Abstract
Our understanding of the origin and functions of human blood CXCR5+ CD4+ T cells found in human blood has changed dramatically in the past years. These cells are currently considered to represent a circulating memory compartment of T follicular helper (Tfh)-lineage cells. Recent studies have shown that blood memory Tfh cells are composed of phenotypically and functionally distinct subsets. Here we review the current understanding of human blood memory Tfh cells and the subsets within this compartment. We present a strategy to define these subsets based on cell surface profiles. Finally, we discuss how increased understanding of the biology of blood memory Tfh cells may contribute insight into the pathogenesis of autoimmune diseases and the mode of action of vaccines.
Tfh cells in lymphoid organs and in the blood
T follicular helper (Tfh) cells are a CD4+ T cell subset specialized in providing help to B cells [1–3]. Tfh cells are essential for the generation of high-affinity memory B cells through the germinal center (GC) reaction. Bona fide Tfh cells are present in GCs in secondary lymphoid organs, and display multiple features associated with their helper functions. Tfh cells express the chemokine receptor CXCR5 [4–7], which guides their migration into B cell follicles. Interleukin-21 (IL-21) secreted by Tfh cells and their precursors [8–10] potently promotes differentiation, and class-switching in B cells [11]. CD40 ligand (CD40L) on the surface of Tfh cells provides signals to B cells through CD40 and induces B cell differentiation and class-switching [12]. The signaling adaptor SLAM-associated protein (SAP) plays an indispensable role for stable T and B cell interactions required for Tfh cell differentiation [13].
Tfh cells express inducible co-stimulator (ICOS), a molecule essential for Tfh cell generation, at high density; ICOS-deficient mice and humans display significantly reduced GC reactions and Tfh cells [14–16]. ICOS-mediated signals are important for Tfh cell differentiation at two levels: The ICOS signals delivered by dendritic cells at the T cell zone induce T cells to express Bcl-6 [16], an transcriptional repressor essential for Tfh cell generation [17–19]. Then ICOS signals are delivered by follicular B cells at the T and B cell border to promote the migration of Tfh precursors into follicles [20]. ICOS also acts as a critical co-stimulatory molecule to induce the production of IL-21 by Tfh cells [10, 21]. The immune-inhibitory receptor PD-1 is also highly expressed in Tfh cells, and appears to regulate the activity of Tfh cells in GCs [22].
CD4+ T cells also provide help to B cells at extrafollicular sites, beyond the GC response in secondary lymphoid organs, inducing B cell differentiation into plasma cells and in this way contributing to the early generation of specific antibodies after antigen challenge [23]. These extrafollicular CD4+ helper cells share the developmental mechanisms, phenotypes, and functional properties with Tfh cells [10, 24–26]. They are thus are considered to belong to the Tfh lineage.
The biology of Tfh cells in secondary lymphoid organs has been extensively studied during the last decade, particularly in mouse models, resulting in significant advances in our understanding of the origin and functions of these cells. In contrast, despite their discovery some 20 years ago, the biology of blood circulating CXCR5+ CD4+ T cells in humans has been largely uncharacterized. These cells have recently come into the spotlight with the publication of a number of studies in the past few years. These studies largely agree with the theory that blood CXCR5+ CD4+ T cells in humans represent a circulating memory compartment of the Tfh-lineage cells. Extensive analyses of these blood memory Tfh cells have further revealed phenotypically and functionally distinct subsets. A major issue, however, is that the combination of markers used in these studies has often differed among the laboratories involved, and accordingly, many different ways to define blood memory Tfh subsets have been proposed. There is to date no consensus as to the cell surface markers that define blood memory Tfh cells in humans. An clear phenotypic definition of memory Tfh cell subsets in the blood is important not only to better understand their biological functions, but also for translational purposes as these circulating cells could serve as potential biomarkers for following antibody responses in vaccinations and infections, and in dysregulated antibody responses in autoimmune diseases.
Here we review the current understanding on blood memory Tfh cells in humans. We discuss the functionally distinct subsets that have been defined using varied phenotypic markers, and propose a unified approach to defining distinct Tfh subsets based on the expression of key cell surface markers. Finally, we discuss how an improved understanding of the biology of these cells can contribute to vaccination strategies and provide insight into the etiology of some autoimmune diseases.
Emerging understanding of the nature of human blood CXCR5+ CD4+ T cells
The presence of CD4+ T cells expressing CXCR5 in human blood was first described in 1994 [27]. As opposed to bona fide Tfh cells in secondary lymphoid organs, human blood CXCR5+ CD4+ T cells were initially proposed to represent recently activated T cells. This hypothesis was built based on several observations.
First, initial reports concluded that blood CXCR5+ CD4+ T cells lack long-lived memory cells, as few or no tetanus toxoid (TT)-specific cells were found within blood CXCR5+ CD4+ T cells in adults, despite a history of multiple TT vaccinations [4, 28]. In these studies, blood CXCR5+ CD4+ T cells were isolated from healthy adults who had not received a TT booster vaccine for at least 8 years, and analyzed for their proliferation upon stimulation with TT-loaded autologous monocytes. However, a later study demonstrated (by using a similar approach) that the TT-specific memory cells can be detectable within blood CXCR5+ CD4+ T cells for 2–4 years after a booster vaccination [29]. The presence of TT-specific memory CXCR5+ CD4+ T cells in blood of healthy adults was also confirmed recently with specific class II tetramers [30]. Therefore, in the case of TT vaccine, antigen-specific memory CXCR5+ CD4+ T cells seem to be maintained in blood for at least several years. Interestingly, the persistence of antigen-specific memory CXCR5+ CD4+ T cells in blood seems to differ among vaccines. In a study of long-lived memory CD4+ T cells specific for smallpox virus, three out of four healthy adults were found to display smallpox-specific memory CXCR5+ CD4+ cells in blood (published in 2004)[29]. Given that routine smallpox vaccination was stopped during the 70’s and that smallpox was globally eradicated in 1980, this observation suggests that antigen-specific memory CXCR5+ CD4+ T cells can be maintained in blood for many decades without restimulation. Although this observation needs to be confirmed in a larger cohort, the difference in the persistence of antigen-specific memory CXCR5+ CD4+ T cells in blood between TT and smallpox vaccines suggests that signals that T cells receive during the priming play major roles in determining their persistence. As smallpox vaccine also induces long-lived memory B cells [31], the signals required to induce long-lived memory Tfh cells and memory B cells might be shared.
Second, the expression of CXCR5 by CD4+ T cells was initially considered transient because human naïve CD4+ T cells activated in vitro via T cell receptor crosslinking rapidly induce expression of CXCR5 but rapidly lose this expression [28]. However, the CXCR5 expression by blood CXCR5+ CD4+ T cells was found to be constitutive, and stable in vitro at least for 20 days without any stimulation [30]. Furthermore, as discussed later, a vast majority of blood CXCR5+ CD4+ T cells do not express other activation markers. These observations suggest that blood CXCR5+ CD4+ T cells have an intrinsic property to maintain CXCR5 expression.
Last, earlier studies suggested that blood CXCR5+ CD4+ T cells do not share functional properties with Tfh cells, because the helper capacity was reported to be similar between blood CXCR5+ and CXCR5− CD4+ T cells [6]. In contrast, multiple recent studies show that blood CXCR5+ CD4+ T cells display a superior capacity to CXCR5− cells in inducing B cells to differentiate into Immunoglobulin (Ig)-producing cells and to undergo class-switching [30, 32–35]. Such discrepancy seems to lie on the differences in the experimental designs. In the recent studies, T cells were cultured with autologous B cells loaded with a super-antigen and thus were promoted to form cognate interactions with B cells. In contrast, in the earlier studies, T cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28, and autologous unloaded B cells were added to the culture. In this condition, T cells are primarily activated by anti-CD3 and CD28 and the cognate interactions between T and B cells occur only by chance. Therefore, the experimental design in the recent studies seems better to simulate physiological cell interactions in vivo. Furthermore, recent studies show that a superior helper capacity of blood CXCR5+ CD4+ T cells is (at least in part) due to secretion of larger amounts of IL-21 and IL-10, cytokines secreted by Tfh cells [32, 33, 36].
Moreover, evidence supporting the relationship between blood CXCR5+ CD4+ T cells and the Tfh-lineage cells in lymphoid organs has been obtained from the studies with samples from primary immunodeficiency subjects. Subjects with severely impaired GC formation due to deficiencies of CD40-ligand, ICOS, STAT3, or IL-12 receptor β1 chain were found to have significantly less blood CXCR5+ CD4+ T cells [15, 37, 38]. Surprisingly, subjects deficient of SAP were found to display normal levels of blood CXCR5+ CD4+ T cells [34], despite severe alterations in the generation of mature Tfh cells in lymphoid organs [16, 39]. A consistent result was obtained from SAP-deficient mouse models [34]. This evidence supports a model in which blood CXCR5+ CD4+ T cells are predominantly generated from cells committed to the Tfh-lineage, but not from bona fide Tfh cells [40].
Collectively, these observations show that blood CXCR5+ CD4+ T cells contain long-lived memory cells and share functional properties with Tfh cells. Accordingly, blood CXCR5+ CD4+ T cells are currently termed blood (or peripheral) memory Tfh cells.
Biological differences between blood and tonsillar Tfh cells
The skepticism regarding the direct relationship between blood memory Tfh cells and the Tfh-lineage cells in lymphoid organs was also derived in part from phonotypic differences. While both blood memory Tfh cells and tonsillar GC Tfh cells express CXCR5, the expression of other markers is largely different. A vast majority of blood memory Tfh cells express CD62L and CCR7, markers associated with central memory CD4+ T cells [4–6]. The ligands of CD62L (including GlyCAM-1) and CCR7 (CCL19 and CCL21) are highly expressed by high endothelial venules of secondary lymphoid organs, and the expression of these molecules likely permit blood memory Tfh cells to go into and patrol secondary lymphoid organs. In contrast, GC Tfh cells do not express CCR7, because CCR7 expression needs to be downregulated when T cells migrate out of the T cell zone and into B cell follicles [41]. Furthermore, in contrast to GC Tfh cells, a vast majority of blood memory Tfh cells in healthy subjects lack the expression of activation markers such as CD69 and ICOS [6, 42, 43]. While tonsillar Tfh cells frequently interact with B cells and dendritic cells in GCs and are in an active state, a majority of blood memory Tfh cells are in a quiescent state. Consistently, blood memory Tfh cells require to be activated to act as functional helpers in vitro [32, 35]. Activated blood memory Tfh cells rapidly upregulate the expression of CD69 and ICOS, and produce IL-21, IL-10, and CXCL13 [32, 33]. These observations show that human blood memory Tfh cells are endowed with multiple features of memory cells.
In contrast to Tfh cells in GCs, Bcl-6 protein expression is absent in blood memory Tfh cells, including ICOS+ subsets [32–34, 36, 43]. While Bcl-6 promotes the expression of many Tfh molecules by human CD4+ T cells including CXCR5, ICOS, PD-1, SAP, CD40L, and CXCL13 [30, 44], the absence of Bcl-6 expression in blood memory Tfh cells suggests that Bcl-6 is dispensable for their maintenance. The molecular mechanisms by which blood memory Tfh cells maintain Tfh characteristics remain largely unknown and will be an important research topic. Nonetheless, gene expression profiling of blood memory Tfh cells has suggested potential mechanisms. Blood memory Tfh cells express higher levels of the transcription factor Maf than CXCR5− CD4+ memory T cells [30, 33, 35]. Because Maf promotes the expression of CXCR5 and IL-21 in human CD4+ T cells [44], high levels of Maf expression may be associated with the maintenance of Tfh phenotype and/or function. Alternatively, the characteristics of blood memory Tfh cells might be associated with the balance between Bcl-6 and its antagonist transcription repressor Blimp-1. Tfh cells in GCs express abundant Bcl-6 but little Blimp-1 [1], and constitutive overexpression of Blimp-1 in CD4+ T cells prevents the differentiation of Tfh cells [18]. This evidence indicates that Bcl-6 needs to be dominant over Blimp-1 during Tfh cell differentiation. Similarly, human blood memory Tfh cells were found to express lower levels of Blimp-1 transcript than CXCR5− CD4+ memory T cells, although the expression of Bcl-6 transcript was similar [32]. Therefore, keeping the balance of the two molecules towards a Bcl-6 dominant state might be associated with the maintenance of Tfh characteristics in memory cells. Last, a recent study in mouse models indicated that the transcription factor achaete-scute homologue 2 (Ascl2) positively regulates the differentiation of Tfh cells as well as the expression of CXCR5 in a Bcl-6 independent manner [45]. While the function of Ascl2 in human CD4+ T cells remains to be established, it is tempting to speculate that Ascl2 might be associated with the biology of blood memory Tfh cells.
Defining distinct subsets of circulating memory Tfh cells in humans
Blood memory Tfh cells constitute approximately 15–25% of memory CD4+ T cells in humans [27, 32, 33, 36, 43]. Recent studies have demonstrated that blood memory Tfh cells are composed of heterogeneous cell populations with different phenotype. Through an extensive in vitro characterization of isolated subpopulations, now we have realized that blood memory Tfh cells are composed of subsets with distinct functional properties. Below we first summarize the distinct features of blood memory Tfh cell subsets, and then discuss a strategy to define these subsets.
The expression of ICOS, PD-1 and CCR7 defines three distinct subsets of Tfh cells
In contrast to Tfh cells in secondary lymphoid organs, ICOS is expressed by only less than 1% of blood memory Tfh cells in healthy subjects [6, 30, 36, 42, 43]. These minor ICOS+ blood memory Tfh cell population co-expresses PD-1 at high levels (thus ICOS+PD-1++ cells) [30, 34–36]. While more than 70% of blood memory Tfh cells do not express either PD-1 or ICOS (ICOS−PD-1− cells), recent studies discovered a population expressing low levels of PD-1 but not ICOS (thus ICOS−PD-1+ cells. ~30% of blood memory Tfh cells)[30, 34, 35]. While ICOS+PD-1++ blood memory Tfh cells express Ki-67, an indicative marker of active cell cycle, ICOS−PD-1+ and ICOS−PD-1− blood memory Tfh cells do not express Ki-67 and thus are in a quiescent state [30, 34]. The ICOS−PD-1+ cells appear to represent a population distinct from the ICOS−PD-1− cells, as PD-1 expression on isolated ICOS−PD-1+ cells is remarkably stable in vitro [30]. These three subpopulations express CCR7 at different levels with a negative correlation with PD-1 expression. CCR7 expression is the lowest on the ICOS+PD-1++ cells, and the ICOS− PD-1+ cells express less CCR7 than the ICOS−PD-1− cells [30, 34]. Of note, CCR7 expression levels by blood memory Tfh cells are generally low among the blood central memory CD4+ T cells, and even the ICOS−PD-1− cells express CCR7 at lower levels than blood CXCR5− central memory CD4+ T cells [30]. Given a coordinated increase in CXCR5 and decrease in CCR7 is required for T cells to migrate into B cell follicles [41], the differential expression levels of CCR7 by these blood memory Tfh cell subsets might reflect their distinct propensity to enter B cell follicles in vivo.
Therefore, blood memory Tfh cells contain three subsets that differentially express ICOS, PD-1, and CCR7: ICOS+PD-1++CCR7lo activated cells, ICOS−PD-1+CCR7int and ICOS−PD-1− CCR7hi quiescent cells.
Expression of CXCR3 and CCR6 defines three subsets of Tfh cells
Blood memory Tfh cells can be subdivided by a different approach. Approximately 30–50% of blood memory Tfh cells express CXCR3 or CCR6, the chemokine receptor preferentially expressed by Th1 and Th17 cells, respectively [46, 47]. Accordingly blood memory Tfh cells can be subdivided into the three major subsets: CXCR3+CCR6− cells, CXCR3−CCR6− cells, and CXCR3−CCR6+ cells [32]. The CXCR3+CCR6− subset expresses the transcription factor T-bet and produces Th1 cytokine IFN-γ; thus resembles to Th1 cells (hereafter called blood memory Tfh1 cells). The CXCR3−CCR6− subset expresses the transcription factor GATA3 and produces Th2 cytokines IL-4, IL-5, and IL-13; thus resembles to Th2 cells (hereafter called blood memory Tfh2 cells). The CXCR3−CCR6+ subset expresses the transcription factor RORγT and produces Th17 cytokines IL-17A and IL-22; thus resembles to Th17 cells (hereafter called blood memory Tfh17 cells)[32].
Thus, the analysis of CXCR3 and CCR6 expression permits the dissection of blood memory Tfh cells into the three major subsets: CXCR3+CCR6− Tfh1 cells, CXCR3−CCR6− Tfh2 cells and CXCR3−CCR6− Tfh17 cells.
Blood memory Tfh subsets display distinct capacities to help B cells
The major task of Tfh cells is to induce antibody responses by providing help to B cells. Therefore, an assessment of helper functions is critical to understand the biology of blood memory Tfh cell subsets. In this context, blood memory Tfh subsets have been found to display distinct helper capacities. An original report indicated that while blood memory Tfh2 and Tfh17 cells (thus the CXCR3− subsets) are able to induce naïve B cells to produce Igs and to switch isotypes through IL-21 secretion, blood memory CXCR3+ Tfh1 cells lack the capacity to help naïve B cells [32]. This observation was largely confirmed by recent studies [30, 35]. Furthermore, while blood memory Tfh2 cells promote IgG and IgE secretion, blood memory Tfh17 cells are efficient at promoting IgG and, in particular, IgA secretion [32]. Thus, blood memory Tfh2, and Tfh17 cells represent efficient helpers among blood memory Tfh cells, and differentially regulate Ig isotype switching.
Recent studies further demonstrated that among blood memory Tfh2 and Tfh17 cells, the ICOS−PD-1+CCR7int population, but not the ICOS−PD-1−CCR7hi population, promptly induce memory B cells to become Ig-producing cells [30, 35]. Furthermore, blood ICOS−PD-1+CCR7int Tfh2 and Tfh17 cells were found to display a gene expression profile resembling to tonsillar Tfh cells and their precursors [30, 35]. Thus, among the quiescent blood memory Tfh subsets, ICOS− PD-1+CCR7int Tfh2 and Tfh17 cells are the closest to tonsillar Tfh-lineage cells in terms of the functions and the gene profiles.
On the other hand, these findings also pose many questions. What are the functions of the ICOS−PD-1−CCR7hi populations in blood memory Tfh2 and Tfh17 cells? Are they also memory Tfh cells or rather closer to central memory cells? Although direct evidence is yet to be shown, recent studies have provided several clues to answer these questions. Upon co-culture with naïve B cells, the ICOS−PD-1−CCR7hi population in blood memory Tfh2 and Tfh17 cells are also capable of inducing B cells to produce Igs, while CXCR5− central memory cells are not [30, 35]. In addition, the ICOS−PD-1−CCR7hi population in blood memory Tfh2 and Tfh17 cells produce CXCL13, the chemokine that human Tfh cells produce [10, 48], while CXCR5− central memory cells do not [30]. Furthermore, in the analysis with class II tetramers, TT-specific memory cells were found in both the ICOS−PD-1+CCR7int and the ICOS−PD-1−CCR7hi populations within blood memory Tfh2 and Tfh17 cells [30]. These observations suggest that the ICOS−PD-1−CCR7hi populations in blood memory Tfh2 and Tfh17 cells likely constitute blood memory Tfh cell subsets that are distinct from the ICOS−PD-1+CCR7int populations. The differences in the ability to help memory B cells suggest that the ICOS−PD-1−CCR7hi populations require more time after activation and/or greater activation signals than the ICOS−PD-1+CCR7int population to differentiate into functional helpers. Whether the ICOS−PD-1+CCR7int populations become the ICOS−PD-1−CCR7hi populations over time or vice versa remains unknown.
Last, what do blood memory Tfh1 cells do? In vitro, the ICOS−PD-1+CCR7int and the ICOS−PD-1−CCR7hi populations within blood memory Tfh1 subsets lack the capacity to help naïve and memory B cells [30, 32, 35, 36]. Yet, like other blood memory Tfh subsets, these subpopulations within blood memory Tfh1 cells produce CXCL13 and contain TT-specific memory cells [30]. Furthermore, upon stimulation with PMA and ionomycin, ICOS−PD-1+CCR7int blood memory Tfh1 cells express IL-21 at an equivalent level with ICOS−PD-1+CCR7int blood memory Tfh2 and Tfh17 cells [30]. These observations suggest that blood memory Tfh1 cells also constitute another subset of blood memory Tfh cells. Supportive yet somewhat surprising evidence came out from studies of influenza vaccine [34, 36]. Influenza vaccination induced a transient increase of the ICOS+PD-1++CCR7lo activated cells exclusively within blood memory Tfh1 subset. Strikingly, the increase of the ICOS+PD-1++CCR7lo cells within Tfh1 cells positively correlated with the generation of protective antibody responses [36]. However, their helper capacity seems limited, because ICOS+PD-1++CCR7lo blood memory Tfh1 cells lack the capacity to help naïve B cells in vitro, while being capable of inducing memory B cells to differentiate into plasma cells [36]. This observation suggests that blood memory Tfh1 cells also contribute to antibody responses, but only when they become ICOS+PD-1++CCR7lo activated cells.
Collectively, these studies show that blood memory Tfh cells can be largely subdivided into non-efficient helpers (Tfh1) and efficient helpers (Tfh2 and Tfh17). The differential expression of ICOS, PD-1, and CCR7 further defines functionally distinct subpopulations within the subsets.
A three-dimensional analysis of blood memory Tfh cell subsets
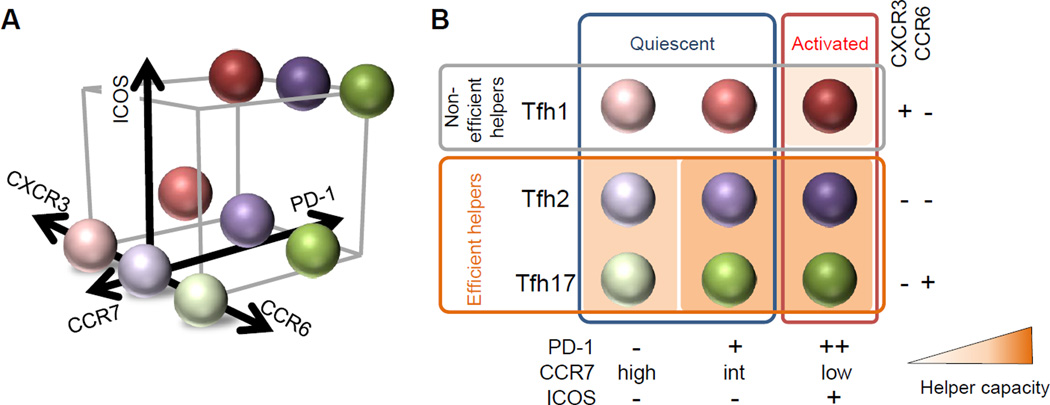
Based on these recent findings, here we would like to propose an approach to define functionally distinct blood memory Tfh subsets. Human blood memory Tfh cells can be analyzed in a three-dimensional approach with distinct parameters (Figure 1). The first parameter comprises CXCR3 and CCR6, which define Tfh1, Tfh2, and Tfh17 subsets. The second parameter includes PD-1 and CCR7, which define the two quiescent populations among blood memory Tfh1, Tfh2, and Tfh17 subsets: the PD-1−CCR7hi and the PD-1+CCR7int cells. The third parameter is ICOS, which defines ICOS+ activated cells within blood memory Tfh1, Tfh2, and Tfh17 subsets. All these markers can be integrated into one flow cytometry panel together with the markers CD3, CD4, and CXCR5. Inclusion of CD45RO (a general marker of memory cells), a damp channel (to exclude contamination of other cells such as CD8+ T cells and CD56+ NK cells) and a viability dye (such as LIVE/DEAD®) will be also appropriate when feasible. When a flow cytometer amenable to more than 10 color staining is not available, CCR7 and CCR6 can be omitted to simplify the panel.
Figure 1. The nine blood memory Tfh cell subsets defined by a three-dimensional analysis.
(A) A three-dimensional analysis of human blood memory Tfh cells. The first parameter comprises CXCR3 and CCR6, which define Tfh1, Tfh2, and Tfh17 subsets. The second parameter includes PD-1 and CCR7, which define the PD-1−CCR7hi and the PD-1+CCR7int quiescent subpopulations within the blood memory Tfh1, Tfh2, and Tfh17 subsets. The third parameter is ICOS, which defines the ICOS+ activated population within the blood memory Tfh1, Tfh2, and Tfh17 subsets. The nine blood memory Tfh subsets defined by these parameters are indicated in a three-dimensional scale.
(B) The nine blood memory Tfh subsets. The markers CXCR3 and CCR6 largely separate non-efficient helpers (Tfh1) and efficient helpers (Tfh2 and Tfh17). ICOS expression defines the quiescent subpopulations and the activated cells in each subset. The helper capacity of Tfh1 cells is limited to the activated ICOS+PD-1++CCR7lo subset that can help only memory B cells. While both quiescent subsets within Tfh2 and Tfh17 cells are capable of helping B cells, the ICOS−PD-1+CCR7int subset provides a prompt help to memory B cells. Tfh2 and Tfh17 cells produce different sets of cytokines, and differentially regulate isotype switching. The intensity of the background orange color of each subset reflects the capacity to provide help to B cells.
This three dimensional analysis permits the determination of 9 distinct blood memory Tfh subsets (6 subsets with the simplified panel)(Figure 1). The markers CXCR3 and CCR6 separate non-efficient helpers (Tfh1) and efficient helpers (Tfh2 and Tfh17). Within blood memory Tfh1 cells, the helper capacity is limited to the activated ICOS+PD-1++CCR7lo subset and their target is limited to memory B cells. In contrast, within blood memory Tfh2 and Tfh17 cells, both quiescent ICOS−PD-1+CCR7int and ICOS−PD-1−CCR7hi subsets are capable of helping B cells, while the ICOS−PD-1+CCR7int subsets can provide a prompt help to memory B cells. Tfh2 and Tfh17 cells produce different sets of cytokines, and differentially regulate isotype switching. The function of the activated ICOS+PD-1++CCR7lo subsets within blood memory Tfh2 and Tfh17 cells is yet to be established, but it is presumable that they are capable of providing help to both naïve and memory B cells.
It is no doubt that this proposed strategy will need to be revised in future according to the increase of our knowledge on human blood memory Tfh cells. However, this global strategy will at least provide an initial framework to facilitate the basic research on human blood memory Tfh cells, and to determine alterations in the blood memory Tfh subsets in clinical studies.
Insights into the mechanisms of antibody responses in clinical studies
The phenotype of human blood memory Tfh cells has been extensively analyzed during the past decade in many studies on autoimmune diseases, infectious diseases, and vaccinations. These studies have already provided valuable insights into the pathogenesis of autoimmune diseases and into the mechanisms for antibody production upon vaccinations.
Many studies focused on the expression of ICOS on blood memory Tfh cells. The frequency ICOS+ cells among blood memory Tfh cells was found to be increased in patients with autoimmune diseases including systemic lupus erythematosus (SLE), Sjogren’s syndrome, rheumatoid arthritis, and autoimmune thyroid diseases, [34, 43, 49–51]. In some of the studies, an increase of ICOS+ blood memory Tfh cells showed a positive correlation with serum autoantibody titers and disease activity and/or severity [34, 43, 49]. These studies suggest that aberrant Tfh responses in patients with autoimmune diseases can be captured by the analysis of blood memory Tfh cells. In the studies on seasonal influenza vaccines, the frequency of ICOS+ cells within blood Tfh cells was shown to increase only transiently after vaccination (peak at day 7) [34, 36]. This kinetics seems synchronized with the emergence of influenza-specific plasmablasts and plasma cells in blood [36, 52, 53]. A transient increase of ICOS+ blood memory Tfh cells was also observed in immunized mice [34]. Importantly, in contrast to a transient appearance of ICOS+ memory Tfh cells in blood, GC response in immunized mice was observed in an extended period [34]. These observations suggest that an increase of ICOS+ memory Tfh cells in blood reflects developing GC responses in lymphoid organs [40]. Of note, a technical difficulty in the analysis of ICOS expression on human blood memory Tfh cells has been the gating strategy to define the ICOS+ population, as their ICOS expression levels are often modest. The integration of PD-1 and CCR7 in a flow cytometry panel will facilitate the detection of this population as ICOS+PD-1++CCR7lo cells.
Accumulating evidence shows that an alteration in the balance of blood memory Tfh1, Tfh2, and Tfh17 cells is also associated with the pathogenesis of autoimmune diseases. In patients with juvenile dermatomyositis [32], adult SLE [54], Sjogren’s syndrome [55], and multiple sclerosis [56], the composition of Tfh1 cells among blood memory Tfh cells was found to decrease, while the composition of Tfh2 and/or Tfh17 cells increased. Furthermore, such alterations were found to correlate with disease activity, serum autoantibody titers, and/or the frequency of blood plasmablasts [32, 54–56]. Therefore, it is presumable that a decrease of Tfh1 subsets and an increase of Tfh2/Tfh17 subsets among blood memory Tfh cells reflect an overall increase of efficient helpers that promote the generation of antibodies in lymphoid organs and/or inflammatory sites in patients with autoimmune diseases. This theory seems to be applicable to studies of infectious diseases. Among HIV-infected subjects, the subjects who have developed broadly neutralizing antibodies against HIV were found to display a higher frequency of ICOS− PD-1+CCR7int memory Tfh2 and Tfh17 subsets in blood [30].
Analyses of blood memory Tfh subsets have also provided insights into the mechanisms of vaccines. As described earlier, in a study of seasonal influenza vaccines, an increase of ICOS+PD-1++CCR7lo blood memory Tfh1 cells at day 7 was found to correlate with a generation of antibody responses [36]. This suggests that current influenza vaccine is largely dependent on the Tfh1 subset that display limited capacity to induce antibody responses. This hypothesis might explain why current influenza vaccines have limited efficacy particularly in children who have a limited repertoire of influenza-specific memory B cells.
Concluding remarks
Remarkable progress has been made in the understanding of the biology of human blood memory Tfh cells in last few years, and has permitted identification of functionally distinct subsets. Now we know that human blood memory Tfh cells contain non-efficient helpers (Tfh1) and efficient helpers (Tfh2 and Tfh17). The activity of blood memory Tfh cells is tightly regulated, and a vast majority of blood memory Tfh cells is in a quiescent state. Among the quiescent subsets, PD-1+CCR7int Tfh2 and Tfh17 cells promptly provide help to B cells. However, whether the analyses of blood memory Tfh subsets faithfully reflect Tfh responses in lymphoid organs remains to be established. In the same line, whether an alteration of blood memory Tfh subsets in autoimmune disease patients mirrors the Tfh response in lymphoid organs and/or inflammatory tissues remains to be shown. Nonetheless, it is no doubt that a systematic analysis of human blood memory Tfh subsets in a standardized manner will significantly increase our knowledge on Tfh responses in health and disease. These studies will further provide valuable insights into novel therapeutic strategies for human autoimmune diseases where Tfh responses need to be down-regulated, and into novel vaccine designs for infectious diseases where durable Tfh responses are desired.
Highlights.
CXCR5+ CD4+ T cells represent a circulating memory compartment of Tfh-lineage cells
Blood memory Tfh cells are composed of phenotypically and functionally distinct subsets
A proposed three-dimensional approach defines nine blood memory Tfh subsets
BOX: The origin of blood memory Tfh cells.
The origin of blood memory Tfh cells remains elusive in humans. Hypothetically, blood memory Tfh cells can be derived from GC Tfh cells that exited GCs and the other Tfh-lineage cells localized outside GCs. Yet, the recent studies using T cells deficient of SAP (the signaling adaptor SLAM-associated protein) in mice and humans suggest that blood memory Tfh cells are predominantly generated from cells committed to the Tfh-lineage, but not from GC Tfh cells [40]. In SAP-deficient mice, GC formation and the generation of mature Tfh cells are severely altered due to the failure in forming stable T and B cell interactions [13]. However, SAP-deficient mice were found to display normal frequency of blood memory Tfh cells [57], suggesting that the development of blood memory Tfh cells do not require bona fide GC formation. This was further confirmed in humans by using blood samples from female carriers of X-linked lymphoproliferative disease, who have both SAP-sufficient and deficient T cells due to random X-inactivation. Within the same individuals, both SAP-sufficient and -deficient CD4+ T cells were found to equally give rise to blood Tfh cells including the ICOS−PD-1+CCR7lo population [57].
There is also evidence supporting that GC Tfh cells might differentiate into memory cells. Upon transfer to naïve mice, GC Tfh cells can survive for more than 1 month in secondary lymphoid organs without antigen stimulation. The surviving memory Tfh cells downregulate the expression of Tfh molecules including Bcl-6, CXCR5, and PD-1, but increase the expression of IL-7 receptor, CCR7, and CD62L, markers associated with central memory cells [58–60]. Nonetheless, upon antigenic challenge in vivo, these memory cells rapidly become GC Tfh cells and promote antibody responses [58, 59]. However, whether these GC Tfh-derived memory cells in lymphoid organs become circulating or not remains unknown.
Acknowledgement
We thank many donors and patients involved in our studies and many staffs at Baylor Institute for Immunology Research. This study was supported by research funding from NIH grants U19-AI057234, U19-AI082715, U19-AI089987, Alliance for Lupus Research, and Baylor Health Care System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 2.King C, et al. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual review of immunology. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 3.Victora GD, Nussenzweig MC. Germinal centers. Annual review of immunology. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 4.Breitfeld D, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaerli P, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. The Journal of experimental medicine. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim CH, et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. The Journal of experimental medicine. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasheed AU, et al. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. European journal of immunology. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 8.Bryant VL, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 9.Chtanova T, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 10.Bentebibel SE, et al. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proceedings of the National Academy of Sciences. 2011;108:E488–E497. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annual review of immunology. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 12.Banchereau J, et al. The CD40 antigen and its ligand. Annual review of immunology. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 13.Qi H, et al. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akiba H, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 15.Bossaller L, et al. ICOS Deficiency Is Associated with a Severe Reduction of CXCR5+CD4 Germinal Center Th Cells. J Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 16.Choi YS, et al. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science (New York N.Y. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science (New York N.Y. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu D, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 21.Paulos CM, et al. The Inducible Costimulator (ICOS) Is Critical for the Development of Human TH17 Cells. Science Translational Medicine. 2010;2:55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cubas RA, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nature medicine. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLennan IC, et al. Extrafollicular antibody responses. Immunological reviews. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 24.Odegard JM, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. The Journal of experimental medicine. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poholek AC, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. The Journal of experimental medicine. 2011;208:1377–1465. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forster R, et al. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- 28.Schaerli P, et al. Cutting edge: induction of follicular homing precedes effector Th cell development. J Immunol. 2001;167:6082–6086. doi: 10.4049/jimmunol.167.11.6082. [DOI] [PubMed] [Google Scholar]
- 29.Rivino L, et al. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. The Journal of experimental medicine. 2004;200:725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locci M, et al. Human Circulating PD-1(+)CXCR3(−)CXCR5(+) Memory Tfh Cells Are Highly Functional and Correlate with Broadly Neutralizing HIV Antibody Responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty S, et al. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 32.Morita R, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevalier N, et al. CXCR5 Expressing Human Central Memory CD4 T Cells and Their Relevance for Humoral Immune Responses. J Immunol. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 34.He J, et al. Circulating Precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T Cells Indicate Tfh Cell Activity and Promote Antibody Responses upon Antigen Reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Boswell KL, et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 2014;10:e1003853. doi: 10.1371/journal.ppat.1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentebibel SE, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Science Translational Medicine. 2013;5:176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitt N, et al. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121:3375–3385. doi: 10.1182/blood-2012-08-448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma CS, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deenick EK, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai LM, Yu D. Follicular helper T-cell memory: establishing new frontiers during antibody response. Immunology and cell biology. 2014;92:57–63. doi: 10.1038/icb.2013.68. [DOI] [PubMed] [Google Scholar]
- 41.Haynes NM, et al. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 42.Ma CS, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunology and cell biology. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 43.Simpson N, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis and rheumatism. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 44.Kroenke MA, et al. Bcl6 and Maf Cooperate To Instruct Human Follicular Helper CD4 T Cell Differentiation. J. Immunol. 2012 doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014 doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sallusto F, et al. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. The Journal of experimental medicine. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature immunology. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 48.Kim CH, et al. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- 49.Zhu C, et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 2012;97:943–950. doi: 10.1210/jc.2011-2003. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, et al. High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis. Clinical and experimental immunology. 2013;174:212–220. doi: 10.1111/cei.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu R, et al. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis research & therapy. 2012;14:R255. doi: 10.1186/ar4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakaya HI, et al. Systems biology of vaccination for seasonal influenza in humans. Nature immunology. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obermoser G, et al. Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity. 2013;38:831–844. doi: 10.1016/j.immuni.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Coz C, et al. Circulating TFH Subset Distribution Is Strongly Affected in Lupus Patients with an Active Disease. PLoS One. 2013;8:e75319. doi: 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li XY, et al. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjogren's syndrome. Biochem Biophys Res Commun. 2012;422:238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 56.Romme Christensen J, et al. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One. 2013;8:e57820. doi: 10.1371/journal.pone.0057820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He J, et al. Circulating precursor CCR7loPD-1hi CXCR5+ CD4 T cells identify active follicular T helper programs in health and disease. Immunity. 2013 [Google Scholar]
- 58.Luthje K, et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nature immunology. 2012;13:491–498. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 59.Weber JP, et al. T-follicular helper cells survive as long-term memory cells. European journal of immunology. 2012;42:1981–1988. doi: 10.1002/eji.201242540. [DOI] [PubMed] [Google Scholar]
- 60.Kitano M, et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]