Abstract
The recent recognition of receptor-mediated ATP signalling as a pathway of epithelial pro-inflammatory cytokine release challenges the ubiquitous role of the TLR4 pathway during urinary tract infection. The aim of this study was to compare cellular responses of renal epithelial cells infected with uropathogenic Escherichia coli (UPEC) strain IA2 to stimulation with ATP-γ-S. A498 cells were infected or stimulated in the presence or absence of apyrase, that degrades extracellular ATP, or after siRNA-mediated knockdown of ATP-responding P2Y2 receptors. Cellular IL-8 release and global gene expression were analysed. Both IA2 and A498 cells per se released ATP, which increased during infection. IA2 and ATP-γ-S caused a ∼5-fold increase in cellular release of IL-8 and stimulations performed in the presence of apyrase or after siRNA knockdown of P2Y2 receptors resulted in attenuation of IA2-mediated IL-8 release. Microarray results show that both IA2 and ATP-γ-S induced marked changes in gene expression of renal cells. Thirty-six genes were in common between both stimuli, and many of these are key genes belonging to classical response pathways of bacterial infection. Functional analysis shows that 88 biological function-annotated cellular pathways were identical between IA2 and ATP-γ-S stimuli. Results show that UPEC-induced release of IL-8 is dependent on P2Y2 signalling and that cellular responses elicited by UPEC and ATP-γ-S have many identical features. This indicates that renal epithelial responses elicited by bacteria could be mediated by bacteria- or host-derived ATP, thus defining a key role of ATP during infection.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-014-9414-7) contains supplementary material, which is available to authorized users.
Keywords: Urinary tract infection, Host response, Adenosine triphosphate, Uropathogenic E. coli, Purinergic P2Y receptors
Introduction
The uroepithelial cells lining the urinary tract constitute a fundamental barrier against invading bacteria. In addition to its mechanical protection, it also displays both specific responses, through activation of Toll-like receptors (TLR) and, more unspecific, through responses to damage (or danger)-associated molecular pattern (DAMP) molecules. DAMPs are molecules that initiate and maintain non-infectious inflammatory response, and many of these are intracellular proteins released or leaked during tissue injury. However, DAMPs also include non-protein molecules such as the nucleotide ATP [1] that are released during stress or damage. Extracellular ATP in the urinary tract function as an endogenous danger signal [2], and urinary levels of ATP are used as a parameter to differentiate bacteriuria from abacteriuria [3]. In the extracellular environment, ATP binds to purinergic P2 receptors, a versatile class of P2Y receptors (P2Y1, 2, 4, 6, 8 and 11–13) and P2X receptors (P2X1–7) [4]. These are present on the surface of many cells, and the urothelium has been shown to express several P2X and P2Y receptors [5]. In addition to being released from uroepithelial cells, ATP has also recently been shown to be released by uropathogenic Escherichia coli (UPEC) per se to the surrounding media during a urinary tract infection (UTI) [6]. Due to rapid diffusion and hydrolysis of ATP [7], the levels of ATP measured in media greatly underestimate quantities that are actually released at the cell surface. ATP-mediated cell activation is generally terminated by surface-bound ectonucleotidases that are expressed on cells [8]. It has been shown that ectonucleotidase-deficient mice and humans with decreased expression exhibit exacerbated inflammatory responses [9, 10]. Evidence that P2 nucleotide receptors directly modulate phagocytosis, chemotaxis and cytokine production [11–13] further augments the importance of ATP in immune responses.
Bacterial lipopolysaccharide is known to activate TLR4 to initiate signalling cascades that ultimately induce expression of various inflammation-associated genes. Recently, a non-TLR pathway has emerged that may function as a more general pathway for chemokine and cytokine release during infection. It was shown that release of IL-8 and IL-6, generally attributed to TLR4 activation during UTI, can be caused by ATP-induced P2Y2 activation [6, 14]. The recognition of this more general or alternative pathway confronts the ubiquitous role of the classical TLR4 pathway for urothelial inflammatory cell recruitment during UTI.
Taken together, it may be hypothesised that UPEC-mediated cellular release of IL-8 and other key chemokines/cytokines during UTI can also be mediated by ATP acting on P2 receptors that detect the presence of bacterial ATP and/or TLR-potentiated release of endogenous ATP. Therefore, the aim of our study were to examine the functional role of ATP in IL-8 release in response to bacterial infection and to study the similarities in global gene expression responses of renal epithelial cells to both UPEC and ATP stimuli.
Material and methods
Renal epithelial cells and bacteria
The human renal epithelial cell line A498 (HTB-44; American Tissue Culture Collection (ATCC), Manassas, VA, USA) was chosen on the basis that P2 receptor expression and ATP-mediated activation of this cell line has previously been characterised [6, 14], with the conclusion of the latter study that P2Y2 receptors are responsible for ATP-mediated release of pro-inflammatory cytokines in these cells. The A498 cell line was routinely checked to be uncontaminated with respect to mycoplasma. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10 % foetal bovine serum (FBS), 2 mM l-glutamine, 1 mM non-essential amino acids, and 100 U/mL penicillin and 100 μg/mL streptomycin (PEST) (all obtained from Invitrogen Ltd, Paisley, UK) at 37 °C in a humidified atmosphere with 5 % CO2. Prior to bacterial infection, confluent cells were incubated with 2 % FBS and gentamicin (50 μg/mL; Invitrogen) for 12 h in order to limit bacterial multiplication during infection. During stimulation, cells were cultured in medium with 2 % FBS without gentamicin.
The uropathogenic E. coli strain IA2, originally isolated from a patient with acute pyelonephritis, was grown on tryptic soy agar (TSA) plates (Becton, Dickinson and Company, Sparks, MD) at 37 °C.
Stimulation and measurement of ATP
Cell culture media from A498 cells was collected after 1 and 2 h of stimulation with IA2 (108 CFU/ml). Uninfected cells were used as controls, and, in order to evaluate ATP levels generated by UPEC per se, IA2 experiments were also performed in the absence of A498 cells. Collected medium was centrifuged at 5,000×g for 5 min, and ATP levels of supernatant determined directly with an ATP Determination Kit (A22066, Invitrogen) measured on a FLUOstar Optima instrument (BMG Labtechnologies, Germany).
Stimulation and measurement of IL-8
Stimulation was performed during 5 h with adenosine 5′-O-(3-thiotriphosphate) (ATP-γ-S; 20 or 100 μM; Roche Diagnostics) alone or in combination with IA2 (108 CFU/mL) or with lipopolysaccharide (LPS; 1 μg/mL; E. coli serotype O127:B8; Sigma-Aldrich) or IL-1β (10 ng/mL; Sigma-Aldrich). Experiments were also conducted in the presence of apyrase (2U; Sigma-Aldrich) and after P2Y2 knockdown. Medium alone, vehicle and scrambled siRNA transfection were used as controls.
Analysis of IL-8 was performed with BD OptEIATM Human IL-8 ELISA kit II (BD Biosciences Pharmingen San Diego, USA) measured in a Multiskan Ascent (Thermo Labsystems, Helsingfors, Finland).
siRNA knockdown
Prior to transfection, human anti-P2Y2 siRNA (P2Y2 Silencer Select Validated; s9966; Ambion, Life Technologies, Carlsbad, CA, USA) or scrambled negative control siRNA (AllStars Negative Control; Qiagen) was incubated with Lipofectamine 2000 (Invitrogen) in antibiotic- and serum-free media at room temperature according to manufacturer instructions. Complexes of siRNA and lipofectamine, with a final concentration of 10 nM siRNA, were added to wells in 24-well plates followed by reverse transfection of A498 cells during 24 h. After transfection, media were replaced followed by incubation for a total of 72 or 96 h before messenger RNA (mRNA) isolation or stimulation.
Real-time RT-PCR
Total RNA was isolated using RNeasy Mini Kit (Qiagen) according to manufacturer instructions. Concentration and purity of RNA were determined with a NanoDrop analyser (NanoDrop ND-1000, Wilmington, USA) and then reverse transcribed to complementary DNA (cDNA) using oligo(dT)16 primers (Applied Biosystems, Forester City, USA) and Omniscript RT Kit (Qiagen). Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed according to manufacturer instructions using SsoFast EvaGreen Supermix with added ROX (Bio-Rad Laboratories, Hercules, CA, USA) and QuantiTect Primer Assays (Qiagen) for P2Y2 (Hs_P2RY2_SG), beta-actin (ACTB; Hs_ACTB_SG) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hs_GAPDH_SG). Real-time RT-PCR was performed in a 7900HT Fast Real-time RT-PCR system (Applied Biosystems) followed by melt curve analysis. The efficiency of all primers was between 105 and 107 %.
Microarray analysis
Integrity of RNA (RIN) was analysed with a 2100 Bioanalyzer in conjunction with RNA 6000 Nano LabChip kit (Agilent). High-quality total RNA (RIN > 9) was used to prepare labelled cRNA with Low Input Quick Amp Labelling Kit (Agilent) according to manufacturer instructions. Dye swap was performed within each dual-colour hybridisation pair and hybridisation to G4112F Whole Human Genome Oligo Microarrays 4×44K (Agilent) was performed in a G2545A Hybridization Oven (Agilent) according to manufacturer instructions. Microarrays were scanned with a G2565 CA array laser scanner (Agilent) followed by image analysis and data extraction with Feature Extraction Software version 10.7.3.1 (Agilent).
Calculations, statistical analysis and microarray data processing
Non-microarray data is shown as mean ± standard error of the mean (SEM) with n indicating number of independent experiments. All statistical analysis was performed on raw data, prior to percentage of control transformation, with analysis of variance (randomised block ANOVA) followed by Holm-Sidak post hoc test (SigmaPlot 11.0, SPSS Science, Chicago, USA). Statistical significance at p ≤ 0.05, ≤0.01 and ≤0.001 is indicated with *, ** and ***, respectively.
Microarray data analysis was performed using GeneSpring GX version 12.1 (Agilent) after per chip and gene normalisation. Significantly different expressions were extracted by volcano plot analysis with a corrected p value <0.05 (t test followed by Benjamini-Hochberg multiple testing correction) and a fold change >2. Significant GO term enrichment was set at a corrected p value <0.05 (Benjamini-Yekutieli multiple testing correction). The direct entity relationship network analysis was generated through Ingenuity Pathway Analysis (IPA, Ingenuity Systems, www.ingenuity.com) with GeneSpring-derived entity lists. IPA interaction analysis of gene entities was restricted to direct interactions between entities. Identified direct relationship networks represent molecular biological relationships, supported by references from literature and canonical information stored in the Ingenuity Knowledge Base (per July 2012).
Results
IA2 and A498 release ATP upon infection
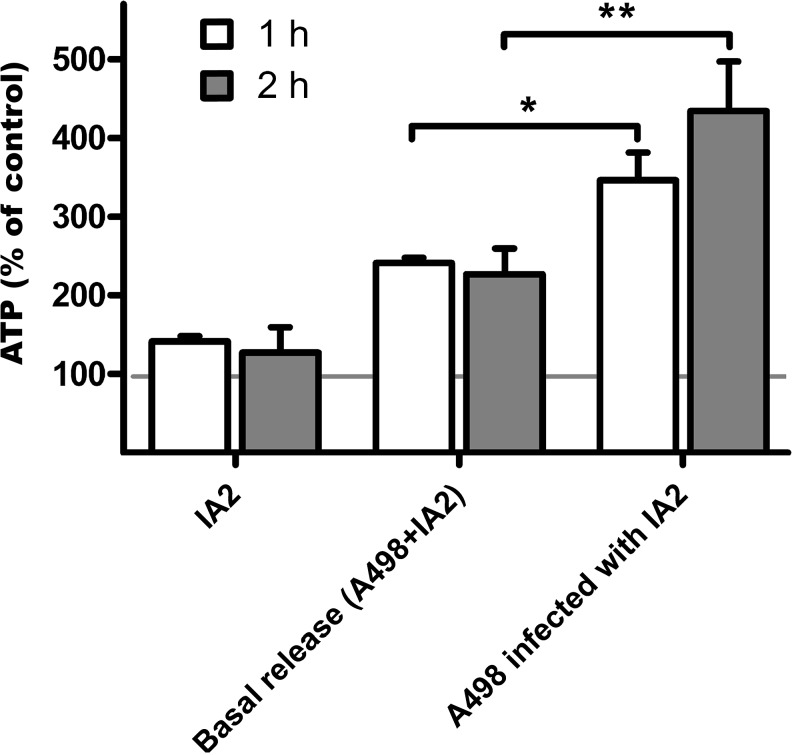
ATP was released by both IA2 and A498 cells alone prior to infection. However, during infection, the release of ATP was higher than the sum of IA2 and A498 cells alone (Fig. 1). The effect of ATP-γ-S stimulation on endogenous release of ATP was masked by ATP-γ-S ability to act as a substrate in the luciferase reaction.
Fig. 1.
ATP release by UPEC strain IA2 and by A498 upon IA2 infection. ATP measured in supernatant after 1 and 2 h IA2 (108 CFU/mL) stimulation of A498 cells. Uninfected A498 cells were used as controls, and IA2 alone, in absence of A498 cells, was used in order to evaluate ATP levels generated by UPEC per se. The basal release is the sum of both uninfected A498 cells and IA2 alone. Data are expressed as mean percentage of control ± SEM with control (uninfected A498 cells) shown as the horizontal line at 100 % corresponding to 1.2 pM ATP. Asterisk represents significance compared to basal release from A498 cells and IA2 alone (n = 4)
IL-8 is released in response to IA2 and/or ATP-γ-S
Stimulation with IA2 or ATP-γ-S alone caused a four- and sixfold increase, respectively, in IL-8 release from A498 cells compared to controls. The combination of IA2 and ATP-γ-S gave a potentiating effect on the release of IL-8 with a 15-fold increase compared to controls (Fig. 2). This effect was higher than the added effects of IA2 and ATP-γ-S; 4,553 ± 782 vs. 2,955 ± 454 pg/mL (p = 0.04; t test).
Fig. 2.
IA2- and/or ATP-γ-S-induced release of IL-8. IL-8 measured in supernatant after 5 h IA2 (108 CFU/mL) and/or ATP-γ-S (20 μM) stimulation of A498 cells in the absence or presence of apyrase (2 U/mL) in the cell culture media. Data are expressed as mean ± SEM. Asterisk represents significance compared to control unless indicated by brackets (n = 3)
P2Y2-receptor is required for IL-8 release
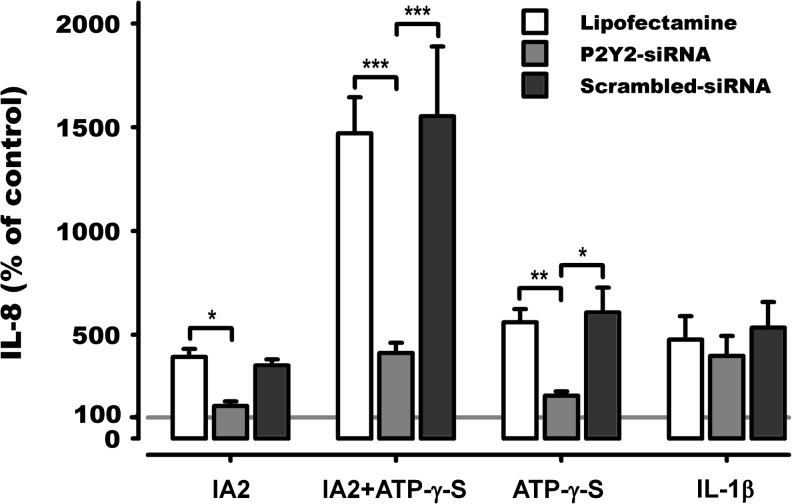
In order to test the effect of released ATP during IA2 stimulation, apyrase, an enzyme that hydrolyses ATP to AMP, was added to the medium during experiments. Apyrase completely removed extracellular ATP (data not shown) and caused a significant decrease in IL-8 released from cells stimulated with the combination of IA2 and ATP-γ-S and with ATP-γ-S alone (Fig. 2).
To further test the influence of ATP signalling during UPEC infection, the effect of IA2-mediated IL-8 release was studied after knockdown of P2Y2 receptors. The lipofectamine-mediated siRNA transfection resulted in 78 ± 4 % knockdown of P2Y2 mRNA after 72 h with no decrease in cell viability compared to lipofectamine alone or lipofectamine/scrambled siRNA transfection. In addition, this knockdown of P2Y2 receptor expression has previously been confirmed to not decrease other candidate receptors for ATP-dependent responses in A498 cells, i.e. P2Y11 and P2X7 [14]. The knockdown resulted in a threefold decrease in the responsiveness to either IA2 or ATP-γ-S alone and a fourfold decrease in the responsiveness to the combination of these (Fig. 3) when compared to lipofectamine alone or lipofectamine/scrambled siRNA-transfected cells. In order to verify that the general responsiveness of transfected cells against non-P2Y2-related stimuli was not affected by the knockdown, cells were stimulated with IL-1β. IL-1β-mediated release of IL-8 was not affected by knockdown of P2Y2 (Fig. 3). Thus, P2Y2 receptor activation had a major influence on IL-8 release during IA2 infection.
Fig. 3.
IA2- and/or ATP-γ-S-induced release of IL-8 after P2Y2 knockdown. IL-8 measured in supernatant after 5 h IA2 (108 CFU/mL) and/or ATP-γ-S (20 μM) or IL-1β (10 ng/mL) stimulation of A498 cells. Cells had, prior to stimulation, been transfected with either lipofectamine alone or in combination with P2Y2 specific siRNA. Data was normalised to obtain the actual effect of P2Y2 knockdown and are expressed as mean percentage of control ± SEM with control shown as the horizontal line at 100 %. Asterisk represents significance between groups indicated by brackets (n = 3)
Gene expression changes in response to IA2 and/or ATP-γ-S
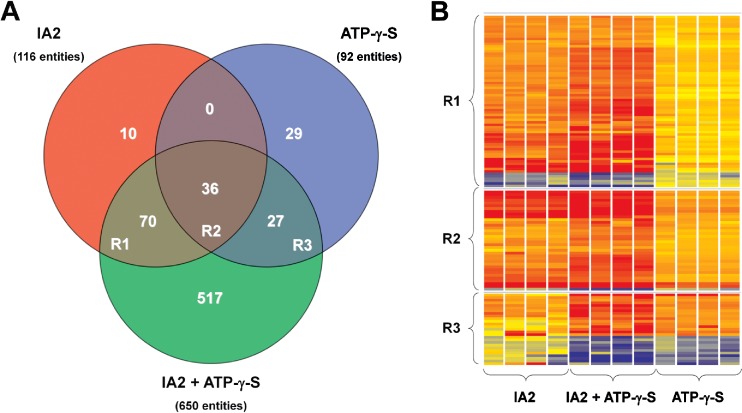
For a more in-depth study of similarities in IA2- and ATP-γ-S-mediated host responses, microarray analysis was performed on mRNA isolated from cells stimulated with either IA2 or ATP-γ-S alone or with the combination. Differentially expressed gene entities within each treatment were categorised using Venn diagram and heat maps as shown Fig. 4, and selections of altered gene entities are shown in Tables 1, 2, 3 and 4. Both IA2 and ATP-γ-S induced distinct changes in gene expressions with 116 and 92 altered gene expressions, respectively (Fig. 4). Of these, 36 gene entities, region R2 of Fig. 4, were shared between IA2 and ATP-γ-S stimulus, and a selection of 24 is shown in Table 1. Twenty of the 36 shared gene entities belong to and were enriched in the gene ontology class “response to chemical stimuli” (p = 8.84E-5), and 12 of these were also enriched in the gene ontology class “response to bacterium” (p = 5.372E-9). In general, IA2 yielded higher fold changes than ATP-γ-S.
Fig. 4.
Venn diagram and heat maps of gene entities with altered expressions. Venn diagram of all altered gene expressions in A498 cells after 4 h IA2 (108 CFU/mL) and/or ATP-γ-S (20 μM) stimulus. The shared entities are referred to in text as R1, R2 and R3 of the Venn diagram (n = 4 in each treatment group)
Table 1.
Gene expression changes induced by IA2, ATP-γ-S or by the combination of IA2 and ATP-γ-S
| Gene symbol | IA2 | IA2 ± ATP-γ-S | ATP-γ-S | Gene name | |||
|---|---|---|---|---|---|---|---|
| FC | p value | FC | p value | FC | p value | ||
| CCL20 | 72.2 | 0.002 | 146.1 | 0.002a, b | 8.2 | 0.003 | Chemokine (C-C motif) ligand 20 |
| IL8 | 35.4 | 0.002 | 49.5 | 0.003b | 7.9 | 0.001 | Interleukin 8 |
| PTX3 | 12.9 | 0.002 | 49.3 | 0.002a, b | 5.2 | 0.000 | Pentraxin 3, long |
| CXCL3 | 22.8 | 0.002 | 28.1 | 0.002b | 3.3 | 0.004 | Chemokine (C-X-C motif) ligand 3 |
| CCL2 | 6.9 | 0.002 | 19.9 | 0.002a, b | 4.7 | 0.001 | Chemokine (C-C motif) ligand 2 |
| LTB | 9.3 | 0.002 | 18.1 | 0.003b | 3.2 | 0.001 | Lymphotoxin beta (TNF superfamily, member 3) |
| TNFAIP3 | 8.6 | 0.002 | 16.8 | 0.002b | 3.7 | 0.001 | Tumour necrosis factor, alpha-induced protein 3 |
| CXCL1 | 13.7 | 0.002 | 16.5 | 0.002b | 3.5 | 0.003 | Chemokine (C-X-C motif) ligand 1 |
| TNF | 19.6 | 0.002 | 14.0 | 0.018b | 2.6 | 0.003 | Tumour necrosis factor |
| SOD2 | 12.2 | 0.002 | 12.5 | 0.002b | 2.3 | 0.004 | Superoxide dismutase 2, mitochondrial |
| INHBA | 4.0 | 0.002 | 12.2 | 0.005a, b | 3.1 | 0.003 | Inhibin, beta A |
| CXCL2 | 13.4 | 0.002 | 12.0 | 0.002b | 2.9 | 0.000 | Chemokine (C-X-C motif) ligand 2 |
| IL6 | 6.7 | 0.002 | 11.2 | 0.002b | 3.2 | 0.001 | Interleukin 6 |
| TRAF1 | 4.4 | 0.003 | 11.1 | 0.002a, b | 3.1 | 0.002 | TNF receptor-associated factor 1 |
| ICAM1 | 4.3 | 0.002 | 9.2 | 0.002b | 2.4 | 0.001 | Intercellular adhesion molecule 1 |
| CSF2 | 5.5 | 0.002 | 8.0 | 0.002 | 3.6 | 0.000 | Colony stimulating factor 2 |
| CXCL10 | 5.0 | 0.006 | 8.0 | 0.002b | 2.2 | 0.004 | Chemokine (C-X-C motif) ligand 10 |
| AQP9 | 2.1 | 0.002 | 6.5 | 0.002a, b | 2.1 | 0.040 | Aquaporin 9 |
| SAA4 | 2.5 | 0.004 | 5.3 | 0.003a, b | 2.1 | 0.014 | Serum amyloid A4, constitutive |
| NFKBIA | 4.1 | 0.002 | 5.1 | 0.003b | 2.2 | 0.001 | Nucl. fact. of κ light polypep. gene enhan. in B-cells inhib, α |
| BIRC3 | 2.6 | 0.003 | 5.0 | 0.002b | 2.3 | 0.002 | Baculoviral IAP repeat containing 3 |
| TNFSF14 | 3.3 | 0.005 | 4.4 | 0.002b | 2.0 | 0.020 | Tumour necrosis factor (ligand) superfamily, member 14 |
| NFKBID | 2.4 | 0.002 | 3.1 | 0.004 | 2.1 | 0.003 | Nucl. fact. of κ light polypep. gene enhan. in B-cells inhib, δ |
| CCL11 | 2.1 | 0.075 | 3.0 | 0.018 | 2.2 | 0.061 | Chemokine (C-C motif) ligand 11 |
FC fold change vs. unstimulated controls, p Benjamini-Yekutieli multiple testing corrected p value
aSignificant vs. IA2; n = 4
bSignificant vs. ATP; n = 4
Table 2.
Gene expression changes induced specifically by IA2
| Gene symbol | FC | p value | Gene name |
|---|---|---|---|
| ICAM4 | 7.4 | 0.003 | Intercellular adhesion molecule 4 |
| SERPINA3 | 7.2 | 0.003 | Serpin peptidase inhibitor, member 3 |
| EGR2 | 4.9 | 0.007 | Early growth response 2 |
| MMP3 | 4.6 | 0.002 | Matrix metallopeptidase 3 |
| SELE | 4.1 | 0.017 | Selectin E |
| VCAM1 | 3.6 | 0.002 | Vascular cell adhesion molecule 1 |
| PTGS2 | 3.6 | 0.002 | Prostaglandin-endoperoxide synthase 2 |
| CEBPD | 3.0 | 0.002 | CCAAT/enhancer binding prot. (C/EBP), delta |
| JUNB | 3.0 | 0.002 | jun B proto-oncogene |
| BDKRB1 | 2.9 | 0.002 | Bradykinin receptor B1 |
| KLF6 | 2.9 | 0.004 | Kruppel-like factor 6 |
| PNRC1 | 2.9 | 0.002 | Proline-rich nuclear receptor coactivator 1 |
| IFNB1 | 2.6 | 0.003 | Interferon, beta 1, fibroblast |
| BDKRB2 | 2.5 | 0.003 | Bradykinin receptor B2 |
| TNIP3 | 2.5 | 0.003 | TNFAIP3 interacting protein 3 |
| SOCS3 | 2.5 | 0.002 | Suppressor of cytokine signalling 3 |
| NCOA7 | 2.4 | 0.004 | Nuclear receptor coactivator 7 |
| CX3CL1 | 2.4 | 0.003 | Chemokine (C-X3-C motif) ligand 1 |
| TNFRSF9 | 2.3 | 0.002 | Tumour necrosis factor receptor superfamily, member 9 |
| MMP1 | 2.2 | 0.007 | Matrix metallopeptidase 1 |
| IRF1 | 2.2 | 0.004 | Interferon regulatory factor 1 |
| DUSP1 | 2.2 | 0.006 | Dual specificity phosphatase 1 |
| NFKBIE | 2.2 | 0.003 | Nuclear fact kappa light gene enh B-cell inhib, eps |
| RELB | 2.1 | 0.002 | v-rel reticuloendotheliosis viral oncog. homolog B |
| CEBPE | 2.1 | 0.002 | CCAAT/enhancer binding prot. (C/EBP), epsilon |
| CLDN1 | 2.0 | 0.002 | Claudin 1 |
| IFNGR1 | 2.0 | 0.011 | Interferon gamma receptor 1 |
FC fold change vs. unstimulated controls, p Benjamini-Yekutieli multiple testing corrected p value (n = 4)
Table 3.
Gene expression changes induced specifically by ATP-γ-S
| Gene symbol | FC | p value | Gene name |
|---|---|---|---|
| NR4A3 | 5.0 | 0.006 | Nuclear receptor subfamily 4, group A, member 3 |
| IL24 | 2.9 | 0.003 | Interleukin 24 |
| BCL2A1 | 2.9 | 0.001 | BCL2-related protein A1 |
| EDN2 | 2.6 | 0.001 | Endothelin 2 |
| IL1B | 2.6 | 0.001 | Interleukin 1, beta |
| PTPRB | 2.4 | 0.013 | Protein tyrosine phosphatase, receptor type, B |
| F3 | 2.4 | 0.012 | Coagulation factor III |
| NFATC2 | 2.3 | 0.019 | Nucl. fact. of activated T cells, cytopl, calcineurin-dep 2 |
| HBEGF | 2.3 | 0.009 | Heparin-binding EGF-like growth factor |
| RASGRP3 | 2.2 | 0.014 | RAS guanyl releasing prot 3 (calcium and DAG-reg) |
| BTG3 | 2.1 | 0.001 | BTG family, member 3 |
| ASB2 | 2.0 | 0.001 | Ankyrin repeat and SOCS box containing 2 |
| PTGER4 | 2.0 | 0.001 | Prostaglandin E receptor 4 (subtype EP4) |
| EGR1 | −2.1 | 0.029 | Early growth response 1 |
| PLCH1 | −2.1 | 0.014 | Phospholipase C, eta 1 |
| VASN | −2.2 | 0.016 | Vasorin |
| BMF | −2.3 | 0.016 | Bcl2 modifying factor |
| AKAP5 | −2.6 | 0.019 | A kinase (PRKA) anchor protein 5 |
| CXXC4 | −3.5 | 0.010 | CXXC finger protein 4 |
FC fold change vs. unstimulated controls, p Benjamini-Yekutieli multiple testing corrected p value (n = 4)
Table 4.
Gene expression changes induced specifically by the combination of IA2 and ATP-γ-S
| Gene symbol | FC | p valuea | p valueb | Gene name |
|---|---|---|---|---|
| MMP13 | 8.3 | 0.013 | 0.015 | Matrix metallopeptidase 13 (collagenase 3) |
| CXCR4 | 4.9 | 0.006 | 0.001 | Chemokine (C-X-C motif) receptor 4 |
| CXCL9 | 4.8 | 0.013 | 0.029 | Chemokine (C-X-C motif) ligand 9 |
| ADORA2A | 4.2 | 0.005 | 0.000 | Adenosine A2a receptor |
| IFNAR2 | 3.9 | 0.003 | 0.001 | Interferon (alpha, beta and omega) receptor 2 |
| ICOSLG | 3.6 | 0.003 | 0.000 | Inducible T cell costimulator ligand |
| IRAK2 | 3.2 | 0.007 | 0.003 | Interleukin-1 receptor-associated kinase 2 |
| PPP3CC | 3.0 | 0.003 | 0.000 | Protein phosphatase 3, catalytic subunit, gamma isozyme |
| RRAD | 2.8 | 0.015 | 0.005 | Ras-related associated with diabetes |
| EFNA1 | 2.8 | 0.005 | 0.000 | Ephrin-A1 |
| GBP1 | 2.7 | 0.003 | 0.003 | Guanylate binding protein 1, interferon-inducible |
| IL12A | 2.7 | 0.009 | 0.038 | Interleukin 12A |
| FGF12 | 2.7 | 0.048 | 0.045 | Fibroblast growth factor 12 |
| SAA1 | 2.6 | 0.005 | 0.001 | Serum amyloid A1 |
| CD274 | 2.5 | 0.003 | 0.004 | CD274 molecule |
| DGKI | 2.5 | 0.009 | 0.006 | Diacylglycerol kinase, iota |
| NFKB1 | 2.5 | 0.002 | 0.005 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 |
| SOCS2 | 2.4 | 0.002 | 0.000 | Suppressor of cytokine signalling 2 |
| FOXO1 | 2.4 | 0.003 | 0.037 | Forkhead box O1 |
| SAA2 | 2.4 | 0.003 | 0.001 | Serum amyloid A2 |
| PLA2G4C | 2.3 | 0.007 | 0.031 | Phospholipase A2, group IVC (cytosolic, calcium-independent) |
| IFNGR2 | 2.3 | 0.008 | 0.029 | Interferon gamma receptor 2 (interferon gamma transducer 1) |
| EFNB2 | 2.3 | 0.005 | 0.028 | Ephrin-B2 |
| FOSL1 | 2.2 | 0.007 | 0.017 | FOS-like antigen 1 |
| CSF1 | 2.2 | 0.006 | 0.009 | Colony stimulating factor 1 |
| NFKB2 | 2.2 | 0.011 | 0.019 | Nuclear factor of kappa light polypeptide gene enhancer in B cells 2 |
| RGS17 | 2.2 | 0.010 | 0.011 | Regulator of G-protein signalling 17 |
| THBS1 | 2.1 | 0.005 | 0.000 | Thrombospondin 1 |
| RIPK2 | 2.1 | 0.004 | 0.040 | Receptor-interacting serine-threonine kinase 2 |
| CCL3 | 2.1 | 0.022 | 0.048 | Chemokine (C-C motif) ligand 3 |
| MAP2K3 | 2.1 | 0.009 | 0.041 | Mitogen-activated protein kinase kinase 3 |
| TNFAIP8 | 2.1 | 0.012 | 0.001 | Tumour necrosis factor, alpha-induced protein 8 |
| TLR1 | 2.1 | 0.004 | 0.001 | Toll-like receptor 1 |
| TFAP2C | 2.1 | 0.006 | 0.027 | Transcription factor AP-2 gamma (activating enhancer binding protein 2 γ) |
| CEBPB | 2.0 | 0.005 | 0.017 | CCAAT/enhancer binding protein (C/EBP), beta |
| IL28B | 2.0 | 0.016 | 0.041 | Interleukin 28B (interferon, lambda 3) |
| NR2F1 | −2.0 | 0.002 | 0.017 | Nuclear receptor subfamily 2, group F, member 1 |
| MAP2K6 | −2.0 | 0.022 | 0.039 | Mitogen-activated protein kinase kinase 6 |
| PPARGC1A | −2.1 | 0.011 | 0.041 | Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha |
| GLI2 | −2.1 | 0.046 | 0.049 | GLI family zinc finger 2 |
| ID2 | −2.2 | 0.004 | 0.041 | Inhibitor of DNA binding 2, dominant negative helix-loop-helix protein |
| CDH6 | −2.4 | 0.010 | 0.005 | Cadherin 6, type 2, K-cadherin (foetal kidney) |
| IGSF5 | −2.5 | 0.004 | 0.003 | Immunoglobulin superfamily, member 5 |
| PPP1R3C | −2.6 | 0.005 | 0.003 | Protein phosphatase 1, regulatory (inhibitor) subunit 3C |
| CAT | −2.8 | 0.030 | 0.034 | Catalase |
| NR3C2 | −2.9 | 0.037 | 0.041 | Nuclear receptor subfamily 3, group C, member 2 |
| GPER | −3.0 | 0.003 | 0.000 | G protein-coupled oestrogen receptor 1 |
| ARHGAP20 | −3.1 | 0.014 | 0.046 | Rho GTPase activating protein 20 |
| OXR1 | −3.2 | 0.031 | 0.044 | Oxidation resistance 1 |
| EPHA4 | −3.2 | 0.007 | 0.001 | EPH receptor A4 |
| CTGF | −3.4 | 0.007 | 0.001 | Connective tissue growth factor |
| CCR6 | −4.2 | 0.012 | 0.021 | Chemokine (C-C motif) receptor 6 |
| SLC17A1 | −4.3 | 0.007 | 0.008 | Solute carrier family 17 (sodium phosphate), member 1 |
| CSRNP3 | −5.2 | 0.018 | 0.009 | Cysteine-serine-rich nuclear protein 3 |
FC fold change vs. unstimulated controls, p Benjamini-Yekutieli multiple testing corrected p value
aSignificance vs. unstimulated controls (n = 4)
bSignificance vs. IA2 or ATP-γ-S stimulation (n = 4)
The combination of IA2 and ATP-γ-S yielded a higher level of stimulation with 650 significantly altered gene expressions (Table 4). Of these, the same 36 gene entities, region R2 of Fig. 4, were shared with both IA2 and ATP-γ-S stimulations; a selection of 24 are shown in Table 1, and 70 and 27 gene entities were specifically shared with IA2 or ATP-γ-S stimulation, respectively (Fig. 4). No gene entities were shared between cells stimulated with IA2 or ATP-γ-S that were not found to be shared with cells stimulated with the combination of IA2 and ATP-γ-S, which validates that the effects seen is indeed related to the actual combination of the two stimuli.
Of the 36 shared gene entities, seven were significantly higher in the IA2 and ATP-γ-S combination compared to the IA2 infection, denoted with the superscript a in Table 1, and 25 were significantly higher compared to the ATP-γ-S stimulation; 21 are shown and denoted with the superscript b in Table 1. Thus, the potentiating effect of IA2 and ATP-γ-S combination seen on IL-8 release (Fig. 2) also applies to other significant cellular responses.
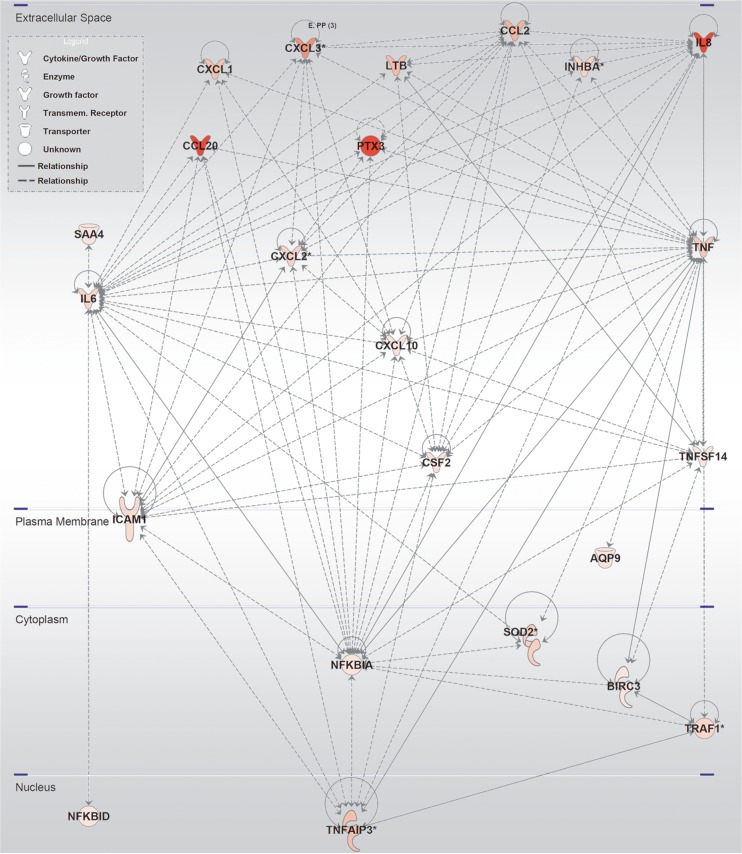
Differentially expressed gene entities were not verified by RT-PCR since the scope of our study was to characterise similarities in response pathways rather than focusing on individual gene expression. Therefore, further characterisation of altered gene entities was performed with IPA Functional Analysis Tools in order to determine significant overrepresentation of gene entities in known canonical pathways. The IPA Functional Analysis reports that a total of 88 biological function-annotated pathways, with a z-score ≥2, were identical and shared between the cells incubated with IA2 or ATP-γ-S or with the combination of these (Table S1). In addition, interactions between corresponding gene products of altered gene entities were mapped in IPA, and networks of known interactions were created. Figure 5 shows direct interactions of proteins associated with the 36 altered gene entities shared by all treatment. Likewise, interactions between gene products of gene entities altered in the individual IA2, ATP-γ-S, and the combination of these treatments were mapped, and IPA-networks of direct interactions were created as is shown in supplemental figures S1–S3.
Fig. 5.
IPA-derived stimulation network of the shared gene entities altered with both IA2 and/or ATP-γ-S stimulations. Molecular biological relationships (lines) are shown between molecules (nodes) with altered gene expression of their corresponding gene entity. The intensity of the node colour indicates the degree of upregulation (red). Nodes are derived from IPA analysis of the 36 shared entities of R2 in Fig. 4
Discussion
Both IA2 and A498 cells released ATP during infection, which is in agreement with previously published results [6]. However, the importance of ATP signalling on host responses during UPEC infection is highlighted in our study. Treatment with apyrase and specific knockdown of P2Y2 receptors both confirm the importance of ATP signalling for UPEC-mediated release of IL-8. Apyrase decreased IL-8 release during IA2 infection. This decrease was also observed during ATP-γ-S stimulation, which could indicate an endogenous release of ATP during ATP-γ-S stimulation or just be an effect of apyrase-mediated hydrolysis of ATP-γ-S. Experiments show a partial hydrolysis of ATP-γ-S in the presence of apyrase (data not shown). Nonetheless, the results are further strengthened by results after specific knockdown of P2Y2 receptors. We have previously reported abrogated cytokine responses to ATP-γ-S after P2Y2 knockdown [14]; however, in the present study, it is shown that knockdown of P2Y2 also abrogates UPEC-mediated release of IL-8. Both LPS-mediated TLR4 activation and ATP-mediated P2 receptor activation have been shown to cause the same activation of the p38 mitogen-activated protein kinase (MAPK) pathway [15]. In addition, when combined, stimulation with LPS and ATP elicited more rapid and prolonged activation than induced by either LPS or ATP alone [15]. The potentiated effects of a combined stimulation with IA2 and ATP-γ-S on release of IL-8 and gene expressions in our study may thus be associated with a stronger activation of signalling pathways.
The microarray approach of our study provides new and detailed information about biological pathways that are activated by UPEC and ATP. ATP-γ-S was chosen in order to minimise activation of other receptors by hydrolysis product of ATP, and even though receptors responding to ATP and ATP-γ-S are similar, they are not identical. However, the predominantly expressed P2Y2 receptor of A498 cells is activated by both ATP and ATP-γ-S [14]. The responsive elements of Table 1 include several entities belonging to classical response pathways of bacterial infections. In the case of IA2, these can of course be attributable to the bacterial infection per se, but many of these elements are also elevated by stimulation with ATP-γ-S alone, and the majority have, to our knowledge, not previously been shown to be induced by ATP-γ-S. Several responding entities of Table 1 belong to the chemokine (CCL2, CCL11, CCL20, CXCL1, CXCL2, CXCL3, CXCL8, CXCL9 and CXCL10) or cytokine (CSF2, IL-6, IL-8 and TNF) superfamily. The expression of chemokines in our study is highly increased by both IA2 and ATP-γ-S and further increased by the combination of these. The secretion of both CXC (CXCL1, CXCL8, CXCL9, CXCL10) and CC (CCL2, CCL3, CCL5) chemokines has previously been reported in UPEC-stimulated A498 cells [16]. CCL20, the most upregulated entity in our study, is a constitutively expressed epithelial chemokine [17] that is upregulated by pro-inflammatory stimuli, such as TNF-α and LPS [18]. CCL20, along with CXCL9 and CXCL10, is known to display antimicrobial activities similar to β-defensins [19]. The secretion profile of chemokines during infection has been shown to vary with bacterial fimbria type [16]. In our study, most of the chemokines induced by IA2 were also induced by ATP-γ-S in the absence of a bacterial stimulus, thus indicating their independence of fimbria type and other bacterial components. ATP-γ-S-mediated release of chemokines may thus have a major influence on infiltration and effector function of immune cells and thereby local host responses of renal epithelia during infection. Neutrophil adhesion to urinary epithelium involves ICAM-1 expressed on uroepithelial cells, and the increased expression of ICAM-1 during IA2 infection in our study is in agreement with previous reports [20]. However, it has also been shown that ICAM-1 expression is induced by ATP [21], and the increase of ICAM-1 during ATP-γ-S stimulation in our study further signifies similarities between bacterial and ATP stimuli.
Amongst the responding genes of Table 1 are several cytokines, i.e. IL-6, IL-8, CSF2 and TNF, that show increased expression by both IA2 and ATP-γ-S and a further increase by the combination of these. The IL-6 and IL-8 responses of A498 cells to IA2 and ATP have previously been reported [14], and the increase of TNF has been shown in human UTI [22]. In addition to IL-8, CSF2 (GM-CSF) is also known to be a strong chemoattractant that enhances neutrophil microbicidal activities. The synthesis of CSF2 is inducible by TNF, and several of the responding entities of Table 1 are connected to TNF signalling. Some of these have previously been recognised during UPEC infections [23, 24, 16], such as TNF-inducible elements coupled to inhibition of cell activation, i.e. TNFAIP3 (A20) and TNFAIP5 (Pentraxin-3); inhibition of apoptosis, i.e. TNFSF14 and BIRC3 [25] and inhibition of NF-κB signalling responses, i.e. TRAF1 and IκBα [26]. Lymphotoxin-beta (LTB or TNF-C), that anchors TNF in membranes [27], TNFAIP3, Pentraxin-3 and BIRC3, an inhibitor of apoptosis, are all increased by both IA2 and ATP-γ-S in our study and may inhibit genes associated with TNF- and LPS-induced cell activation by blocking mechanism involving transcription factors NF-κB and AP-1 [28, 29]. UPEC infection has been shown to inhibit NF-κB signalling in host cells [30], and the alterations that are seen in our study may describe countermeasures of renal host cells to intervene invasion and apoptosis. Consequently, both IA2 and ATP-γ-S can inhibit NF-κB-induced transcription and thereby apoptosis.
Conclusions
Taken together, results show an increased release of ATP during infection that may explain many of the observed similarities between host cell responses elicited by a bacterial IA2 stimulus and an inflammatory ATP-γ-S stimulus. In addition, microarray results show that responses to both IA2 infection and ATP-γ-S stimuli appear to reflect a sense of balance between host responses that drive inflammation and concurrent responses that restrain inflammatory responses.
Electronic supplementary material
IPA-derived IA2-associated stimulation networks. Molecular biological relationships (lines) are shown between molecules (nodes) with altered gene expression of their corresponding gene entity. The intensity of the node colour indicate the degree of up (red) or down (blue) regulation. Nodes are derived from IPA-analysis of all 116 entities altered by IA2-treatment. Bold purple lines and nodes correspond to relationship networks between the 80 (10 + 70) IA2-specific entities of figure 4. (GIF 488 kb)
IPA-derived ATP-γ-S-associated stimulation networks. Molecular biological relationships (lines) are shown between molecules (nodes) with altered gene expression of their corresponding gene entity. The intensity of the node colour indicate the degree of up (red) or down (blue) regulation. Nodes are derived from IPA-analysis of all 92 entities altered by ATP γ S treatment. Bold purple lines and nodes correspond to relationship networks between the 56 (29 + 27) ATP-γ-S-specific entities of figure 4. (GIF 442 kb)
IPA-derived IA2 + ATP-γ-S -associated stimulation networks. Molecular biological relationships (lines) are shown between molecules (nodes) with altered gene expression of their corresponding gene entity. The intensity of the node colour indicate the degree of up (red) or down (blue) regulation. Nodes are derived from IPA-analysis of all 650 entities altered by the IA2 + ATP-γ-S -treatment. Bold purple lines and nodes correspond to relationship networks between the 517 IA2 + ATP-γ-S -specific entities of figure 4. (GIF 936 kb)
Acknowledgments
This project was supported by the Magnus Bergvalls Foundation and the Faculty of Medicine and Health at Örebro University.
References
- 1.Boeynaems JM, Communi D. Modulation of inflammation by extracellular nucleotides. J Invest Dermatol. 2006;126(5):943–944. doi: 10.1038/sj.jid.5700233. [DOI] [PubMed] [Google Scholar]
- 2.la Sala A, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Alerting and tuning the immune response by extracellular nucleotides. J Leukoc Biol. 2003;73(3):339–343. doi: 10.1189/jlb.0802418. [DOI] [PubMed] [Google Scholar]
- 3.Osterberg E, Hallander HO, Kallner A, Lundin A, Aberg H. Evaluation of the adenosine triphosphate test in the diagnosis of urinary tract infection. Eur J Clin Microbiol Infect Dis. 1991;10(2):70–73. doi: 10.1007/BF01964410. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Introduction: P2 receptors. Curr Top Med Chem. 2004;4(8):793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- 5.Shabir S, Cross W, Kirkwood LA, Pearson JF, Appleby PA, Walker D, Eardley I, Southgate J. Functional expression of purinergic P2 receptors and transient receptor potential channels by the human urothelium. Am J Physiol Renal Physiol. 2013;305(3):F396–F406. doi: 10.1152/ajprenal.00127.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Säve S, Persson K. Extracellular ATP and P2Y receptor activation induce a proinflammatory host response in the human urinary tract. Infect Immun. 2010;78(8):3609–3615. doi: 10.1128/IAI.00074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278(26):23331–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- 8.Kukulski F, Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, Kirley TL, Sévigny J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005;1(2):193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23(2):473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DJ, Künzli BM, A-Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106(39):16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabel CA. P2 purinergic receptor modulation of cytokine production. Purinergic Signal. 2007;3(1–2):27–38. doi: 10.1007/s11302-006-9034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kukulski F, Ben Yebdri F, Lecka J, Kauffenstein G, Lévesque SA, Martín-Satué M, Sévigny J. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46(2):166–170. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314(5806):1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 14.Kruse R, Säve S, Persson K. Adenosine triphosphate induced P2Y2 receptor activation induces proinflammatory cytokine release in uroepithelial cells. J Urol. 2012;188(6):2419–2425. doi: 10.1016/j.juro.2012.07.095. [DOI] [PubMed] [Google Scholar]
- 15.Harada K, Hide I, Seki T, Tanaka S, Nakata Y, Sakai N. Extracellular ATP differentially modulates Toll-like receptor 4-mediated cell survival and death of microglia. J Neurochem. 2011;116(6):1138–1147. doi: 10.1111/j.1471-4159.2011.07170.x. [DOI] [PubMed] [Google Scholar]
- 16.Godaly G, Otto G, Burdick MD, Strieter RM, Svanborg C. Fimbrial lectins influence the chemokine repertoire in the urinary tract mucosa. Kidney Int. 2007;71(8):778–786. doi: 10.1038/sj.ki.5002076. [DOI] [PubMed] [Google Scholar]
- 17.Fujiie S, Hieshima K, Izawa D, Nakayama T, Fujisawa R, Ohyanagi H, Yoshie O. Proinflammatory cytokines induce liver and activation-regulated chemokine/macrophage inflammatory protein-3alpha/CCL20 in mucosal epithelial cells through NF-kappaB [correction of NK-kappaB] Int Immunol. 2001;13(10):1255–1263. doi: 10.1093/intimm/13.10.1255. [DOI] [PubMed] [Google Scholar]
- 18.Scapini P, Crepaldi L, Pinardi C, Calzetti F, Cassatella MA. CCL20/macrophage inflammatory protein-3alpha production in LPS-stimulated neutrophils is enhanced by the chemoattractant formyl-methionyl-leucyl-phenylalanine and IFN-gamma through independent mechanisms. Eur J Immunol. 2002;32(12):3515–3524. doi: 10.1002/1521-4141(200212)32:12<3515::AID-IMMU3515>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, Oppenheim JJ. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74(3):448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 20.Säve S, Mohlin C, Vumma R, Persson K. Activation of adenosine A2A receptors inhibits neutrophil transuroepithelial migration. Infect Immun. 2011;79(8):3431–3437. doi: 10.1128/IAI.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai Y, Kaidoh M, Ohhashi T. MDA-MB-231 produces ATP-mediated ICAM-1-dependent facilitation of the attachment of carcinoma cells to human lymphatic endothelial cells. Am J Physiol Cell Physiol. 2008;295(5):C1123–C1132. doi: 10.1152/ajpcell.00247.2008. [DOI] [PubMed] [Google Scholar]
- 22.Engel D, Dobrindt U, Tittel A, Peters P, Maurer J, Gütgemann I, Kaissling B, Kuziel W, Jung S, Kurts C. Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect Immun. 2006;74(11):6100–6107. doi: 10.1128/IAI.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem. 2002;277(9):7412–7419. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- 24.Godaly G, Bergsten G, Hang L, Fischer H, Frendéus B, Lundstedt AC, Samuelsson M, Samuelsson P, Svanborg C. Neutrophil recruitment, chemokine receptors, and resistance to mucosal infection. J Leukoc Biol. 2001;69(6):899–906. [PubMed] [Google Scholar]
- 25.Kuai J, Nickbarg E, Wooters J, Qiu Y, Wang J, Lin LL. Endogenous association of TRAF2, TRAF3, cIAP1, and Smac with lymphotoxin beta receptor reveals a novel mechanism of apoptosis. J Biol Chem. 2003;278(16):14363–14369. doi: 10.1074/jbc.M208672200. [DOI] [PubMed] [Google Scholar]
- 26.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9(22):2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 27.Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O’Brine-Greco B, Foley SF, Ware CF. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72(6):847–856. doi: 10.1016/0092-8674(93)90574-A. [DOI] [PubMed] [Google Scholar]
- 28.Cooper JT, Stroka DM, Brostjan C, Palmetshofer A, Bach FH, Ferran C. A20 blocks endothelial cell activation through a NF-kappaB-dependent mechanism. J Biol Chem. 1996;271(30):18068–18073. doi: 10.1074/jbc.271.30.18068. [DOI] [PubMed] [Google Scholar]
- 29.Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84(10):3483–3493. [PubMed] [Google Scholar]
- 30.Billips BK, Forrestal SG, Rycyk MT, Johnson JR, Klumpp DJ, Schaeffer AJ. Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli. Infect Immun. 2007;75(11):5353–5360. doi: 10.1128/IAI.00922-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IPA-derived IA2-associated stimulation networks. Molecular biological relationships (lines) are shown between molecules (nodes) with altered gene expression of their corresponding gene entity. The intensity of the node colour indicate the degree of up (red) or down (blue) regulation. Nodes are derived from IPA-analysis of all 116 entities altered by IA2-treatment. Bold purple lines and nodes correspond to relationship networks between the 80 (10 + 70) IA2-specific entities of figure 4. (GIF 488 kb)
IPA-derived ATP-γ-S-associated stimulation networks. Molecular biological relationships (lines) are shown between molecules (nodes) with altered gene expression of their corresponding gene entity. The intensity of the node colour indicate the degree of up (red) or down (blue) regulation. Nodes are derived from IPA-analysis of all 92 entities altered by ATP γ S treatment. Bold purple lines and nodes correspond to relationship networks between the 56 (29 + 27) ATP-γ-S-specific entities of figure 4. (GIF 442 kb)
IPA-derived IA2 + ATP-γ-S -associated stimulation networks. Molecular biological relationships (lines) are shown between molecules (nodes) with altered gene expression of their corresponding gene entity. The intensity of the node colour indicate the degree of up (red) or down (blue) regulation. Nodes are derived from IPA-analysis of all 650 entities altered by the IA2 + ATP-γ-S -treatment. Bold purple lines and nodes correspond to relationship networks between the 517 IA2 + ATP-γ-S -specific entities of figure 4. (GIF 936 kb)