Abstract
Giant cell tumors of bone (GCTB) are generally benign neoplasms, but recently, some authors consider them to be low-grade malignant neoplasms because they have a relatively high rate of recurrence and at least some potential for metastases. The majority of GCTB are unifocal, and less than 1 % are multicentric. We report a rare case of a multicentric GCTB arising simultaneously in the non-dominant fourth and fifth metacarpals of a 25-year-old female. The patient underwent ray amputation of the two involved digits, and the surgical margins were histologically negative for tumor. The tumor had the classic histologic appearance of a benign GCTB. A year after the amputation, the patient developed pulmonary metastasis which was treated with pulmonary lobe resection. She is currently over 2.5 years postsurgical treatment of the primary lesion with no evidence of local recurrence or distant metastasis.
Keywords: Giant cell, Multicentric, Metacarpal, Tumor
Introduction
Giant cell tumors of bone (GCTB) have been historically classified as benign neoplasms; however, some recent classifications consider them to be low-grade malignant neoplasms, with a significant rate of local recurrence and an infrequent development of metastases, even in the most histologically benign-appearing cases [5, 8, 15, 21]. Simultaneous involvement of two adjacent metacarpals by a GCTB is very rare and, to our knowledge, has not been reported in the literature.
GCTB make up approximately 5 % of the primary bone tumors in the USA and up to 20 % of the primary bone tumors in Asia [8, 19]. They usually occur in young adults (ages 25–40), have a slight female predominance, and are very rare in children [1]. The tumors are usually found in the epiphysis, with or without extension into the metaphysis, of the distal femur, proximal tibia, distal radius, proximal humerus, or sacrum. They are rare in the hand, and if they do occur in the hand, they, most commonly, involve the metacarpals [2, 9, 11]. GCTB are unifocal in 99 % of the cases, and less than 1 % of cases are multicentric. The multicentric tumors tend to occur in younger patients, have a propensity for the small bones of hands and feet, and may act more aggressively [7–9]. In extremely rare cases, GCTB may transform into a frank sarcoma that is almost always high grade. When this occurs, the tumor is then referred to as a “malignant GCTB” and has a high risk of metastases and a poor prognosis [5, 16]. Benign GCTB, primary malignant GCTB, and secondary malignant GCTB are all distinct different lesions. The use of the phrase “malignant GCTB” should not detract from the fact that every conventional GCTB has the ability to metastasize.
Grossly, GCTB are tan brown, soft, and solid with fibrous bands; often surrounded by a thin partial rim of reactive bone; and may be associated with fracture. The classic histologic appearance of GCTB is a combination of osteoclast-like giant cells with two types of mononuclear cells. The two types of mononuclear cells are round and spindled. The round mononuclear cells, together with the osteoclast-like giant cells, are reactive specialized benign cells derived from a circulating monocyte lineage and are recruited to the tumor by the spindled mononuclear cells. The spindled mononuclear cells are the only true neoplastic cells in GCTB and are probably derived from primitive mesenchymal stromal cells that develop fibroblast/osteoblast-like features [21]. The primary signaling mechanism that controls tumor growth includes receptor activator of nuclear factor kappa B (RANK) and RANK ligand (RANKL) [8, 15, 18]. Targeted therapy for GCTB consists of monoclonal antibodies to RANKL (denosumab) [1]. The most common chromosomal abnormality is a bonding together of telomeres (“telomeric fusion”) from various separate chromosomes [8, 15].
Case Report
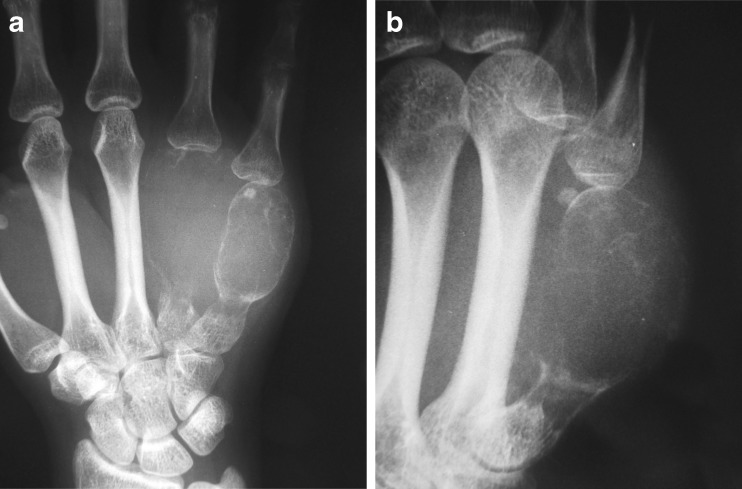
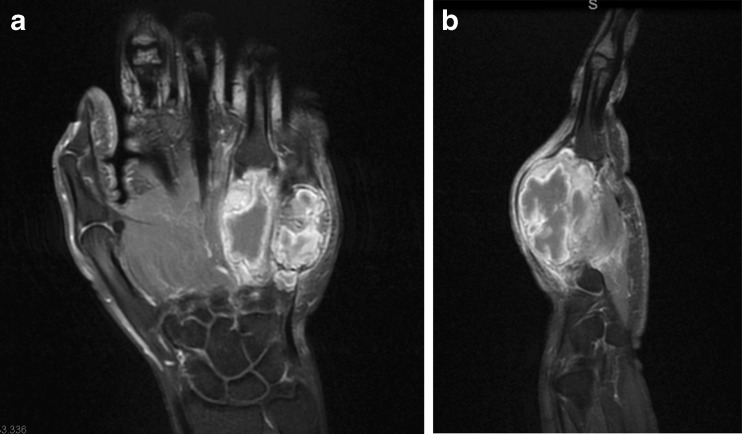
A 25-year-old right-hand dominant woman presented with severe swelling and pain of the dorsal aspect of the left non-dominant hand 1 week after an injury that she had sustained at home. The patient denied any swelling or discomfort of her hand prior to that injury. Clinical examination revealed a large, tender swelling of the dorsoulnar aspect of the left hand (Fig. 1). Plain radiographs demonstrated large osteolytic lesions occupying most of the ring and little finger metacarpals with marked cortical expansion. The tumors extended to the metacarpophalangeal joints (Fig. 2). An MRI of the hand revealed mixed signal intensity and multiple fluid–fluid levels within the expansile destructive metacarpal lesions which also showed peripheral and septal contrast enhancement (Fig. 3). The initial radiological impression was that of an anuerysmal bone cyst or telangiectatic osteosarcoma.
Fig. 1.
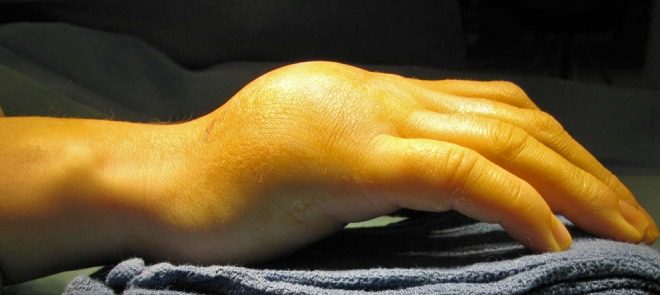
Large mass on the dorsoulnar aspect of the non-dominant left hand
Fig. 2.
Plain radiography. a PA and b oblique magnified views demonstrating markedly lytic and expansile, central juxta-articular tumors of the fourth and fifth metacarpal bones
Fig. 3.
MRI of the a coronal and b sagittal regions. Contrast-enhanced fat-suppressed T1-weighted images show intensely enhancing solid and cystic metacarpal tumors
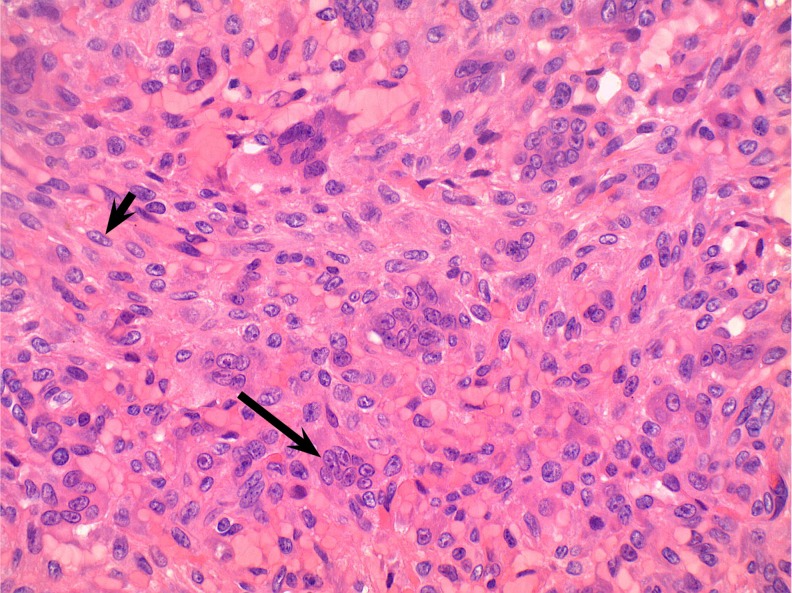
An incisional biopsy identified the presence of giant cell tumor. Several options were discussed with the patient including wide local excision with reconstruction and ray amputation. Because of the destructive behavior of the tumor and the simultaneous involvement of two metacarpals, we made the decision to proceed with ray amputation of the two involved digits. Intraoperatively, the fourth and fifth metacarpals were found to be almost completely replaced by a cystic tumor full of bloody fluid mixed with friable soft tissue (Fig. 4). The tumor invaded the heads of the metacarpals including the articular surfaces, but parts of the bases of the metacarpals were still intact. The third metacarpal was completely normal. Pathological examination of the specimen identified a giant cell tumor with the classic histologic appearance with no evidence of malignancy (Fig. 5). The patient did very well postoperatively and returned to her original work as a custodian 4 weeks postoperatively. Additional work-up including PET scan and CT scan of the chest did not show any evidence of anymore lesions in the bones or lungs.
Fig. 4.
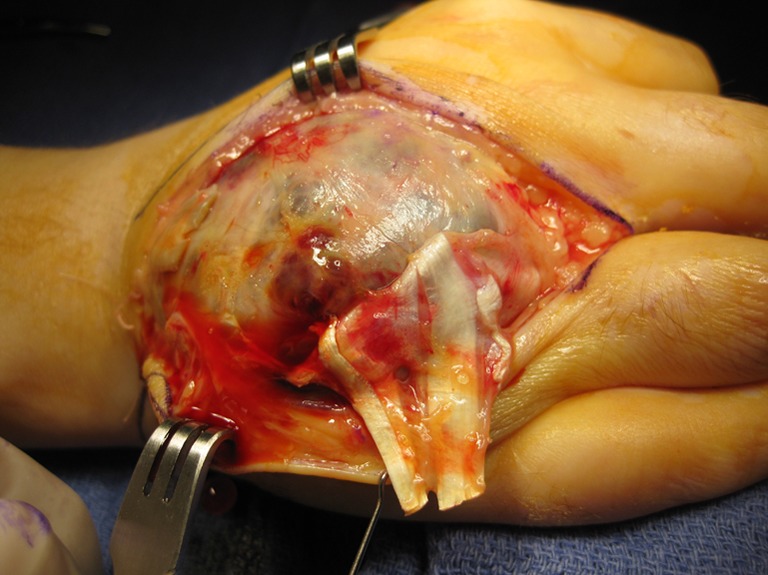
Intra-operative photograph showing the large, mostly cystic, tumors of the fourth and fifth metacarpals
Fig. 5.
Histologic appearance of the giant cell tumor of bone. Long arrow multinucleated giant cell. Short arrow spindled mononuclear neoplastic cell (×400)
One year postoperatively, the patient developed two pulmonary lesions identified on PET and computed tomography imaging. Open video-assisted thoracic surgery (VATS) biopsy confirmed the presence of giant cell tumor that was histologically similar to the primary hand lesion. The patient was treated with the RANKL inhibitor denosumab followed by VATS resection of the two pulmonary nodules. The patient is now 2 years and 7 months after her original surgery with no evidence of local recurrence or metastatic disease as evidenced by MRI of the hand, chest X-rays, CT scan, and PET scan.
Discussion
This case represents a very rare involvement of two adjacent metacarpals with giant cell tumor. In general, the behavior of individual GCTB is notoriously difficult to predict. Unlike many other tumors in the body, a single isolated atypical histologic feature (e.g., nuclear atypia, focal pleomorphism, necrosis, increased mitotic rate, or tumor masses within blood vessels) cannot predict tumor behavior [8, 15].
Surgical management of GCTB involving the metacarpals ranges from curettage to wide local excision to amputation. Curettage is associated with a high incidence of recurrence and is not recommended [3]. Reconstruction following wide local excision may be performed with autogenous vascularized [6] or non-vascularized bone grafts [2, 14], or allografts [13]. Radiation therapy has also been described with encouraging results [17], although transformation to sarcoma in some cases can occur many years after radiation therapy. In our patient, wide local excision and reconstruction was not deemed to be adequate, given the aggressive nature of the lesions and the simultaneous involvement of two metacarpals.
A significant number of GCTB recur locally. The type of initial surgical procedure, such as curetting or wide local excision, is the most important predictor of recurrence, with recurrence rates of 34–37 % and 5–7 % respectively [9, 10].
Approximately 2–5 % of GCTB will metastasize, most often to the lungs [14]. Almost all of the cases with metastases have been associated with previous surgery, raising the possibility that surgical procedures themselves can cause mechanical vascular disruption, allowing tumor cells to “seed” the lungs [12, 15]. For this reason, the presence of a GCTB pulmonary metastasis does not have the same clinical significance as most other metastatic neoplasms. Many GCTB lung metastases are indolent and stable (sometimes called “benign pulmonary implants”). Other GCTB lung metastases may spontaneously regress, whereas still other GCTB lung metastases may rapidly progress and can cause death.
One of the main features of GCTB is osteoclast-mediated bone resorption. Therefore, inhibition of osteoclast differentiation and function is a primary therapeutic target. RANKL expression is increased in GCTB stromal cells. Denosumab, a fully human monoclonal antibody against RANKL, inhibits RANKL-mediated bone resorption. By binding to RANKL, it disrupts the cycle of bone destruction. Clinical studies have demonstrated a promising effect on controlling the disease and the symptoms with the use of denosumab especially in advanced or metastatic disease [19, 20].
Various classifications of GCTB have been proposed over the years. Some classifications have been based upon the histologic appearance of the tumors, whereas others have been based upon the clinical and radiologic appearance of the tumors. The histologic grading systems have had limited usefulness due to their inability to accurately predict tumor behavior in the vast majority of cases. On the other hand, initial staging classifications may provide useful prognostic information [4]. Campanacci et al. proposed one of the most common classifications, which divided GCTB into three grades: grade I refers to intraosseous tumors, grade II refers to larger intraosseous tumors with a thin (but still intact) bony cortex, and grade III refers to tumors that have invaded through the cortex into surrounding soft tissue [6].
The initial grade of GCTB correlates well with rates of local recurrence and metastases, with higher grades associated with higher rates of local recurrence and metastases. It is very important for GCTB to be initially excised with negative surgical margins, followed by careful pathologic examination of multiple sections to exclude a malignant transformation within the tumor. In addition, clinical follow-up is advisable for every GCTB, regardless of the histologic appearance, to monitor for local recurrence or metastases. The use of MRI, PET scans, and CT scans of the chest would be helpful to rule out local recurrence or distant metastasis.
Acknowledgments
Conflict of interest
Nash H. Naam declares that he has no conflict of interest.
Steven L. Jones declares that he has no conflict of interest.
Justin Floyd declares that he has no conflict of interest.
Esat I. Memisoglu declares that he has no conflict of interest.
Statement on human rights
We hereby testify that all procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from the patient for being included in the study.
Informed consent
A written informed consent for publication was obtained from the patient mentioned in this case report.
References
- 1.Arroud M, Afifi MA, Chbani L, Bouabdallah Y. Giant-cell tumor of the fourth metacarpal bone in children: case report. J Pediatr Orthop B. 2010;19(1):86–89. doi: 10.1097/BPB.0b013e328332b8a5. [DOI] [PubMed] [Google Scholar]
- 2.Athanasian EA, Wold LE, Amadio PC. Giant cell tumors of the bones of the hand. J Hand Surg [Am] 1997;22(1):91–98. doi: 10.1016/S0363-5023(05)80187-X. [DOI] [PubMed] [Google Scholar]
- 3.Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg [Am] 1980;5(1):39–50. doi: 10.1016/S0363-5023(80)80042-6. [DOI] [PubMed] [Google Scholar]
- 4.Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520–2529. doi: 10.1002/cncr.11359. [DOI] [PubMed] [Google Scholar]
- 5.Bertoni F, Present D, Enneking WE. Giant cell tumor of bone with pulmonary metastases. J Bone Joint Surg Am. 1985;67(6):890–900. [PubMed] [Google Scholar]
- 6.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106–114. [PubMed] [Google Scholar]
- 7.Dhillon MS, Prasad P. Multicentric giant cell tumour of bone. Acta Orthop Belg. 2007;73(3):289–299. [PubMed] [Google Scholar]
- 8.Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization classification of tumours: pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. pp. 310–313. [Google Scholar]
- 9.Hoch BL, Inwards C, Sundaram M, Rosenberg AE. Multicentric giant cell tumor of bone: clinicopathologic analysis of thirty cases. J Bone Joint Surg Am. 2006;88(9):1998–2008. doi: 10.2106/JBJS.E.01111. [DOI] [PubMed] [Google Scholar]
- 10.McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235–242. [PubMed] [Google Scholar]
- 11.Minhas MS, Mehboob G, Ansari I. Giant cell tumours in hand bones. J Coll Physicians Surg Pak. 2010;20(7):460–463. [PubMed] [Google Scholar]
- 12.Moon JC, Kim SR, Chung MJ, Lee YC. Multiple pulmonary metastases from giant cell tumor of the hand. Am J Med Sci. 2012;343(2):171–173. doi: 10.1097/MAJ.0b013e31823483e1. [DOI] [PubMed] [Google Scholar]
- 13.Patradul A, Kitidumrongsook P, Parkpian V, Ngarmukos C. Allograft replacement in giant cell tumour of the hand. Hand Surg. 2001;6(1):59–65. doi: 10.1142/S0218810401000552. [DOI] [PubMed] [Google Scholar]
- 14.Rock MG, Pritchard DJ, Unni KK. Metastases from histologically benign giant-cell tumor of bone. J Bone Joint Surg Am. 1984;66(2):269–274. [PubMed] [Google Scholar]
- 15.Rosai J. Rosai and Ackerman's surgical pathology. 10. Edinburgh: Mosby Elsevier; 2011. pp. 2043–2046. [Google Scholar]
- 16.Sanerkin NG. Malignancy, aggressiveness, and recurrence in giant cell tumor of bone. Cancer. 1980;46(7):1641–1649. doi: 10.1002/1097-0142(19801001)46:7<1641::AID-CNCR2820460725>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Singhal RM, Mukhopadhyay S, Tanwar RK, Pant GS, Julka PK. Case report: giant cell tumour of metacarpals: report of three cases. Br J Radiol. 1994;67(796):408–410. doi: 10.1259/0007-1285-67-796-408. [DOI] [PubMed] [Google Scholar]
- 18.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20(3):345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 19.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, Dansey R, Jun S. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 20.Thomas DM, Skubitz T. Giant cell tumors of bone. Curr Opin Oncol. 2009;21(4):338–344. doi: 10.1097/CCO.0b013e32832c951d. [DOI] [PubMed] [Google Scholar]
- 21.Wülling M, Engels C, Jesse N, Werner M, Delling G, Kaiser E. The nature of giant cell tumor of bone. J Cancer Res Clin Oncol. 2001;127(8):467–474. doi: 10.1007/s004320100234. [DOI] [PMC free article] [PubMed] [Google Scholar]