Abstract
Motivational impairment is a critical factor that contributes to functional disability in schizophrenia and undermines an individual’s ability to engage in and adhere to effective treatment. However, little is known about the developmental trajectory of deficits in motivation and whether these deficits are present prior to the onset of psychosis. We assessed several components of motivation including anticipatory versus consummatory pleasure (using the Temporal Experience of Pleasure Scale (TEPS)), and behavioral drive, behavioral inhibition, and reward responsivity (using the Behavioral Inhibition Scale/Behavioral Activation Scale (BIS/BAS)). A total of 234 participants completed study measures, including 60 clinical high risk (CHR) participants, 60 recent-onset schizophrenia participants (RO), 78 chronic schizophrenia participants (SZ) and 29 healthy controls (HC) age matched to the CHR group. CHR participants endorsed greater deficits in anticipatory pleasure and reward responsivity, relative to HC comparison participants and individuals diagnosed with schizophrenia. Motivational deficits were not more pronounced over the course of illness. Depressed mood was uniquely associated with impairments in motivation in the CHR sample, but not the schizophrenia participants. The results suggest that CHR individuals experience multiple contributors to impaired motivation, and thus multiple leverage points for treatment.
Keywords: Schizophrenia, Motivation, Avolition, Anticipatory Pleasure, Psychosis, Reward, Duration of illness
1. Introduction
Dating back to the earliest descriptions of schizophrenia by Kraepelin and Bleuler, amotivation/avolition was observed to be central to the phenomenology and course of schizophrenia (McGlashan, 2011). Amotivation and negative symptoms more broadly are resistant to current treatment options and often lead to substantial functional impairment (Foussias et al., 2011; Foussias and Remington, 2010). Motivation, or goal-directed behavior, is reliant on several component processes, such as the anticipation of reward (e.g., wanting), the hedonic experience of rewards (e.g., liking), the ability to develop and sustain a representation of reward, and guiding and planning behavior towards that future reward (in the face of competing stimuli; e.g., learning (Gold et al., 2012; Gold et al., 2008). In schizophrenia, patients have demonstrated deficits in anticipatory pleasure despite intact in-the-moment hedonic experiences (e.g., wanting vs. liking discrepancy) (Cohen and Minor, 2010; Foussias and Remington, 2010; Gard et al., 2007), as well as difficulty maintaining cognitive representation of rewarding experiences and redirecting behavior back to rewarding experiences (Barch and Dowd, 2010).
While much of the research on motivation in schizophrenia has focused heavily on wanting vs. liking, and on the use of rewards to guide behavior, there is also a large literature on underlying traits linked to the likelihood to pursue rewards—behavioral approach (driven by appetitive motives) and behavioral avoidance (driven by aversive motives). Individuals with a high degree of approach motivation are more likely to pursue new and potentially rewarding experiences, while those with behavioral avoidance are more likely to anticipate punishment and as such avoid such experiences. The behavioral inhibition system is hypothesized to be sensitive to threat cues and to activate responses of avoidance via noradrenergic and serotonergic activity (Cools et al., 2005; Depue and Iacono, 1989; Harre and Parrott, 1996), while the behavioral activation system controls reward sensitivity via dopaminergic activity (Sutton and Davidson, 1997). Only two studies to date have examined the behavioral inhibition and behavioral activation systems (BIS/BAS) in schizophrenia, despite important implications for negative symptoms and motivation specifically. In the first study, Scholten and colleagues (2006) found that individuals with schizophrenia were more sensitive to threat than healthy controls, but no differences were detected between patients and controls in the behavioral activation system. In a more recent study (Engel, et al., 2013), the BAS system was found to be negatively associated with more severe negative symptoms, suggesting heterogeneity within schizophrenia samples. Thus far, most studies on motivation and reward processing in schizophrenia have been conducted on individuals who have been persistently ill for most of their adult lives. It is therefore unknown whether motivational deficits worsen over the course of schizophrenia. The stability of negative symptoms generally, however, has been examined and while it appears they might be stable in severity across the course of psychosis, some studies suggest that as the duration of untreated psychosis increases, individuals with schizophrenia experience worsening negative symptoms over time (Chang et al., 2013). Although motivational deficits are often considered within the context of negative symptoms and reward processing, the presence of mood and anxiety symptoms are also linked to motivational capacity in schizophrenia. In particular, depressed mood is associated with decreased hedonic capacity while anxiety symptom severity is associated with greater threat sensitivity/avoidance behavior (Barch et al., 2008). These studies raise questions about the degree of behavioral activation and avoidance within schizophrenia samples that vary in the degree of negative symptom severity, as well as other factors, such as the duration of illness, and the presence of comorbid depressive and anxiety symptoms. In the current study, we examined negative symptom severity, mood and anxiety symptoms, and motivational deficits across the course of schizophrenia, using a cross-sectional design.
The primary aim of this study was to examine several behavioral components of motivation (wanting, liking, approach and avoidance) in individuals at various stages of experiencing psychosis: those at clinical high risk (CHR) of developing a psychotic disorder, those within the first 5 years of onset of schizophrenia (Recent Onset; RO), and those with persistent schizophrenia or schizoaffective disorder (SZ). We tested the following hypotheses: 1) Individuals with persistent schizophrenia will demonstrate greater motivational impairments than those at-risk or with a recent onset of schizophrenia, 2) Mood and anxiety symptoms will influence the degree of motivational impairments at all stages of illness, such that more severe anxiety and depression will be positive correlated with avoidance and negatively correlated with wanting, liking, and approach motivation in all participant groups.
2. Methods
2.1 Participants
The study included 234 participants:, 60 clinical high risk (CHR), 60 recent-onset schizophrenia (RO), 78 chronic schizophrenia (SZ) participants and 29 healthy controls (HC) who were recruited for randomized controlled trials of cognitive training (ClinicalTrials.gov NCT00655239, NCT00694889, and NCT00312962). The HC participants were age-matched to the CHR participants. Participants in the HC, CHR and RO groups were drawn from two research programs at the University of California, San Francisco (UCSF) and University of California at Davis (UCD) and persistent SZ participants from a research program at the San Francisco VA Medical Center (SFVAMC). Patient participants were recruited from community mental health centers, outpatient clinics, local schools and universities, and HC participants were recruited via advertisement. CHR status was ascertained using the Structured Interview for Prodromal Syndromes (SIPS version 4.0(Miller et al., 2002). All CHR participants met one of the following prodromal syndromes on the SIPS/SOPS,: 1) the presence of attenuated positive, psychotic symptoms, occurring at least weekly with onset or worsening in the past year, 2) brief intermittent psychotic symptoms, which must have begun in the past three months, or 3) a 30% decline in GAF score over the past year, plus either a diagnosis of schizotypal personality disorder or a first-degree relative with a psychotic disorder. Recent onset participants were included based on a diagnosis of schizophrenia, schizophreniform, or schizoaffective disorder with an onset within the past five years as determined by the Structured Clinical Interview for the DSM-IV TR Axis I disorder interview (SCIP-I; First, 1996). Onset of illness was defined by the date diagnostic criteria were first met, as assessed by the SCID. Chronic schizophrenia participants were diagnosed using the SCID-I. Healthy controls did not meet DSM-IV criteria for an Axis I psychiatric disorder as determined by the SCID-I or meet criteria for a prodromal syndrome, and had no first-degree relatives with psychosis, based on participant and collateral informant reports during the SIPS interview. CHR, RO and SZ participants were clinically stable at the time of testing (no hospitalization within the past 3 months and stable dose of medication over the past month), as per the requirements for the parent cognitive training study. Other inclusion criteria included: 1) Good general physical health; 2) Fluent and proficient in English; 3) IQ ≥ 70 (WASI, 1st edition, 2-subtest version: Vocabulary and Matric Reasoning); 4) No neurological disorder; 5) No substance dependence or significant use that would interfere with study participation.
2.2 Procedure
Advanced graduate students, predoctoral interns, postdoctoral fellows, and trained bachelor-level research assistants administered the measures described below in the context of a larger battery of cognitive and clinical assessments. All participants gave written informed consent or assent for the study and were compensated for their participation in all assessments. Parental informed consent for minors was also obtained. After an intake evaluation that determined study eligibility, participants underwent a structured diagnostic clinical interview and completed self-report measures of motivation and clinician ratings of symptom severity. Only baseline, cross sectional data were included in this study.
2.3 Measures
We assessed several components of motivation including negative symptom severity and mood and anxiety using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987), anticipatory (wanting) versus consummatory (liking) pleasure using the Temporal Experience of Pleasure Scale (TEPS; Gard, et al., 2006), and behavioral drive, behavioral inhibition, and reward responsivity using the Behavioral Inhibition Scale/Behavioral Activation Scale (BIS/BAS; Carver and White, 1994). The TEPS includes eighteen items and are rated on a scale of 1 (very flase for me) to 6 (very true for me). The BIS/BAS measure is comprised of twenty-four items rated on scale of 1 (very true for me) to 4 (very false for me), of which seven items represent the BIS. The BAS scales were designed to measure approach motivation traits while the BIS scale was designed to measure aversive motivation traits. Examples of TEPS and BIS/BAS items are in Table 1. The PANSS is clinician-administered, while the TEPS and BIS/BAS are self-report measures.
Table 1.
Examples of items from the Temporal Experience of Pleasure Scale (TEPS) and the Behavioral Inhibition/Behavioral Activation Scale (BIS/BAS).
| TEPS-Anticipatory pleasure | TEPS- Consummatory pleasure | Behavioral Inhibition | Behavioral Drive | Reward Responsivity |
|---|---|---|---|---|
| When something exciting is coming up in my life, I really look forward to it | I have enjoyed experiences like taking deep breaths of fresh air when I walk outside | I feel pretty worried or upset when I think or know somebody is angry at me | When I want something, I usually go all-out to get it | When I get something I want, I feel excited and energized |
| I have noticed that looking forward to a pleasurable experience is in itself pleasurable | The smell of freshly cut grass (or the outdoors) has been enjoyable to me | I worry about making mistakes | I go out of my way to get things I want | When good things happen to me, it affects me strongly |
2.4 Data Analytic Plan
First, data were inspected for normality and outliers were windsorized at a level of 95%. Less than 5% of the data on these measures, within each group, were adjusted. One-way ANOVAs and Chi-Square Tests were conducted to test for demographic differences. To better understand the relationship between the TEPS and BIS/BAS scales, we used Pearson correlation analyses. BIS/BAS scores were reverse coded, such that higher ratings represented greater degrees of approach motivation. To test hypothesis 1, a series of one-way ANOVAs were used to compare the mean differences in anticipatory and consummatory pleasure and approach and aversive motivation between groups. In order to test Hypothesis 2, mood and anxiety symptoms were examined in relation to motivation, using Pearson correlation analyses. If significant, the PANSS mood and/or anxiety ratings were included as covariates in the main analyses (ANCOVAs). Post hoc analyses were conducted using Tukey tests.
3. Results
The demographic characteristics of the study participants are included in Table 2. The HC participants were matched to the CHR subjects by age, gender, education, and IQ. The RO and SZ participants included significantly more males and a lower IQ than the HC and CHR samples. The SZ sample was significantly more educated than the HC and CHR participants, likely due to age differences. Negative symptoms were more severe in the schizophrenia samples (RO and SZ) than the CHR sample and there was no significant difference in negative symptom severity between RO and SZ. Demographic characteristics (age, gender, education, and IQ) were not associated with the TEPS and BIS/BAS ratings in each sample.
Table 2.
Demographic characteristics of study participants
| Healthy Controls (n=29) | Clinical High Risk (n=60) | Recent Onset (n=67) | Schizophrenia (n=78) | |
|---|---|---|---|---|
| Age | 17.6 (3.7) | 18.6 (4.6) | 21.7 (4.5)* | 42.9 (10.9)** |
| Male (%) | 48% | 52% | 70%* | 70%* |
| Caucasian (%) | 63% | 57% | 60% | 55% |
| Subject education (mean years, SD) | 11.4 (2.9) | 11.5 (3.1) | 12.8 (1.9) | 13.8 (2.1)* |
| WASI-IQ | 110.9 (13.5) | 109.6 (16.0) | 102.8 (12.8)** | 100.3 (17.0)* |
| Months of illness (mean, SD) | -- | -- | 21.9 (2.5)** | 267.8 (128.2)** |
| Negative symptoms (mean, SD) | -- | 4.68 (5.0)** | 17.89 (6.1)** | 16.87 (5.8)** |
| Depression (mean, SD) | -- | 3.18 (1.8)* | 2.50 (1.6)* | 3.14 (1.6)* |
| Anxiety (mean, SD) | -- | 3.36 (1.3) | 2.85 (1.5) | 3.26 (1.3) |
Note.
p<.05;
p<.01.
Recent onset and schizophrenia participants were significantly older, more represented by males, more educated, had a lower IQ than healthy control and clinical high risk participants. Recent onset and schizophrenia participants had more severe negative symptoms and less severe depressive symptoms than clinical high risk participants.
The interrelationships between TEPS and BIS/BAS scales (Table 3) demonstrated a strong, positive relationship between reward responsivity and the anticipatory pleasure scale in all samples. Reward responsivity was also significantly associated to the consummatory pleasure scale in HC, RO, and SZ samples, but not in the CHR sample. Behavioral drive was related to anticipatory and consummatory pleasure in the RO sample, while only related to consummatory pleasure in HCs.
Table 3.
Interrelationships between TEPS anticipatory and consummatory pleasure and BIS/BAS behavioral approach and inhibition in HC, CHR, RO, and SZ
| Behavioral Inhibition | Reward Responsivity | Behavioral Drive | |
|---|---|---|---|
| Healthy control | |||
| Anticipatory pleasure | .254 | .548** | .347 |
| Consummatory pleasure | −.174 | .379* | .481** |
| Clinical High Risk | |||
| Anticipatory pleasure | .022 | .459** | .128 |
| Consummatory pleasure | −.149 | .081 | .014 |
| Recent Onset | |||
| Anticipatory pleasure | .050 | .591** | .389** |
| Consummatory pleasure | .022 | .477** | .273* |
| Schizophrenia | |||
| Anticipatory pleasure | .263* | .424** | .181 |
| Consummatory pleasure | .185 | .424** | .151 |
Note.
p<.05,
p<.01
Due to their potential relationships with motivation, depression and anxiety severity scores were examined in each sample. Depressed mood was strongly, negatively correlated with anticipatory pleasure in CHR participants (r = −.430, p = .002), but not in the RO or SZ samples. Interestingly, there was no significant difference in the severity of depressed mood between CHR and RO; however, the RO and SZ participants exhibited more severe negative symptom severity, relative to the CHR participants. Depressed mood was unrelated to behavioral approach (drive or reward responsivity) or behavioral inhibition in each sample; however, anxiety was found to be strongly related to behavioral inhibition in the CHR (r = .564, p < .001), RO (r =.232, p =.03), and SZ (r =.377, p =.001) samples. Due to the significant relationship between depressed mood and anticipatory pleasure in the CHR group, depressed mood was included as a covariate in the main analysis comparing group differences in anticipatory pleasure. Anxiety severity was also included in the main analysis comparing group differences in behavioral inhibition.
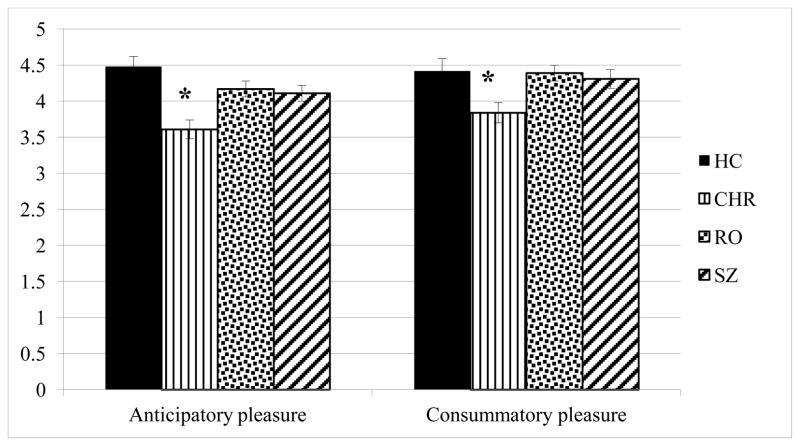
The overall between-group differences in anticipatory and consummatory pleasure were significant (F (3,224) = 7.33; p = .001; Figure 1). This result remained significant when depressed mood was entered as a covariate. Post hoc comparisons using the Tukey test indicated that this results was driven by CHR participants who reported significantly less anticipatory (M = 3.61, SD = .73) and consummatory pleasure (M = 3.84, SD = 1.1), than HC (anticipatory pleasure: M = 4.48, SD = .73; consummatory pleasure: M = 4.42, SD = .91), RO (anticipatory pleasure: M = 4.17, SD = .89; consummatory pleasure: M = 4.39, SD = .80), and SZ participants (anticipatory pleasure: M = 4.11, SD = .93; consummatory pleasure: M = 4.31, SD = .1.1). The RO and SZ samples endorsed intact anticipatory and consummatory pleasure, relative to the young HC sample.
Figure 1.
Between group differences of anticipatory and consummatory pleasure
Note. *p<.05. CHR participants endorsed significantly less anticipatory and consummatory pleasure, relative to the HC, RO, and SZ samples.
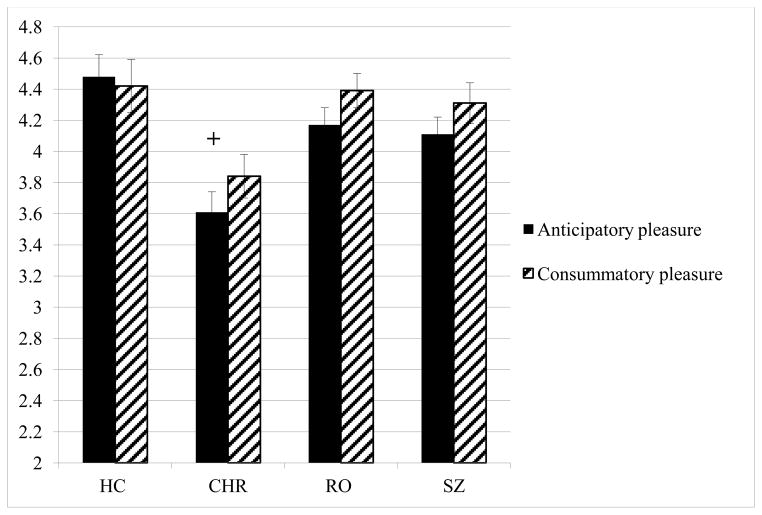
The within-group differences in anticipatory versus consummatory pleasure were examined in each of the samples (Figure 2). No significant difference in anticipatory versus consummatory pleasure was observed in HCs (t = .47, p = .64) . In both the RO and SZ samples, their anticipatory pleasure was reported to be significantly lower than consummatory pleasure (RO: t = −2.33, p = .02; SZ: t = −2.00, p = .05). In CHR participants, this difference was at trend level (t = −1.85, p = .07). The results remained significant for the RO and SZ groups when depressed mood was entered as a covariate.
Figure 2.
Within-group differences in anticipatory and consummatory pleasure
Note: + p=.07; *p<.05 Anticipatory pleasure was significantly lower in RO and SZ participants relative to consummatory pleasures. This difference was at trend level in CHR participants.
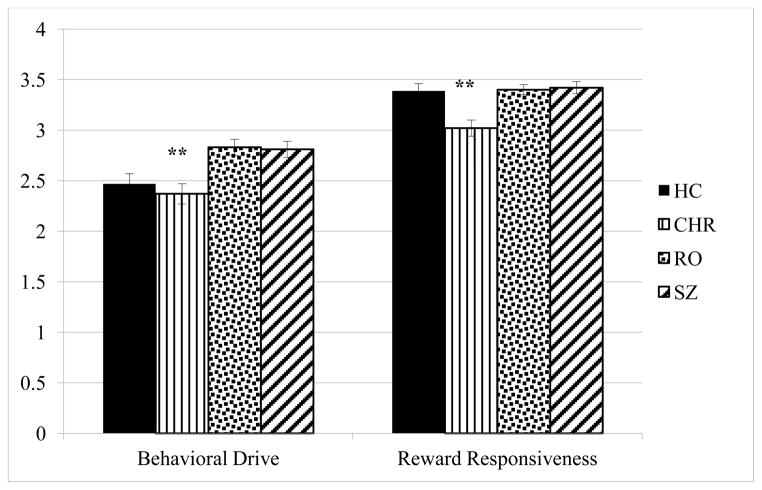
Between-group differences in behavioral drive and reward responsivity (approach motivation traits) were tested (Figure 3). The overall differences in behavioral drive and reward responsivity were significantly different between the groups (behavioral drive: F (3, 230) = 7.46l p = .001; reward responsivity: F (3, 230) = 9.17; p =.001)(. Consistent with the directionality of the anticipatory and consummatory pleasure data, CHR participants endorsed lower behavioral drive (M = 2.37, SD = .73), relative to RO (M = 2.83, SD = .63) and SZ (M = 2.81, SD = .68) participants, and lower reward responsivity (M = 3.03, SD = .58) relative to the HC, (M = 3.40, SD = .35) RO (M = 3.40, SD = .41) or SZ (M = 3.42, SD = .49) participants. There was no difference in the approach motivation traits between any of the other paired participant groups. Depression and anxiety were not entered as covariates since these were unrelated to drive and reward responsivity in each of the samples.
Figure 3.
Between group differences in behavioral drive and reward responsivity
Note. **p<.01, *p<.05. CHR endorsed significantly less drive relative to RO and SZ, and were significantly less responsive to reward relative to HC, RO, and SZ participants.
Behavioral inhibition was significantly different between the groups (F (3,230) = 3.39; p = .02). Consistent with the pattern in the behavioral approach data, CHR participants endorsed greater behavioral inhibition (M = 3.04, SD = .66) than the HCs (M = 2.68, SD = .42), but no differences were observed between any other paired groups. The results remained the same when anxiety was entered as a covariate.
There were no significant differences between the RO and SZ participants on any of the motivational items, suggesting no decline in anticipatory pleasure, reward responsivity, drive or inhibition across the duration of illness. Further, the correlations between months of illness and anticipatory and consummatory pleasure, behavioral approach and inhibition were not significant (all r’s < .100).
4. Discussion
This is the first study, to our knowledge, to investigate motivational deficits across individuals with psychosis of varying illness duration. Notably, CHR participants endorsed more severe deficits in anticipatory pleasure, consummatory pleasure, and reward responsivity, relative to age-matched healthy comparison subjects, and, contrary to our hypotheses, also relative to individuals already diagnosed with early-course and later-course schizophrenia. CHR participants also showed lower behavioral drive relative to individuals with early and later-course schizophrenia. This is surprising, considering that overall negative symptoms were more pronounced in the established schizophrenia samples. Our hypothesis that motivational deficits would be more severe in individuals living with schizophrenia for a greater duration was also not supported. CHR participants endorsed greater motivational deficits across a range of motivational factors compared to early illness and chronic schizophrenia participants, even after controlling for depressed mood.
The degree and extent of motivational impairments endorsed by CHR participants exemplifies the multifarious sequelae of the CHR syndrome. Individuals who are at imminent risk for psychosis are phenotypically complex. Many CHR individuals experience a multitude of problems, including attenuated psychotic symptoms, depressed mood, anxiety, social withdrawal, and functional deficits, all of which might reflect the behavioral correlates of a reward system that is impaired. Further, the degree to which the reward system appears to be impaired has predictive validity for this population. Less severe negative symptoms and depressed mood in CHR individuals significantly increases the likelihood of recovering from an at-risk state and experiencing improved functional outcomes (Addington et al., 2011; Schlosser et al., 2012). Further, it is possible that motivational deficits in CHR individuals who go on to develop psychosis might be a precursor to the development of negative symptoms, if they are not yet present for an individual. Taken together, this suggests that motivational deficits should be a target of vigorous preemptive treatment.
The wanting vs liking discrepancy that has been previously reported in the literature was observed amongst the RO and SZ samples and was at trend level in the CHR group, suggesting that a characteristic feature of schizophrenia may indeed be an impairment in the anticipation of rewarding stimuli and experiences. Also consistent with the literature, RO and SZ participants reported intact consummatory pleasure. However, CHR participants reported significantly less consummatory and anticipatory pleasure. Interestingly there was no difference in the degree of discrepancy in wanting vs liking and in the degree of drive and reward responsivity as the duration of illness increased; furthermore, both RO and SZ participants were rated as having similar levels of negative symptom severity. Taken together, these data suggest that motivational deficits may be relatively stable shortly after illness onset, but are more prominent prior to the onset of the first episode of psychosis.
The degree to which CHR participants endorsed impairments in drive, reward responsivity, and sensitivity to punishment is significant, especially when put in the context of negative symptom severity. The RO and SZ samples were rated as having more negative symptoms than the CHR group, yet the degree of motivational impairment was more pronounced in the CHR sample. The CHR group also reported more impaired motivational impairment than age-matched HCs, suggesting that age or developmental stage alone does not explain their degree of suppressed sensitivity to reward. Importantly, both depressed mood and anxiety appear to play an important role. Although depressed mood did not account for the differences in motivational impairment between the CHR group and the schizophrenia samples, it was strongly associated with anticipatory pleasure in the CHR sample, possibly indicating a useful leverage point for early intervention. For example, it is possible that alleviating depressive symptoms might enhance reward anticipation (Fulford et al., 2013). In addition, anxiety was significantly related to behavioral inhibition in CHR, RO and the SZ samples, suggesting that anxiety plays a key role in heightening sensitivity to punishing stimuli across the course of psychosis and may also represent an important therapeutic target.
The current study was limited due to the use of cross-sectional data, an exploratory approach, and the reliance on self-report measures of motivation. Although it is crucial to understand the experience and perspective of patients’ sense of their own degree of motivation, self-reports may have biases built into them (e.g., the ability to represent the stimuli that are being presented in the measures (Gold et al., 2008). Nonetheless, the self-report measures used in this study have been well-validated and are associated with neural circuits that subserve reward processing(De Pascaliso et al., 2010; Simon et al., 2010), suggesting that self-reported motivational states give some insight into reward processing functionality. Future studies should explore motivational deficits across the course of illness using observational and laboratory-based measures of motivation, and whenever possible, obtain longitudinal assessments in relation to functional outcome. It is likely that motivational states fluctuate over time and with the course of illness, and as such, are ideally suited to be studied longitudinally.
In summary, our findings indicate that adolescents in a high-risk clinical state may experience significant impairments in motivation, potentially related to the unique constellation of psychological and neurocognitive challenges that occur in the early phase of psychotic illness. We posit that this in turn leads them to avoid effort-related goals and to withdraw from perceived psycho-social challenges. Both of these processes likely contribute to a failure to engage in growth-promoting socio-affective experiences and thus to increasing social isolation, rendering them even more vulnerable to heightened stress responsivity when facing the normal developmental challenges of adolescence. It is not difficult to conceive that such a vulnerable state increases the risk for onset of a first-episode of psychosis. It is also not difficult to conceive that such a state may contribute to the development of negative symptoms, as suggested by Beck and colleagues (Beck et al., 2013). The fact that negative symptoms were greater in the RO and chronic patients compared to the CHR individuals lends some support to this model. It appears that CHR individuals might be on a trajectory with multiple contributors to impaired motivation, and thus multiple leverage points for treatment: depressed mood, anxiety, reduced drive towards rewarding stimuli, and heightened sensitivity to aversive stimuli (resulting in fear of failure), all culminating in social withdrawal and decreased stress resilience. Importantly, these leverage points may be responsive to straightforward behavioral treatments, which hopefully would provide long-lasting health-promoting effects.
Acknowledgments
Role of Funding Source
This research was supported by the following grants: NIMH K23 MH097795-01 (Schlosser); UCSF CTSI-SOS NIH UL1 TR0000004 (Schlosser); NIMH R34 MH100399 (Schlosser/Vinogradov); and (Ford: T32 MH089920). The funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We would like to thank the participants and their families for contributing to this research. This research was supported by the following funding sources: NIH/NIMH (Schlosser: K23 MH097795), UCSF CTSI-SOS NIH UL1 TR0000004 (Schlosser); (Vinogradov/Schlosser R34 MH100399), and (Ford: T32 MH089920).
Footnotes
Contributors
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Cornblatt BA, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Heinssen R. At clinical high risk for psychosis: Outcome for nonconverters. Am J Psychiatry. 2011;168(8):800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Yodkovik N, Sypher-Locke H, Hanewinkel M. Intrinsic motivation in schizophrenia: Relationships to cognitive function, depression, anxiety, and personality. J Abnorm Psychol. 2008;117(4):776–787. doi: 10.1037/a0013944. [DOI] [PubMed] [Google Scholar]
- Beck AT, Grant PM, Huh GA, Perivoliotis D, Chang NA. Dysfunctional attitudes and expectancies in deficit syndrome schizophrenia. Schizophr Bull. 2013;39(1):43–51. doi: 10.1093/schbul/sbr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, Hui CLM, Tang JYM, Wong GHY, Chan SKW, Lee EHM, Chen EYH. Impacts of duration of untreated psychosis on cognition and negative symptoms in first-episode schizophrenia: a 3-year prospective follow-up study. Psychol Med. 2013;43(09):1883–1893. doi: 10.1017/S0033291712002838. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36(1):143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Calder AJ, Lawrence AD, Clark L, Bullmore E, Robbins TW. Individual differences in threat sensitivity predict serotonergic modulation of amygdala response to fearful faces. Psychopharmacology. 2005;180(4):670–679. doi: 10.1007/s00213-005-2215-5. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Varriale V, D’Antuono L. Event-related components of the punishment and reward sensitivity. Neuropsychsiol Clin. 2010;121(1):60–76. doi: 10.1016/j.clinph.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annu Rev Psychol. 1989;40(1):457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Engel M, Fritzsche A, Lincoln TM. Anticipatory pleasure and approach motivation in schizophrenia-like negative symptoms. Psychiatry Res. 2013;210(2):422–426. doi: 10.1016/j.psychres.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Foussias G, Mann S, Zakzanis KK, van Reekum R, Agid O, Remington G. Prediction of longitudinal functional outcomes in schizophrenia: the impact of baseline motivational deficits. Schizophr Res. 2011;132(1):24–27. doi: 10.1016/j.schres.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36(2):359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford D, Niendam TA, Floyd EG, Carter CS, Mathalon DH, Vinogradov S, Stuart BK, Loewy RL. Symptom dimensions and functional impairment in early psychosis: more to the story than just negative symptoms. Schizophr Res. 2013;147(1):125–131. doi: 10.1016/j.schres.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D, Gard M, Kring A, John O. Anticipatory and consummatory components of the experience of pleasure: A scale development study. J Res Pers. 2006;40:1086–1102. [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, Collins AG, Frank MJ. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch Gen Psychiatry. 2012;69(2):129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harre R, Parrott WG. The Emotions: Social, Cultural and Biological Dimensions. SAGE; 1996. [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- McGlashan TH. Eugen Bleuler: centennial anniversary of his 1911 publication of Dementia Praecox or the group of schizophrenias. Schizophr Bull. 2011;37(6):1101–1103. doi: 10.1093/schbul/sbr130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159(5):863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Schlosser DA, Jacobson S, Chen Q, Sugar CA, Niendam TA, Li G, Bearden CE, Cannon TD. Recovery from an at-risk state: clinical and functional outcomes of putatively prodromal youth who do not develop psychosis. Schizophr Bull. 2012;38(6):1225–1233. doi: 10.1093/schbul/sbr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten MRM, van Honk J, Aleman A, Kahn RS. Behavioral inhibition system (BIS), behavioral activation system (BAS) and schizophrenia: relationship with psychopathology and physiology. J Psychiatr Res. 2006;40(7):638–645. doi: 10.1016/j.jpsychires.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, Friederich H-C, Stippich C, Weisbrod M, Kaiser S. Neural reward processing is modulated by approach- and avoidance-related personality traits. Neuroimage. 2010;49(2):1868–1874. doi: 10.1016/j.neuroimage.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal Brain Asymmetry: A Biological Substrate of the Behavioral Approach and Inhibition Systems. Psychol Sci. 1997;8(3):204–210. [Google Scholar]