HL086548, HL119195 (M.K.J.), American Heart Association Established Investigator Award (M.K.J.), Visconsi Scholarship (G.B.A.), Case Western Reserve University School of Medicine Vision Funds (G.B.A.).
Footnotes
Disclosure
The authors report no conflicts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AGRAWAL S, ANDERSON P, DURBEEJ M, VAN ROOIJEN N, IV, ARS F, OPDENAKKER G, SOROKIN LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–19. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AGRAWAL SM, SILVA C, TOURTELLOTTE WW, YONG VW. EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci. 2011;31:669–77. doi: 10.1523/JNEUROSCI.3659-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AGUZZI A, BARRES BA, BENNETT ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–61. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATKINS GB, SIMON DI. Interplay between NF-kappaB and Kruppel-like factors in vascular inflammation and atherosclerosis: location, location, location. J Am Heart Assoc. 2013;2:e000290. doi: 10.1161/JAHA.113.000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAS H, KUMAR A, LIN Z, PATINO WD, HWANG PM, FEINBERG MW, MAJUMDER PK, JAIN MK. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc Natl Acad Sci U S A. 2006;103:6653–8. doi: 10.1073/pnas.0508235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATE D, DAS R, NARLA G, SIMON DI, JAIN MK, MAHABELESHWAR GH. Kruppel-like transcription factor 6 regulates inflammatory macrophage polarization. J Biol Chem. 2014;289:10318–29. doi: 10.1074/jbc.M113.526749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID S, KRONER A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–99. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- ELLRICHMANN G, THONE J, LEE DH, RUPEC RA, GOLD R, LINKER RA. Constitutive activity of NF-kappa B in myeloid cells drives pathogenicity of monocytes and macrophages during autoimmune neuroinflammation. J Neuroinflammation. 2012;9:15. doi: 10.1186/1742-2094-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIAO X, SHARMA N, KAPADIA F, ZHOU G, LU Y, HONG H, PARUCHURI K, MAHABELESHWAR GH, DALMAS E, VENTECLEF N, FLASK CA, KIM J, DOREIAN BW, LU KQ, KAESTNER KH, HAMIK A, CLEMENT K, JAIN MK. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–49. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHABELESHWAR GH, KAWANAMI D, SHARMA N, TAKAMI Y, ZHOU G, SHI H, NAYAK L, JEYARAJ D, GREALY R, WHITE M, MCMANUS R, RYAN T, LEAHY P, LIN Z, HALDAR SM, ATKINS GB, WONG HR, LINGREL JB, JAIN MK. The myeloid transcription factor KLF2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity. 2011;34:715–28. doi: 10.1016/j.immuni.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTOVANI A, BISWAS SK, GALDIERO MR, SICA A, LOCATI M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–85. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- MANTOVANI A, SICA A, SOZZANI S, ALLAVENA P, VECCHI A, LOCATI M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- MARTINEZ FO, HELMING L, GORDON S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- MCCONNELL BB, YANG VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–81. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY AC, LALOR SJ, LYNCH MA, MILLS KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–51. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- NOSEWORTHY JH, LUCCHINETTI C, RODRIGUEZ M, WEINSHENKER BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- O'GRADY E, MULCAHY H, ADAMS C, MORRISSEY JP, O'GARA F. Manipulation of host Kruppel-like factor (KLF) function by exotoxins from diverse bacterial pathogens. Nat Rev Microbiol. 2007;5:337–41. doi: 10.1038/nrmicro1641. [DOI] [PubMed] [Google Scholar]
- PAHAN K, SCHMID M. Activation of nuclear factor-kB in the spinal cord of experimental allergic encephalomyelitis. Neurosci Lett. 2000;287:17–20. doi: 10.1016/s0304-3940(00)01167-8. [DOI] [PubMed] [Google Scholar]
- RAIVICH G, BANATI R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev. 2004;46:261–81. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- RAWJI KS, YONG VW. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin Dev Immunol. 2013;2013:948976. doi: 10.1155/2013/948976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS A, LAURIE C, MOSLEY RL, GENDELMAN HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- SENBANERJEE S, LIN Z, ATKINS GB, GREIF DM, RAO RM, KUMAR A, FEINBERG MW, CHEN Z, SIMON DI, LUSCINSKAS FW, MICHEL TM, GIMBRONE MA, JR, GARCIA-CARDENA G, JAIN MK. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–15. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHECHTER R, SCHWARTZ M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. J Pathol. 2013;229:332–46. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- SHIJIE J, TAKEUCHI H, YAWATA I, HARADA Y, SONOBE Y, DOI Y, LIANG J, HUA L, YASUOKA S, ZHOU Y, NODA M, KAWANOKUCHI J, MIZUNO T, SUZUMURA A. Blockade of glutamate release from microglia attenuates experimental autoimmune encephalomyelitis in mice. Tohoku J Exp Med. 2009;217:87–92. doi: 10.1620/tjem.217.87. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI H, MIZUNO T, ZHANG G, WANG J, KAWANOKUCHI J, KUNO R, SUZUMURA A. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem. 2005;280:10444–54. doi: 10.1074/jbc.M413863200. [DOI] [PubMed] [Google Scholar]
- TOFT-HANSEN H, NUTTALL RK, EDWARDS DR, OWENS T. Key metalloproteinases are expressed by specific cell types in experimental autoimmune encephalomyelitis. J Immunol. 2004;173:5209–18. doi: 10.4049/jimmunol.173.8.5209. [DOI] [PubMed] [Google Scholar]
- VAN LOO G, DE LORENZI R, SCHMIDT H, HUTH M, MILDNER A, SCHMIDT-SUPPRIAN M, LASSMANN H, PRINZ MR, PASPARAKIS M. Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol. 2006;7:954–61. doi: 10.1038/ni1372. [DOI] [PubMed] [Google Scholar]
- YONG VW, POWER C, FORSYTH P, EDWARDS DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–11. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Myeloid KLF2 deficient mice have no significant alteration in expression of KLF4 and KLF6. Peritoneal macrophages from MY-K2-KO and Control mice were harvested for total RNA and qPCR analysis performed for (A) KLF4 and (B) KLF6. (n=5 per group).
Fig S2. EAE induction/pertussis toxin injection does not result in significant change in expression of KLF2 and KLF6. Lumbar spinal cords from Control mice before EAE induction and after EAE induction/pertussis toxin injection were harvested for total RNA and qPCR analysis performed for (A) KLF2 and (B) KLF6. (n=3–5 per group).