Abstract
Background
Difficulty recognizing facial emotions is an important social-cognitive deficit associated with psychotic disorders. It also may reflect a familial risk for psychosis in schizophrenia-spectrum disorders and bipolar disorder.
Objective
The objectives of this study from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium were to: 1) compare emotion recognition deficits in schizophrenia, schizoaffective disorder and bipolar disorder with psychosis, 2) determine the familiality of emotion recognition deficits across these disorders, and 3) evaluate emotion recognition deficits in nonpsychotic relatives with and without elevated Cluster A and Cluster B personality disorder traits.
Method
Participants included probands with schizophrenia (n=297), schizoaffective disorder (depressed type, n=61; bipolar type, n=69), bipolar disorder with psychosis (n=248), their first-degree relatives (n=332, n=69, n=154, and n=286, respectively) and healthy controls (n=380). All participants completed the Penn Emotion Recognition Test, a standardized measure of facial emotion recognition assessing four basic emotions (happiness, sadness, anger and fear) and neutral expressions (no emotion).
Results
Compared to controls, emotion recognition deficits among probands increased progressively from bipolar disorder to schizoaffective disorder to schizophrenia. Proband and relative groups showed similar deficits perceiving angry and neutral faces, whereas deficits on fearful, happy and sad faces were primarily isolated to schizophrenia probands. Even non-psychotic relatives without elevated Cluster A or Cluster B personality disorder traits showed deficits on neutral and angry faces. Emotion recognition ability was moderately familial only in schizophrenia families.
Conclusions
Emotion recognition deficits are prominent but somewhat different across psychotic disorders. These deficits are reflected to a lesser extent in relatives, particularly on angry and neutral faces. Deficits were evident in non-psychotic relatives even without elevated personality disorder traits. Deficits in facial emotion recognition may reflect an important social-cognitive deficit in patients with psychotic disorders.
Keywords: schizophrenia, schizoaffective disorder, bipolar disorder, psychosis, emotion recognition, family study
1. Introduction
The diagnostic boundaries of psychotic disorders including schizophrenia (SCZ)-spectrum disorders and psychotic bipolar disorder (BD-P) are increasingly recognized as more porous than traditionally depicted in diagnostic systems (Thaker, 2000). Identifying distinctive and shared features of these disorders can help to inform psychiatric classification and to understand factors that contribute to their overlapping symptoms (Carpenter, 2014). Affective features are common across psychotic disorders, including varying levels of apathy and depression (Majadas et al., 2012). In contrast to many domains of psychopathology, disturbances in social cognition (i.e., cognitive processes involved in understanding other people and oneself) (Fiske and Taylor, 2013) and their familiality have rarely been systematically investigated in large patient samples across the psychotic spectrum.
Deficits in facial emotion recognition have been reported in SCZ-spectrum disorders, but their prevalence in BD patients with a history of psychosis is less established (Hill et al., 2008; Kohler et al., 2010b; Samame et al., 2012). These deficits are apparent at the first episode of psychotic illness in both disorders, and are relatively independent of mood state and antipsychotic treatment (Daros et al., 2014). In SCZ, deficits in emotion recognition are believed to affect a wide range of emotions (Kohler et al., 2010a). Whether emotion recognition deficits are similar in severity or in selectivity for different emotions across psychotic disorders is an important question about social cognition that remains to be systematically addressed (Craddock et al., 2009).
Non-psychotic individuals at familial risk for SCZ-spectrum disorders have significant but less severe difficulties in emotion recognition than patients themselves (Bediou et al., 2007; Eack et al., 2010; Erol et al., 2010; Goghari et al., 2011; Kee et al., 2004; Li et al., 2010). Less is known about emotion recognition abilities in relatives of patients with affective psychosis, though relatives of nonpsychotic BD individuals may show similar but more subtle deficits than those found in patients (Brotman et al., 2008a; Brotman et al., 2008b; Seidel et al., 2012). Differences in severity of emotion recognition deficits and the degree to which these deficits co-aggregate in SCZ-spectrum and BD-P families, however, have yet to be investigated.
The five-site Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study consortium (Maryland Psychiatric Research Center, University of Illinois at Chicago/University of Chicago, University of Texas Southwestern, Wayne State University/Harvard University, and the Institute of Living/Yale University) was organized to address questions about diagnostic boundaries and familiality of candidate intermediate phenotypes (Hill et al., 2013; Ivleva et al., 2013; Skudlarski et al., 2013) for SCZ, schizoaffective disorder (SAD), and BD-P. Probands with these disorders and their available first-degree biological relatives were recruited from the community using the same eligibility criteria and completed identical testing procedures (Tamminga et al., 2013).
The current article reports on facial emotion recognition data from the B-SNIP study. The Penn Emotion Recognition Task (ER-40) (Gur et al., 2002) was administered to all participants to examine the ability to identify four basic emotions (happiness, sadness, anger, and fear) and neutral expressions. The primary aims were to (1) contrast emotion recognition deficits in SCZ, SAD, and BD-P; (2) determine the familiality of these deficits; and (3) evaluate emotion recognition deficits in nonpsychotic relatives with and without elevated Cluster A and Cluster B personality disorder traits.
2. Materials and Methods
2.1. Participants
2.1.1. Probands
Patients with one of the target disorders (SCZ [n=297], SAD depressed type [SAD-D; n=61] and bipolar type [SAD-B; n=69], or BD-P [n=248]) were recruited through advertisements, community organizations and hospital clinics. Patients were recruited if they had at least one available first-degree biological relative 15-65 years of age willing to participate in the study. Probands were required to have a DSM-IV diagnosis of SCZ, SAD, or BD-P, which was determined at consensus diagnostic meetings using all available information including findings from the Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P)(First et al., 2002). Diagnostic reliability data are described in Tamminga et al. (2013). Clinical symptoms were rated using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1986), the Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), and the Young Mania Rating Scale (YMRS) (Young et al., 1978), and functional status was assessed with the Social Functioning Scale (SFS) (Birchwood et al., 1990). To assess a dimension of psychotic illness ranging from prototypical SCZ to BD, ratings were made using the Schizo-Bipolar Scale (Keshavan et al., 2011).
2.1.2. Relatives
First-degree biological relatives of probands with SCZ (n=332), SAD-D (n=69], SAD-B (n=154), or BD-P (n=286) were assessed with the SCID-I/P and the Structured Interview for DSM-IV Personality (SIDP-IV) (Pfohl et al., 1995). Given that individuals with Cluster A (odd or eccentric) and Cluster B (dramatic, emotional or erratic) personality disorders have deficits in emotion recognition (Daros et al., 2013; Dickey et al., 2011), one objective of this work was to evaluate the relationship of personality features to emotion recognition ability in the relative groups. Relatives were identified as having elevated Cluster A and Cluster B personality disorder features when they either met criteria for a disorder or were one criterion short of the diagnostic threshold as in our prior work (Hill et al., 2013). Individuals identified according to this procedure were those with Cluster A personality disorder features, including paranoid (n=21), schizoid (n=7), and schizotypal (n=3), and Cluster B personality disorder features, including antisocial (n=5), borderline (n=5), histrionic (n=1), and narcissistic (n=9).
2.1.3. Healthy Comparison Participants
Healthy volunteers (n=380) were recruited through advertisements and research registries. They completed the SCID-I/P and SIDP-IV and were required to have no personal history of psychosis, BD or recurrent major depression, and no known immediate family history of these disorders.
Details of participant recruitment are provided in Tamminga et al. (2013). All participants had no history of seizures or head injury with loss of consciousness greater than 10 minutes; had a negative urine toxicology screen for common drugs of abuse on the day of testing; had no diagnosis of substance abuse in the past 30 days or substance dependence in the past six months; had no change in psychotropic medication and were generally clinically stable over the past month; had no history of systemic medical or neurological disorder likely to affect cognition that would interfere with performance on the emotion recognition task; had an age-corrected Wide-Range Achievement Test—4th Edition (Wilkinson and Robertson, 2006) reading test standard score greater than 65; and were sufficiently fluent in English to understand task instructions. Additional information about participant recruitment and BSNIP study methodology is detailed elsewhere (Tamminga et al., 2013). Demographic and clinical characteristics of probands and first-degree relatives are presented in Tables 1 and 2, respectively.
Table 1.
Demographic and Clinical Data for Healthy Comparison Participants and Probands with Schizophrenia, Schizoaffective Disorder Depressed and Bipolar Types, and Psychotic Bipolar Disorder.
| Healthy comparisona (n=380) | Schizophreniab (n=297) | Schizoaffective Depressed Typec (n=61) | Schizoaffective Bipolar Typed (n=69) | Psychotic Bipolar Disordere (n=248) | Post Hoc† | |
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | ||
| Age (years) | 37.71 (12.69) | 35.79 (12.72) | 38.38 (11.42) | 36.32 (12.12) | 36.22 (12.72) | - |
| Education (years) | 14.86 (2.56) | 12.75 (2.28) | 12.56 (2.05) | 13.19 (2.14) | 14.14 (2.40) | a, e>b, c, d |
| WRAT | 103.26 (13.94) | 94.19 (15.57) | 92.45 (15.49) | 98.65 (14.20) | 101.78 (14.06) | a, e> b, c; a>d; d>e |
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Male | 178 (47) | 202 (68) | 30 (49) | 47 (38) | 91 (37) | b>a, c, d, e; a>e |
| Race | ||||||
| Caucasian | 233 (61) | 136 (46) | 31 (51) | 65 (52) | 186 (75) | e>a, b, c, d; a>b |
| African-American | 112 (30) | 138 (47) | 26 (43) | 52 (42) | 48 (19) | b, c, d>a>b |
| Other | 35 (9) | 23 (7) | 4 (6) | 7 (6) | 14 (6) | - |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | ||
| PANSS | ||||||
| Positive subscale | - | 16.80 (5.70) | 16.80 (5.39) | 18.63 (4.99) | 12.96 (4.65) | d>b, c>e |
| Negative subscale | - | 16.51 (5.83) | 16.39 (5.59) | 15.63 (4.87) | 11.90 (3.96) | b, c, d>e |
| YMRS | - | 5.52 (5.78) | 5.52 (6.03) | 8.43 (6.72) | 6.11 (6.99) | d>b, c, e |
| MADRS | - | 8.12 (7.96) | 15.07 (9.64) | 14.08 (10.35) | 10.16 (9.41) | c, d>b, e |
Abbreviations: WRAT, Wide Range Achievement Test-IV: Reading test; PANSS, Positive and Negative Syndrome Scale; YMRS, Young Mania Rating Scale; MADRS, Montgomery Asberg Depression Rating Scale
Post hoc tests were computed when the omnibus F test was significant at p<0.05.
Table 2.
Demographics and Personality Disorder Traits for Healthy Comparison Participants, First-Degree Relatives of Probands with Schizophrenia, Schizoaffective Disorder, and Psychotic Bipolar Disorder.
| Healthy Comparisona (n=380) | Relatives of Schizophrenia Probandsb (n=332) | Relatives of Schizoaffective Depressed Type Probandsc (n=69) | Relatives of Schizoaffective Bipolar Type Probandsd (n=154) | Relatives of Psychotic Bipolar Disorder Probandse (n=286) | Post Hoc† | |
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | ||
| Age (years) | 37.71 (12.69) | 42.65 (14.93) | 41.04 (16.07) | 39.97 (16.29) | 40.25 (15.98) | a<b |
| Education (years) | 14.86 (2.56) | 14.04 (2.56) | 14.06 (2.83) | 13.82 (2.95) | 14.54 (2.71) | a>b, c, d |
| WRAT | 103.26 (13.94) | 97.48 (15.33) | 96.74 (17.21) | 101.23 (15.21) | 103.08 (14.15) | a, e>b, c; d>b, c |
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Male | 178 (47) | 100 (30) | 19 (27) | 49 (32) | 104 (36) | a>b, c, d, e |
| Race | ||||||
| Caucasian | 233 (61) | 179 (54) | 40 (58) | 99 (64) | 229 (80) | e>a, b, c, d |
| African-American | 112 (30) | 135 (41) | 28 (41) | 45 (29) | 45 (16) | b, c>a, d, e |
| Other | 35 (9) | 18 (5) | 1 (1) | 10 (7) | 12 (4) | a, b>c, e |
Abbreviations: WRAT, Wide Range Achievement Test-IV: Reading test
Post hoc tests were computed when the omnibus F test was significant at p<0.05.
2.2. Procedures
All participants completed the ER-40, a standardized measure of facial emotion recognition from the University of Pennsylvania Computerized Neurocognitive Test Battery. The ER-40 is a computerized task that presents 40 color photographs of faces displaying expressions for four basic emotions (happiness, sadness, anger, and fear) and no emotion (neutral). Photographs were created by asking experienced actors to portray evoked facial expressions of emotion. Intended emotions displayed in photographs are consistent with those reported by healthy raters viewing the photographs (Gur et al., 2002). Stimuli included in the ER-40 were balanced for the actor's gender, age, and ethnicity.
Participants were asked to examine the faces and decide what emotion the person is showing, or to select “No Emotion” if the person is not showing any emotion. Accuracy and median response time (RT, log-transformed) for each emotion category were recorded.
2.3. Statistical Analyses
Prior to analysis, scores were standardized (mean=0, SD=1) relative to the healthy control group by regressing age, race, and sex on each outcome variable. The resulting unstandardized beta weights, constants, and standard errors were used to calculate predicted scores were then subtracted from each participant's actual score. Extremely low scores were truncated to z-score=-4.0. Hierarchical linear modeling (HLM) with SAS (version 9.2, SAS Institute Inc., Cary, NC) was used to examine group differences in emotion recognition performance. Because participants were nested within families, family membership was treated as a random effect. Planned comparisons tested for differences among proband groups and controls, and relative groups and controls. Statistical significance was set at p<0.05 for all analyses. All proband and most relative groups showed slower response times (RT) than controls (p's<0.001), but controlling for the longer time observing faces before making emotion judgments did not change the patterns of results.
To estimate familiality of emotion recognition deficits, a heritability analysis was performed using the SOLAR (Sequential Oligogenic Linkage Analysis Routine) software package (Almasy and Blangero, 1998). In this family study design, an estimate of familiality (h2) represents the portion of phenotypic variance accounted for by family membership. To test for the significance of familiality, a maximum likelihood ratio test compared a model in which phenotypic variation is explained by family membership to a model assuming that no variation is explained by familial factors. A correction was applied to account for ascertainment bias because families were recruited through the identification of a psychotic proband and not a representative community sample (Beaty and Liang, 1987). Because of the larger sample sizes and the primary focus on capturing both the traditional diagnostic dichotomy of primary interest, familiality estimates and group comparisons among relatives were restricted to SCZ, SAD and BD-P.
3. Results
3.1.1 Proband Comparisons on Emotion Recognition Accuracy
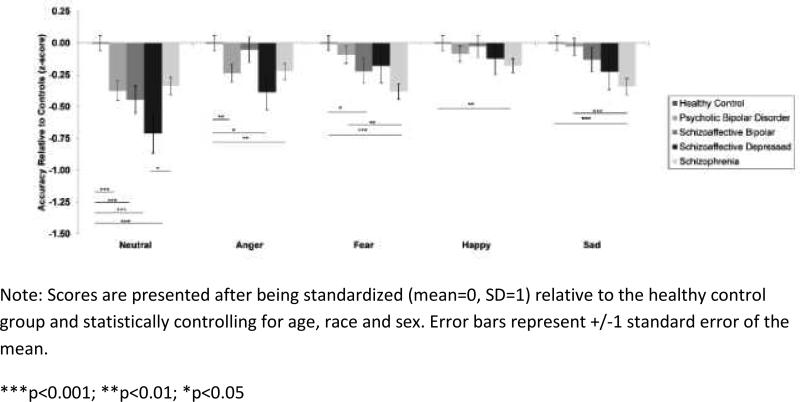
Participant data on the ER-40 (accuracy, RT) are provided in Supplementary Material (Table S1). On the ER-40, groups differed in their recognition of emotional expressions (p's<0.001), with all probands correctly identifying fewer emotions than healthy controls when collapsing across emotion categories (SCZ: B=−0.62, SE=0.09, p<0.001; SAD-D: B=−0.72, SE=0.16, p<0.0001; SAD-B: B=−0.39, SE=0.12, p=0.001; BD-P: B=−0.35, SE=0.12, p<0.001). SCZ and SAD-D probands showed poorer emotion recognition than probands with BD-P (SCZ: B=−0.27, SE=0.10, p=0.007; SAD-D: B=−0.37, SE=0.16, p=0.03).
Regardless of proband group, neutral faces were the most difficult emotion category to identify (p<0.001). A proband group × emotion category interaction revealed that probands with SCZ (B=−0.22, SE=0.08, p=0.009), SAD-D (B=−0.39, SE=0.15, p=0.01), and BD-P (B=−0.24, SE=0.09, p=0.008) were less accurate at recognizing angry facial expressions than healthy controls. For fear and sadness, SCZ probands had greater difficulties recognizing emotions than both healthy controls (fear: B=−0.38, SE=0.08, p<0.0001; sadness: B=−0.34, SE=0.08, p<0.0001) and probands with BD-P (sadness: B=−0.31, SE=0.09, p<0.0001). Proband groups also differed from each other in recognition of happy faces (p=0.02), with only SCZ probands less accurate than healthy controls (B=−0.20, SE= 0.07, p=0.007). Figure 1 displays accuracy on each emotion category for all proband groups and healthy controls.
Figure 1.
Facial emotion recognition accuracy for probands with schizophrenia-spectrum disorders and psychotic bipolar disorder.
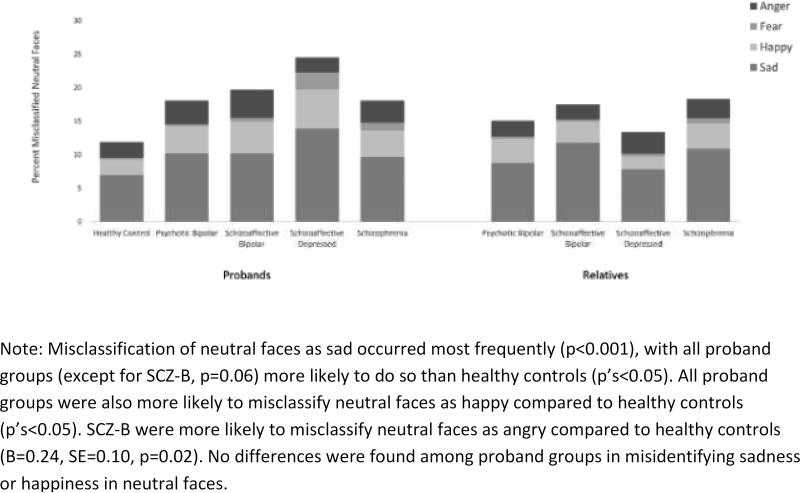
All proband groups were more likely to perceive an emotional expression in neutral faces than healthy controls (p's<0.001; see Figure 3). Probands with SAD-D were especially poor at recognizing neutral faces, and they were significantly less accurate even than SCZ probands (B=−0.37, SE=0.17, p=0.03). Misclassification of neutral faces as sad occurred most frequently (p<0.001), with all proband groups (except for SAD-B, p=0.06) more likely to do so than healthy controls (p's<0.05). All proband groups were also more likely to misclassify neutral faces as happy compared to healthy controls (p's<0.05). SADB were more likely to misclassify neutral faces as angry compared to healthy controls (B=0.24, SE=0.10, p=0.02). No differences were found among proband groups in misidentifying sadness or happiness in neutral faces.
Figure 3.
Misclassification of neutral faces as anger, fear, happy or sad in probands and their first-degree relatives.
3.1.2. Emotion Recognition Accuracy in Family Members
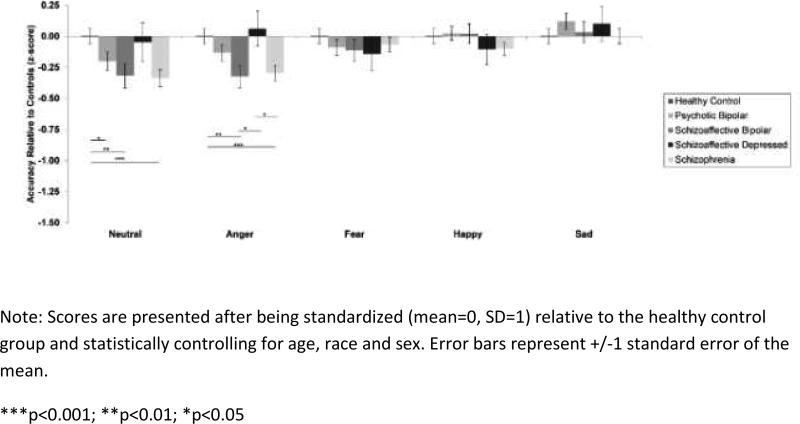
Collapsing across emotion categories, relatives showed a similar pattern of group differences as probands (p=0.001) (see Figure 2). Relatives of probands with SCZ (B=−0.32, SE=0.11, p=0.005) and SADB (B=−0.32, SE=0.11, p=0.005) recognized significantly fewer emotional expressions than healthy controls, and BD-P relatives showed better emotion recognition than SCZ relatives (B=0.21, SE=0.09, p=0.03). SAD-D relatives (B=-0.04, SE=0.15, p=0.77) and BD-P relatives (B=-0.13, SE=0.09, p=0.16) did not show difficulties with emotion recognition relative to healthy controls. As with probands, neutral facial expressions were most difficult for relatives to recognize (p<0.001). A significant relative group × emotion category interaction (p<0.001) indicated that relatives of probands with SCZ and SAD-B showed greater difficulties recognizing angry faces as compared to SAD-D relatives (SCZ: B=−0.36, SE=0.15, p=0.01; SAD-B: B=-0.38, SE=0.16, p=0.02) and healthy controls (SCZ: B=−0.29, SE=0.08, p<0.001; SAD-B: B=−0.32, SE=0.10, p=0.002). No differences among relative groups were found for facial expressions of fear, sadness or happiness (p's>0.17). Figure 2 displays accuracy on each emotion category for all relative groups and healthy controls.
Figure 2.
Facial emotion recognition accuracy for relatives of probands with schizophrenia-spectrum disorders and psychotic bipolar disorder.
On neutral faces, relatives of probands with SCZ (B=−0.34, SE=0.09, p<0.001), SAD-B (B=−0.32, SE=0.12, p=0.005), and BD-P relatives (B=-0.21, SE=0.09, p=0.03) were more likely than controls to perceive emotions in the faces that were not intended and are not seen by healthy individuals (see Figure 3). SCZ relatives were more likely than controls to misclassify neutral faces as sad (B=0.26, SE=0.09, p=0.004), as were SAD-B relatives in comparison with SAD-D relatives (B=0.35, SE=0.11, p=0.002). SCZ relatives were also more likely than controls to misclassify neutral faces as happy (B=0.21, SE=0.09, p=0.02). Relatives groups did not differ from controls in misclassifying neutral faces by perceiving fear or anger (p's>0.14).
3.2. Comparisons of Nonpsychotic Relatives with and without Personality Trait Elevations
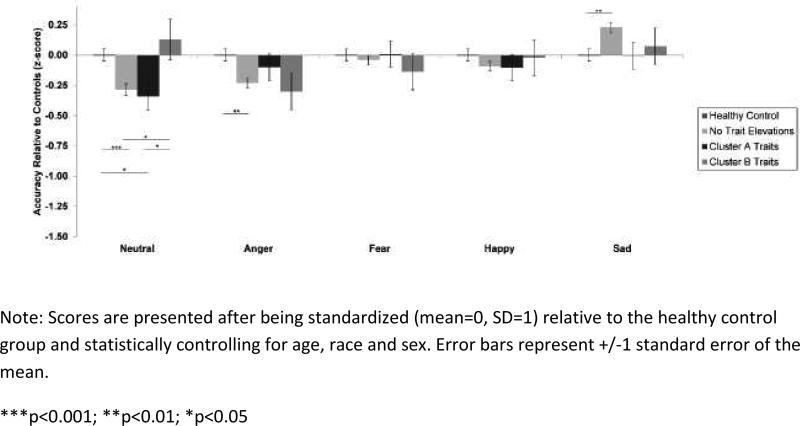
Non-psychotic relatives from all diagnostic groups without elevated Cluster A or Cluster B personality disorder traits showed difficulties recognizing angry and neutral faces compared to controls (p's<0.001). Relatives with elevated Cluster A traits also had deficits on neutral faces compared both to relatives with elevated Cluster B traits and controls (p's<0.05). Aside from difficulties recognizing neutral faces compared to relatives with Cluster B traits, relatives without trait elevations did not differ from relatives with Cluster A or Cluster B trait elevations on any other emotion. Figure 4 displays accuracy on each emotion category for relatives with and without personality disorder trait elevations.
Figure 4.
Facial emotion recognition accuracy for all non-psychotic relatives (pooled) with Cluster A or Cluster B personality disorder trait elevations.
3.3. Familiality of Emotion Perception Accuracy
Collapsing across emotion categories, emotion recognition ability significantly aggregated in SCZ families (h2=0.11, p=0.03). Familiality estimates for SAD and BD-P families were not statistically significant (see Table 3).
Table 3.
Familial Aggregation of Emotion Recognition Ability in Schizophrenia, Schizoaffective and Psychotic Bipolar Disorder Families.
| All Emotions | Neutral | Anger | Fear | Happy | Sad | |
|---|---|---|---|---|---|---|
| Schizophrenia | h2=0.11 (0.06) p=0.03a | h2=0.07 (0.06) p=0.15a | h2=0.10 (0.05) p=0.06s | h2=0.05 (0.05) p=0.20a | h2=0.08(0.05) p=0.08r | h2=0.11 (0.05) p=0.05a,r |
| Schizoaffective | h2=0.00 (.--) p=0.50a | h2=0.00 (.--) p=0.50a | h2=0.06 (0.05) p=0.32 | h2=0.05 (0.06) p=0.33a | h2=0.12 (0.05) p=0.16a | h2=0.11 (0.05), p=0.18a |
| Bipolar Disorder | h2=0.08 (0.05) p=0.20r | h2=0.15(0.06) p=0.08a | h2=0.00 (.--) p=0.50r | h2=0.00 (.--) p=0.50a,r | h2=0.00 (.--) p=0.50 | h2=0.07 (0.05), p=0.30a |
| All Families | h2=0.11 (0.06) p=0.03a | h2=0.07 (0.06) p=0.15a | h2=0.10 (0.06) p=0.06s | h2=0.05 (0.07) p=0.20a | h2=0.08 (0.06) p=0.08r | h2=0.11 (0.06), p=0.05a,r |
Note: Values represent h2 with the standard error indicated in parentheses. Statistically significant estimates are bolded and italicized. Schizoaffective disorder subtype groups were pooled for familiality analyses to increase statistical power.
Age
Sex
Race as significant (p<.05) covariates
3.4. Relationships with Clinical Symptoms, Social Functioning and Cognition
Emotion recognition deficits were associated with negative symptoms on the PANSS for SCZ probands (r=-0.21, p<0.001) and manic symptoms on the YMRS for BD-P probands (r=-0.20, p=0.002). No significant correlations for emotion recognition accuracy or misclassification on neutral faces were found with depression (MADRS), positive symptoms (PANSS), or social functioning (SFS) (p's>0.08).
Antipsychotic dosage in chlorpromazine equivalents (Andreasen et al., 2010) was not significantly associated with emotion recognition accuracy for probands (r=-0.06, p=0.13). Among probands, there was a minimal correlation between ER-40 performance and the Schizo-Bipolar Scale (r=−0.09, p=0.01). Total score on the Brief Assessment of Cognition in Schizophrenia (Keefe et al., 2004) showed a modest correlation with overall ER-40 accuracy (r=0.29, p<0.001; see Supplementary Material, Table S2, for correlations by group).
4. Discussion
Here, we report on results from the B-SNIP study examining facial emotion recognition in probands with SCZ-spectrum disorders and BD-P and their biological relatives. Compared to healthy controls, probands had significant emotion recognition deficits, especially in judging neutral and angry faces. Overall, there was a consistent pattern indicating more pronounced emotion recognition deficits for probands receiving diagnoses characterized by comparatively less frequent mood disturbances and more persistent psychosis. The exception was on angry faces for which probands with SAD-D showed the greatest difficulties in emotion recognition, which may reflect a unique confluence of social-cognitive and depressive mood-related factors influencing recognition of facial expressions conveying threat in this proband group (Green et al., 2003). On fearful, happy and sad faces, however, probands with SCZ performed the poorest and BD-P were most accurate, with SAD-D and SAD-B largely falling intermediate to these proband groups. All proband groups tended to misperceive neutral faces as sad; otherwise, there were no consistent biases in emotion perception across proband groups.
Similarities and differences in emotion recognition between SCZ and BD-P probands may reflect overlapping and distinct neurophysiology underlying facial affect processing in these disorders. Neuroimaging studies of SCZ and BD show common areas of increased neural activation for emotional stimuli in areas such as the amygdala, inferior frontal gyrus, and precuneus, as well as decreased activation in anterior cingulate cortex (Marwick and Hall, 2008; Wegbreit et al., in press). On the other hand, divergent patterns of neural activation on emotion tasks have primarily been isolated to areas showing higher activation in BD than SCZ in medial temporal lobe structures (e.g., hippocampus, parahippocampal gyrus, and mid-cingulate cortex) (Whalley et al., 2012). Electrophysiological studies also suggest that patients with SCZ and less so BD have deficits in structural encoding of faces, whereas both patient groups may show less efficient decoding of facial affect features (Wynn et al., 2013; Wynn et al., 2008). These neurophysiological findings may partly account for similarities between SCZ and BD-P in recognizing specific facial expressions (e.g., anger, neutral) but greater deficits for SCZ in judging other emotions (e.g., sadness, happiness).
Deficits in facial emotion recognition were related to negative symptoms in probands with SCZ but not in BD-P, consistent with research showing similar findings early in the course of these psychotic disorders (Daros et al., 2014; Edwards et al., 2001). On the other hand, elevated manic symptoms (albeit below clinical thresholds for a manic episode) were related to poorer emotion recognition among BD-P probands. These findings converge with other data indicating that manic mood (potentially in combination with associated cognitive difficulties) may interfere with emotion recognition, which to date has primarily been reported with non-psychotic patients (Lembke and Ketter, 2002; Lennox et al., 2004). Comparably accurate emotion recognition performance on fearful, happy and sad faces between BD-P probands and controls was unexpected considering findings of poorer fear recognition in BD patients not in a manic or depressive episode (Martino et al., 2011; Venn et al., 2004), although some research suggests that emotion perception may indeed be relatively intact in euthymic BD patients (Bora et al., 2005; Summers et al., 2006). In contrast to prior work (e.g., Hooker and Park, 2002), emotion recognition was not broadly associated with social functioning in proband groups, although the relationship between these competencies may involve a more complex interplay among factors related to chronicity of illness and cognition (Addington et al., 2006; Mueser et al., 1996).
Relative groups showed a similar pattern of deficits in emotion recognition as probands but to a lesser degree. SCZ and SAD-B relatives were generally the least accurate, particularly on angry and neutral faces. BD-P relatives performed worse than controls only on neutral faces. Unlike the more general finding in probands, only SCZ relatives were more likely than controls to misclassify neutral faces as sad. Even non-psychotic relatives without elevated Cluster A or Cluster B personality disorder traits committed more errors than controls on neutral and angry faces, whereas relatives with Cluster A trait elevations were only worse than controls on neutral faces. It is important to note, however, that smaller sample sizes of relatives with and without personality disorder trait elevations limit statistical power for examining potential differences between groups. Familial aggregation of emotion recognition ability was significant only in SCZ families, not SAD or BD-P families.
Several limitations should be considered when interpreting the results of this study. First, we did not examine the full range of emotional expressions (e.g., disgust, surprise), instead opting to investigate specific basic emotions that have been extensively studied in patients with psychotic disorders. Second, patients included in this study may not be representative of typical individuals with these disorders because they were selected to be clinically stable, report no current significant substance abuse, and have at least one first-degree relative who was willing and able to participate. Third, this study did not evaluate emotion recognition in patients with non-psychotic mood disorders (e.g., non-psychotic bipolar disorder) or those with other psychotic mood disorders (e.g., psychotic unipolar depression), groups which may show distinct patterns of emotion recognition deficits. Fourth, there was limited evidence for effects of medications on emotion recognition deficits. While this is consistent with other research on SCZ and BD-P showing trait-like biases in facial emotion perception after treatment with antipsychotic medications (Daros et al., 2014), it remains possible that treatments impacting mood state may produce or reduce mood-congruent biases in facial emotion perception, especially on ambiguous facial expressions (i.e., neutral faces). Fifth, given that cognition was modestly correlated with emotion recognition ability, more research is needed to delineate specific areas of overlap between these domains across the schizophrenia-spectrum and BD. Sixth, further research may clarify other factors that could contribute to difficulties with facial emotion recognition, including using a gender identification condition to control for more rudimentary facial processing difficulties. Finally, it is important to note that analyses involving relatives with and without Cluster A or Cluster B personality disorder trait elevations were based on considerably smaller samples than the primary analyses.
In summary, the results of the present study are consistent with prior findings from the B-SNIP study demonstrating common and divergent cognitive and neurobiological deficits along a psychosis continuum from SCZ and SAD to BD-P in both probands and relatives (Hill et al., 2013; Ivleva et al., 2013; Narayanan et al., 2013; Reilly et al., 2013). Facial emotion recognition deficits may reflect critical social-cognitive limitations that affect patients with psychotic disorders as well as their non-psychotic relatives. Importantly, difficulties for probands and most relatives in recognizing neutral faces suggested a possible negative attribution bias toward perceiving sadness in faces intended to convey no emotion. This suggests that social interaction difficulties may in part be related to misperceiving emotional cues, perhaps in ways reflecting inner mood states exerting a more robust impact on perceptual processes. For SCZ, emotion recognition ability was modestly familial, consistent with the view that deficits in social cognition may represent fundamental features of SCZ (Penn et al., 2008). As with cognitive deficits (Hill et al., 2013; Reilly et al., 2013), the results of this study indicate that emotion recognition deficits are more severe in SCZ but extend to other psychotic disorders. These emotion recognition deficits appear minimally related to medications and relatively independent of mood state during periods of clinical stabilization, although the overlap of emotion recognition deficits with cognitive impairments and important illness-related characteristics (e.g., negative symptoms, chronicity of illness) requires further investigation. Finally, it will be important for future research to understand how difficulties in emotion perception may impact day-to-day social functioning across the spectrum of psychotic disorders.
Supplementary Material
Acknowledgment
This work was supported by the National Institute of Mental Health at the National Institutes of Health (grant numbers MH078113, MH077945, MH077852, MH077851, MH077862, MH072767, and MH083888), Canadian Institutes of Health Research (New Investigator Salary Award, grant number MSH-130177 to ACR), Social Sciences and Humanities Research Council of Canada (Joseph-Armand Bombardier CGS Doctoral Scholarship to ARD), and the Brain and Behavior Research Foundation (NARSAD Young Investigator Grant to LHR).
The authors thank Gunvant K. Thaker for his collaboration in the design and implementation of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
ACR drafted the manuscript. JLR completed familiality analyses. LHR undertook the statistical analysis, drafted the statistical analysis and results sections, and prepared all tables and figures. ARD managed the literature searches. RCG created the emotion recognition task and consulted on the manuscript. JLR, CAT, GDP, MSK and JAS designed the study and wrote the protocol. All authors contributed to and have approved the final manuscript.
Conflict of Interest
Dr. Tamminga has served as a consultant to Astallas, Eli Lilly Pharmaceuticles, Kaye Scholer LLC, and Lundbeck, Inc., and on an advisory board for Intra-cellular Therapies (ITI, Inc.). Dr. Pearlson has served on an advisory panel for Bristol-Myers Squibb. Dr. Keshavan has received research support from Sunovion and GlaxoSmithKline. Dr. Sweeney has been on advisory boards for Bristol-Myers Squibb, Eli Lilly, Pfizer, Roche, and Takeda and has received grant support from Janssen. Dr. Gur has served as consultant to the Brain Resource Company and received grant support from AstraZeneca and Merck. The other authors report no financial relationships with commercial interests.
References
- Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophr. Res. 2006;85(1-3):142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty TH, Liang KY. Robust inference for variance components models in families ascertained through probands: I. Conditioning on proband's phenotype. Genet. Epidemiol. 1987;4(3):203–210. doi: 10.1002/gepi.1370040305. [DOI] [PubMed] [Google Scholar]
- Bediou B, Asri F, Brunelin J, Krolak-Salmon P, D'Amato T, Saoud M, Tazi I. Emotion recognition and genetic vulnerability to schizophrenia. Br. J. Psychiatry. 2007;191:126–130. doi: 10.1192/bjp.bp.106.028829. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. The British Journal of Psychiatry. 1990;157(6):853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Bora E, Vahip S, Gonul AS, Akdeniz F, Alkan M, Ogut M, Eryavuz A. Evidence for theory of mind deficits in euthymic patients with bipolar disorder. Acta Psychiatr. Scand. 2005;112(2):110–116. doi: 10.1111/j.1600-0447.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, Pine DS, Leibenluft E. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. A. J. Psychiatry. 2008a;165(3):385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Skup M, Rich BA, Blair KS, Pine DS, Blair JR, Leibenluft E. Risk for bipolar disorder is associated with face-processing deficits across emotions. J. Am. Acad. Child Adolesc. Psychiatry. 2008b;47(12):1455–1461. doi: 10.1097/CHI.0b013e318188832e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter WT. Porous diagnostic boundaries: a new emphasis for the bulletin. Schizophr. Bull. 2014;40(1):1–2. doi: 10.1093/schbul/sbt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, O'Donovan MC, Owen MJ. Psychosis Genetics: Modeling the Relationship Between Schizophrenia, Bipolar Disorder, and Mixed (or “Schizoaffective”) Psychoses. Schizophr. Bull. 2009;35(3):482–490. doi: 10.1093/schbul/sbp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daros AR, Ruocco AC, Reilly JL, Harris MS, Sweeney JA. Facial emotion recognition in first-episode schizophrenia and bipolar disorder with psychosis. Schizophr. Res. 2014 doi: 10.1016/j.schres.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daros AR, Zakzanis KK, Ruocco AC. Facial emotion recognition in borderline personality disorder. Psychol. Med. 2013;43(9):1953–1963. doi: 10.1017/S0033291712002607. [DOI] [PubMed] [Google Scholar]
- Dickey CC, Panych LP, Voglmaier MM, Niznikiewicz MA, Terry DP, Murphy C, Zacks R, Shenton ME, McCarley RW. Facial emotion recognition and facial affect display in schizotypal personality disorder. Schizophr. Res. 2011;131(1-3):242–249. doi: 10.1016/j.schres.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Mermon DE, Montrose DM, Miewald J, Gur RE, Gur RC, Sweeney JA, Keshavan MS. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophr. Bull. 2010;36(6):1081–1088. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr. Res. 2001;48(2-3):235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- Erol A, Mete L, Sonmez I, Unal EK. Facial emotion recognition in patients with schizophrenia and their siblings. Nord J Psychiatry. 2010;64(1):63–67. doi: 10.3109/08039480903511399. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis I disorders — patient edition (SCID-I/P, 11/2002 revision) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Fiske ST, Taylor SE. Social cognition: From brains to culture. Sage; 2013. [Google Scholar]
- Goghari VM, Macdonald AW, 3rd, Sponheim SR. Temporal lobe structures and facial emotion recognition in schizophrenia patients and nonpsychotic relatives. Schizophr. Bull. 2011;37(6):1281–1294. doi: 10.1093/schbul/sbq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MJ, Williams LM, Davidson D. Visual scanpaths to threat-related faces in deluded schizophrenia. Psychiatry Res. 2003;119(3):271–285. doi: 10.1016/s0165-1781(03)00129-x. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J. Neurosci. Methods. 2002;115(2):137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Hill SK, Harris MS, Herbener ES, Pavuluri M, Sweeney JA. Neurocognitive allied phenotypes for schizophrenia and bipolar disorder. Schizophr. Bull. 2008;34(4):743–759. doi: 10.1093/schbul/sbn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. A. J. Psychiatry. 2013;170(11):1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112(1):41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D, Moates AF, Lu H, Francis AN, Tandon N, Schretlen DJ, Sweeney JA, Clementz BA, Tamminga CA. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). A. J. Psychiatry. 2013;170(11):1285–1296. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Multi-Health Systems; North Tonawanda, NY: 1986. [Google Scholar]
- Kee KS, Horan WP, Mintz J, Green MF. Do the siblings of schizophrenia patients demonstrate affect perception deficits? Schizophr. Res. 2004;67(1):87–94. doi: 10.1016/s0920-9964(03)00217-2. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004;68(2-3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, Eack SM, Tamminga C. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr. Res. 2011;133(1-3):250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull. 2010a;36(5):1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull. 2010b;36(5):1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. A. J. Psychiatry. 2002;159(2):302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol. Med. 2004;34(5):795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- Li H, Chan RC, Zhao Q, Hong X, Gong QY. Facial emotion perception in Chinese patients with schizophrenia and non-psychotic first-degree relatives. Prog. Neuropsychopharmacol. Bol. Psychiatry. 2010;34(2):393–400. doi: 10.1016/j.pnpbp.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Majadas S, Olivares J, Galan J, Diez T. Prevalence of depression and its relationship with other clinical characteristics in a sample of patients with stable schizophrenia. Compr. Psychiatry. 2012;53(2):145–151. doi: 10.1016/j.comppsych.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Strejilevich SA, Fassi G, Marengo E, Igoa A. Theory of mind and facial emotion recognition in euthymic bipolar I and bipolar II disorders. Psychiatry Res. 2011;189(3):379–384. doi: 10.1016/j.psychres.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Marwick K, Hall J. Social cognition in schizophrenia: a review of face processing. Br. Med. Bull. 2008;88(1):43–58. doi: 10.1093/bmb/ldn035. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British Journal of Psychiatry. 1979;134(4):382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Doonan R, Penn DL, Blanchard JJ, Bellack AS, Nishith P, DeLeon J. Emotion recognition and social competence in chronic schizophrenia. J. Abnorm. Psychol. 1996;105(2):271–275. doi: 10.1037//0021-843x.105.2.271. [DOI] [PubMed] [Google Scholar]
- Narayanan B, O'Neil K, Berwise C, Stevens MC, Calhoun VD, Clementz BA, Tamminga CA, Sweeney JA, Keshavan MS, Pearlson GD. Resting State Electroencephalogram Oscillatory Abnormalities in Schizophrenia and Psychotic Bipolar Patients and Their Relatives from the Bipolar and Schizophrenia Network on Intermediate Phenotypes Study. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr. Bull. 2008;34(3):408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured interview for DSM-IV personality. SIDP-IV. The University of Iowa; Iowa City, IA: 1995. [Google Scholar]
- Reilly JL, Frankovich K, Hill S, Gershon ES, Keefe RS, Keshavan MS, Pearlson GD, Tamminga CA, Sweeney JA. Elevated Antisaccade Error Rate as an Intermediate Phenotype for Psychosis Across Diagnostic Categories. Schizophr. Bull. 2013 doi: 10.1093/schbul/sbt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samame C, Martino DJ, Strejilevich SA. Social cognition in euthymic bipolar disorder: systematic review and meta-analytic approach. Acta Psychiatr. Scand. 2012;125(4):266–280. doi: 10.1111/j.1600-0447.2011.01808.x. [DOI] [PubMed] [Google Scholar]
- Seidel EM, Habel U, Finkelmeyer A, Hasmann A, Dobmeier M, Derntl B. Risk or resilience? Empathic abilities in patients with bipolar disorders and their first-degree relatives. J. Psychiatr. Res. 2012;46(3):382–388. doi: 10.1016/j.jpsychires.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, Tamminga CA, Clementz BA, O'Neil K, Pearlson GD. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. A. J. Psychiatry. 2013;170(8):886–898. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- Summers M, Papadopoulou K, Bruno S, Cipolotti L, Ron MA. Bipolar I and bipolar II disorder: cognition and emotion processing. Psychol. Med. 2006;36(12):1799–1809. doi: 10.1017/S0033291706008804. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). A. J. Psychiatry. 2013;170(11):1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker GK. Defining the schizophrenia phenotype. Curr Psychiatry Rep. 2000;2(5):398–403. doi: 10.1007/s11920-000-0022-6. [DOI] [PubMed] [Google Scholar]
- Venn HR, Gray JM, Montagne B, Murray LK, Michael Burt D, Frigerio E, Perrett DI, Young AH. Perception of facial expressions of emotion in bipolar disorder. Bipolar Disord. 2004;6(4):286–293. doi: 10.1111/j.1399-5618.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- Wegbreit E, Cushman GK, Puzia ME, Weissman AB, Kim KL, Laird AR, Dickstein DP. Developmental meta-analyses of the functional neural correlates of bipolar disorder. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2014.660. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley HC, Papmeyer M, Sprooten E, Lawrie SM, Sussmann JE, McIntosh AM. Review of functional magnetic resonance imaging studies comparing bipolar disorder and schizophrenia. Bipolar Disorders. 2012;14(4):411–431. doi: 10.1111/j.1399-5618.2012.01016.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson G. Wide Range Achievement Test (WRAT4) Psychological Assessment Resources; Lutz: 2006. [Google Scholar]
- Wynn JK, Jahshan C, Altshuler LL, Glahn DC, Green MF. Event-related potential examination of facial affect processing in bipolar disorder and schizophrenia. Psychol. Med. 2013;43(1):109–117. doi: 10.1017/S0033291712001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JK, Lee J, Horan WP, Green MF. Using event related potentials to explore stages of facial affect recognition deficits in schizophrenia. Schizophr. Bull. 2008;34(4):679–687. doi: 10.1093/schbul/sbn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.