Abstract
ATP is released from cells in response to various stimuli. Our previous studies on pancreas indicated that pancreatic acini could be major stores of secreted ATP. In the present study, our aim was to establish the role of the vesicular nucleotide transporter (VNUT), SLC17A9, in storage and release of ATP. Freshly prepared acini from mice and AR42J rat acinar cells were used in this study. We illustrate that in AR42J cells, quinacrine (an ATP store marker) and Bodipy ATP (a fluorescent ATP analog) co-localized with VNUT-mCherry to vesicles/granules. Furthermore, in acini and AR42J cells, a marker of the zymogen granule membranes, Rab3D, and VNUT co-localized. Dexamethasone treatment of AR42J cells promoted formation of acinar structures, paralleled by increased amylase and VNUT expression, and increased ATP release in response to cholinergic stimulation. Mechanical stimulus (pressure) and cell swelling also induced ATP release, but this was not influenced by dexamethasone, most likely indicating different non-zymogen-related release mechanism. In conclusion, we propose that VNUT-dependent ATP release pathway is associated with agonist-induced secretion process and downstream purinergic signalling in pancreatic ducts.
Keywords: Pancreas, ATP release, VNUT, SLC17A9, AR42J, Pancreatitis, Mechanical stress
Introduction
Purinergic receptors are well expressed in both endocrine and exocrine pancreas, particularly in β-cells, duct cells, and stellate cells [1]. ATP is released from most cells, and the release mechanism is currently an intensive research field, where the focus is on identifying whether ATP is released via ion channels/transporters, pannexin or connexins, or via vesicular transport [2, 3]. In the exocrine pancreas, it has been well documented that acini release ATP in response to physiological signals, such as cholinergic agonists, cholecystokinin, and neurotensin [4–6]. Acinar cells also secrete digestive enzymes and nucleotide-converting enzymes [5, 7], and our earlier studies indicated that zymogen granules and fluorescent markers for ATP stores are co-localized [4]. Sawada and coworkers published their discovery of a putative ATP transporter (SLC17A9), named vesicular nucleotide transporter (VNUT), which was expressed in enterochromaffin granules [8]. Soon after, we provided evidence that VNUT is expressed in pancreatic zymogen granules and is engaged in ATP uptake [9]. Since then, expression of VNUT has been demonstrated in several cell types indicating the general importance of VNUT for ATP release [10–15].
Renewed interest in ATP release in pancreas in relation to health and disease was sparked by our recent finding that pancreatic stellate cells (PSC), which are involved in pancreatitis and pancreatic cancer, express the P2X7 receptor that can lead to cytolytic processes and stimulate cell proliferation [16]. Therefore, it is relevant to ask whether pancreatic acini, which lie in a close proximity to pancreatic stellate cells, release ATP into the lumen as well as towards the insterstitium. Our aim was to apply a molecular biological approach to determine whether VNUT is involved in ATP release in pancreatic acinar cells. In particular, we wished to determine whether physiological stimuli, such as cholinergic stimulus, could release ATP and whether VNUT was involved. In addition, we tested effects of potential pathophysiological stimuli, such as mechanical and hypotonic stresses. For this study, we used online ATP measurements in freshly isolated mouse pancreatic acini and rat acinar cell line AR42J, as well as expression of recombinant VNUT coupled to a fluorescent reporter, in combination with other functional and biochemical assays. We show that ATP is released with various stimuli and some depend on VNUT.
Methods
Acini isolation
Female C57BL/6JBomTac mice approx. 20 g were kept with free access to water and standard chow, and procedures were approved by the Danish Animal Experiment Inspectorate (Dyreforsøgstilsynet). Acini were prepared with slight modifications by collagenase digestion, as described earlier [4, 17]. In brief, the pancreas was removed from mice that were killed with cervical dislocation. The pancreas was trimmed free of connective tissue and fat and washed in buffer, and subsequently, the pancreas was cut into 2-mm3 pieces. The pancreatic pieces were added to 3-ml isolation media that contained dulbecco’s modified eagle medium (DMEM) 1:1 F12, 10 mM HEPES (4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid), 1 mg/ml albumin, 2 mM CaCl2, 5 mM glycine, 1 mg/ml trypsin inhibitor, and 4 mg collagenase V (Sigma). The samples were placed in a water bath at 37 °C with constant gassing with 5 % CO2/95 % O2, and pH of media was 7.4. After 10 min, the pancreas pieces were dispersed using a Pasteur pipette and incubated for further 10 min. The digestion was terminated with the addition of cold DMEM media and washed 1× before they were filtered through a 70-μm mesh, and then washed twice. The final wash was with physiological saline buffer, which contained 10 mg/ml of trypsin inhibitor. These procedures enabled preparation of “pure” acini as verified by routine microscopic examinations. The ionic composition of the solution also used for further functional experiments was as follows (in mmol/l): 145 NaCl, 1 MgCl2, 1.5 CaCl2, 0.4 KH2PO4, 1.6 K2HPO4, 5.0 glucose, and 10 HEPES; pH was 7.4. The solution included also 1 mg/ml of trypsin inhibitor for luciferase experiments on fresh acini.
Cell culture of AR42J cells
Rat acinar cell line AR42J (CRL-1492, ATCC, passages 15–25) was cultured in RPMI media supplemented with 10 % fetal bovine serum (FBS) and penicillin/streptomycin. Cells were cultured in humidified incubators at 37 °C, with 5 % CO2 in air. Cells were split every 4 days. In order to increase zymogen granule formation [18], dexamethasone (50 nM) was added 24 or 48 h before experiments.
Immunocytochemistry
AR42J cells were allowed to attach to glass coverslips 24 h before staining, and 50 nM of dexamethasone was added. Acini were attached for 15 min. The cells were subsequently fixed in 4 % paraformaldehyde in phosphate buffered saline (PBS) for 15 min, treated with 0.2 M TRIS-glycine (pH 7.4) for 15 min, and then rinsed in PBS and permeabilized for 10 min in PBS 0.5 % TritonX-100. The samples were blocked with 10 % bovine serum albumin (BSA) in PBS for 45 min and then incubated with the primary antibodies for VNUT (1:400), which were used in previous studies [8, 13], and Rab3D (1:100; Santa Cruz Sc-26559) for 2 h. The slides were then washed in PBS and incubated for 1 h with 1:400 dilution of appropriate secondary antibody conjugated to Alexa 488 or Alexa 568. For nuclear staining, DAPI (4',6-diamidino-2-phenylindole) was used (1:400). Finally, the coverslips were washed in distilled water and mounted with DAKO fluorescent mounting medium on the slides. Fluorescence was examined with 40× PL APO CS (NA 1.3) objective in Leica (TCS SP 5X) confocal laser scanning microscope (Leica Microsystems, Heidelberg).
Western blot
Protein lysates were created by adding five times lysis buffer (250 mM TrisBase, 1.25 M NaCl, 50 mM EDTA, 5 % Triton X-100, and 20 mM NaF) to the AR42J cells grown in 50 nM of dexamethasone at 0, 24, or 48 h. Cell lysates were centrifuged at 15,000 g for 15 min. Western blot samples were denatured by heating to 70 °C in 50 mM dithiothreitol for 10 min and run on precast gels from Invitrogen. The membranes were blocked overnight at 4 °C in 0.5 % milk powder and 1 % BSA. Primary antibody for VNUT (1:1,000 rabbit, see Immunocytochemistry section), amylase (1:500 rabbit, Sigma A8273), and β-Actin (1:1000 mouse monoclonal C4, Santa Cruz, Sc-47778) were added in blocking buffer for 1.5 h. The appropriate secondary antibody conjugated to horse-radish peroxidase (1:2,500) was added in blocking buffer, for 1 h. Enzyme substrate was added, and blots were viewed on Fusion FX Vilber Lourmat, and band intensity was calculated using the inbuilt software.
VNUT expression plasmid
The two original templates were the VNUT plasmid [8, 14] and the Clontech mCherry (from pmCherry-C1 vector) together with plasmid pPAP7160. Plasmid pPAP7160 was generated by homologous recombination in yeast strain PAP1500 [19] between a polymerase chain reaction (PCR) fragment encompassing nucleotides 1–2,580 from pEGFP-N1 (Clontech, USA) and pEMBLyex4 [20]. The resulting plasmid can replicate in Escherichia coli and Saccharomyces cerevisiae and carries the elements from pEGFP-N1 required for expression in mammalian cells and EGFP. The VNUT-mCherry expression plasmid was generated by in vivo homologous recombination by transformation PAP1500 with BamHI, NotI digested pPAP7160, a VNUT PCR fragment, and an mCherry PCR product. The 5′ VNUT fragment carried a 35-nucleotide sequence identical to pPAP7160 while the 3′ VNUT PCR product contained a 35-nucleotide sequence identical to the 5′ end of the mCherry PCR fragment. The 3′ mCherry PCR product carried a 35-nucleotide long fragment identical to pPAP7160. AccuPol DNA polymerase (VWR, Denmark) was used to generate all PCR products. The correct nucleotide sequence of the VNUT-mCherry expression plasmid was confirmed by DNA sequencing.
The following primers were used for PCR amplifications:
Cherryup5′ATGGTGAGCAAGGGCGAG3′
Cherryplasmid5′AAATGTGGTATGGCTGATTATGATCTAGAGTCGCTTATCTAGATCCGGTGGATC3′
VNUTrecup5′ATATAAGCAGAGCTGGTTTAGTGAACCGTCAGATCGCCGCCCCCTTCACCATG3′
VNUTreccherry 5′ATGGCCATGTTATCCTCCTCGCCCTTGCTCACCATGAGGTCCTCATGGGTAGAG3′
Transfection of AR42J cells
AR42J cells were transfected using the FuGENE HD kit from Promega. Cells were plated the day before transfection at a density of 40,000 cells per well in 35-mm Wilco dishes in RPMI media+ 10 % FBS. The DNA was mixed with serum-free medium up to the desired concentration, and FuGENE HD reagent was added and incubated for 15 min. This mix was added to the cells, and transfection rate of 30–50 % was achieved. After 24 h of transfection, 50 nM of dexamethasone was added.
Experiments on co-localization of ATP stores
Before imaging was performed, cells were incubated with quinacrine (5 μM) for 25 min or Bodipy FL ATP (15 μM) for 2 h. Incubation solution was the physiological solution defined in “acini isolation.” Cells were washed, and dishes were kept at 37 °C. For imaging, Leica SP 5X MP confocal microscope (Leica Microsystems) was used with argon laser and white light laser. VNUT-mCherry was exited with 594 nm, and emission collected at 600–700 nm; quinacrine excitation was 458, and emission was 466–578 nm; for Bodipy FL ATP, excitation was 488 nm, and emitted light collected at 500–550 nm. Images were collected simultaneously with frame averaging and with a 40× PL APO CS (NA 1.3) objective; sequential scan with high resolution was not possible due to vesicle motility. For analysis, Leica LAS software was used, and original images and overlays are presented.
ATP release
Freshly isolated mouse acinar cells (50,000/well) were plated on 96 well NUNC white plates and used for in situ live cell luminometry. AR42J cells were plated in similar plates (10,000/well); they were grown for 48 h (doubling time ~24 h) and treated with 50 nM of dexamethasone for 48 h before the experiment. For the experiments with mechanical stimulation, the following were used: 50 μl of acini suspension (1.000 cells/μl), or AR42J cells grown in the plate where the media were removed and replaced with 50 μl of physiological saline. These were mixed with 50 μl of luciferase/luciferin. For carbachol and hypotonic stimulation experiments, 65 μl of physiological saline with acini (770 cells/μl) or 65 μl physiological saline replaced the media of AR42J cells, and 25 μl of 2x concentrated luciferin/luciferase were mixed gently. Luciferin/luciferase mix was made from ATP kit SL 144–041 (BioThema) dissolved in physiological saline. Cells were left to rest for 30 min or until the luminescence dropped below at least 500 relative luminescence units (RLU). Plate mode was chosen, and stimulants were added manually. For mechanical stimulation, 50 μl of physiological saline was added using an injection pump at the indicated speeds. For carbachol, 10 μl of 1 M carbachol (in 1 % BSA and 1 % trypsin inhibitor) was added. For hypotonic stimuli, 45 μl of MilliQ water was added to cells/cell suspensions. The osmolality was changed from 310 to 205 mOsm/kg (i.e., 33 % decrease in tonicity and denoted as 33 % hypotonic load in Results section). Luminescence was recorded at 20-s sampling rate with 1-s integration in FLUOstar Optima (BMG, Labtech). Samples were run in duplicates. Experiments on freshly isolated acini were done at 25 °C, to minimize breakdown of luciferase by digestive enzymes. AR42J cells were incubated at 37 °C. For each experimental protocol, standard curves were made with ATP concentrations ranging from 10−9 to 10−5 M. Concentrations of ATP released from fresh acinar cells or AR42J cells were corrected for a cytokrit of one million of cells per milliliter. Cell numbers were determined by manual counting and/or cell counting kit—8 (DOJINDO).
Chemicals and statistics
All chemicals/kits were purchased from Sigma-Aldrich unless otherwise stated. Data are shown as means ± standard error of the mean (SEM); n denotes a number of experiments on cells isolated from different animals or AR42J cultures. Student’s paired t test was applied when comparing two samples from the same animal or cell cultures and P < 0.05 was accepted as significant. Data were analyzed in GraphPad or Microsoft Excel.
Results
VNUT expression
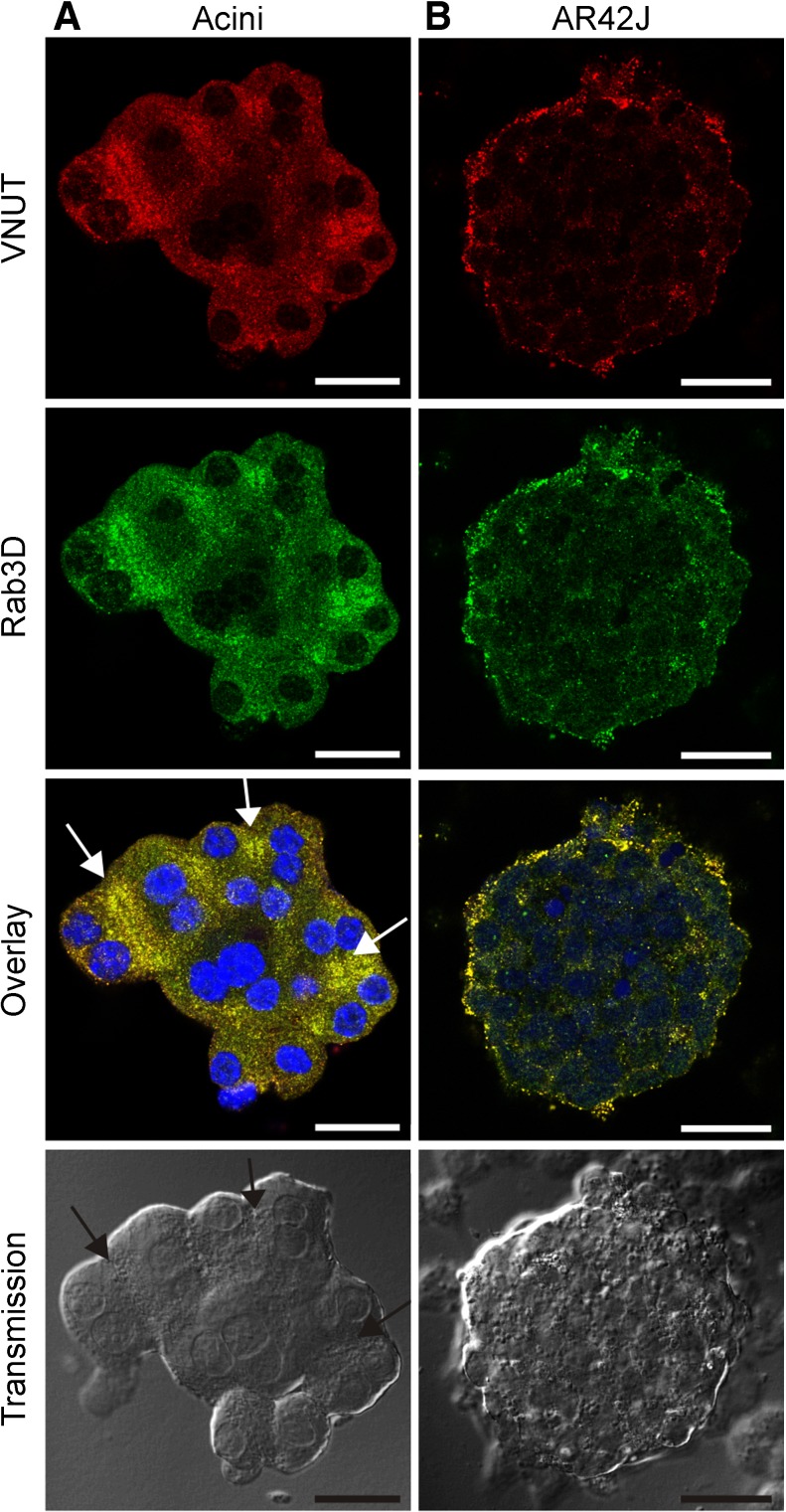
Using biochemical and functional assays, we have previously described that VNUT is a part of an ATP uptake mechanism in zymogen granules isolated from rat acini [9]. In the first part of the study, we used several approaches to determine whether VNUT was expressed in intact acinar cells and in acini cell model, AR42J cells (rat carcinoma cell line). Here, we showed that isolated mouse pancreatic acini and AR42J cells express VNUT, as determined by immunolocalization (Fig. 1). We also showed that VNUT co-localizes with Rab3D, which is a low-molecular weight GTP-binding protein that associates with secretory granules in exocrine glands, e.g., zymogen granules (ZGs) in pancreatic acini [9, 21] (Fig. 1a, b). The co-localization of the two proteins, quantified as Pearson’s co-localization coefficient, is 0.91 ± 0.07 in acini and 0.86 ± 0.05 in AR42J cells (mean ± SD, n = 5 and 4, respectively). Notably, acini showed high density of granules in apical poles of cells forming acinar lumens (Fig. 1a). AR42J cells did not show pronounced polarity (apical and basolateral poles), compared to the freshly isolated acini, and granules labeled with VNUT and Rab3D were more dispersed in peripheral regions of cells.
Fig. 1.
Co-localization of VNUT and Rab3D. Acini isolated from mouse pancreas are shown in the first column (a), and AR42J cells treated with dexamethasone are shown in the second column (b). Both cell preparations were stained with VNUT and Rab3D antibodies. Secondary antibodies were conjugated to Alexa 568 (red) for VNUT and Alexa 488 (green) for Rab3D; nuclear stain was DAPI (blue). Transmission images are shown in the last row. Arrows in overlay and transmission images in a indicate acinar lumens. Representative images are of three independent experiments. Scale bars are 25 μm
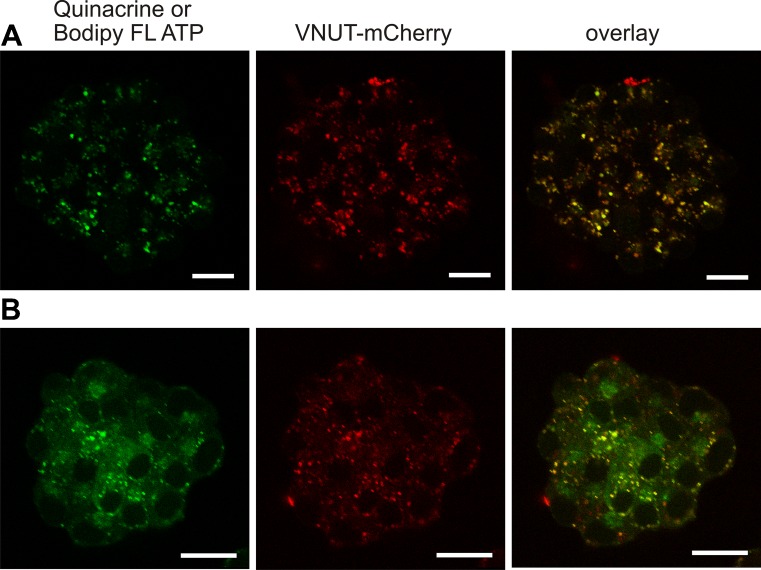
In another series of experiments, we generated a VNUT-mCherry expression plasmid in order to study VNUT localization and function in pancreatic cells. The VNUT-mCherry expression plasmid was transfected into AR42J cells, and Fig. 2 shows that the fluorescent protein localizes to intracellular vesicles. Most of these vesicles also take up the dye quinacrine (Fig. 2a), known to be associated with ATP stores in pancreatic acini [4]. We have also used Bodipy FL ATP, which does not bleach as fast as MANT-ATP that we have used previously [4]. Again, Bodipy FL ATP localized to vesicles, but there was also a weak fluorescence staining of the cytoplasm as expected for cytosolic ATP (Fig. 2b). The overlay images in Fig. 2 clearly indicate that VNUT-mCherry is expressed in vesicles containing ATP markers in AR42J cells and many of these are most likely in ZGs (compare to Fig. 1).
Fig. 2.
Co-localization of VNUT-mCherry and markers of ATP stores in living cells. AR42J cells were transfected with VNUT-mCherry, and cells were incubated either with 5 μM quinacrine (first row) (a) or 15 μM Bodipy FL ATP (second row) (b). The following excitation/emission wavelengths were used: VNUT-mCherry 594/600–700 nm (red), quinacrine 458/466–578 nm (green), and Bodipy FL ATP 488/500–550 nm (green); respective red and green images and overlay images are shown. Representative images are of three independent experiments. Scale bars are 25 μm
AR42J cell differentiation involves upregulation of VNUT
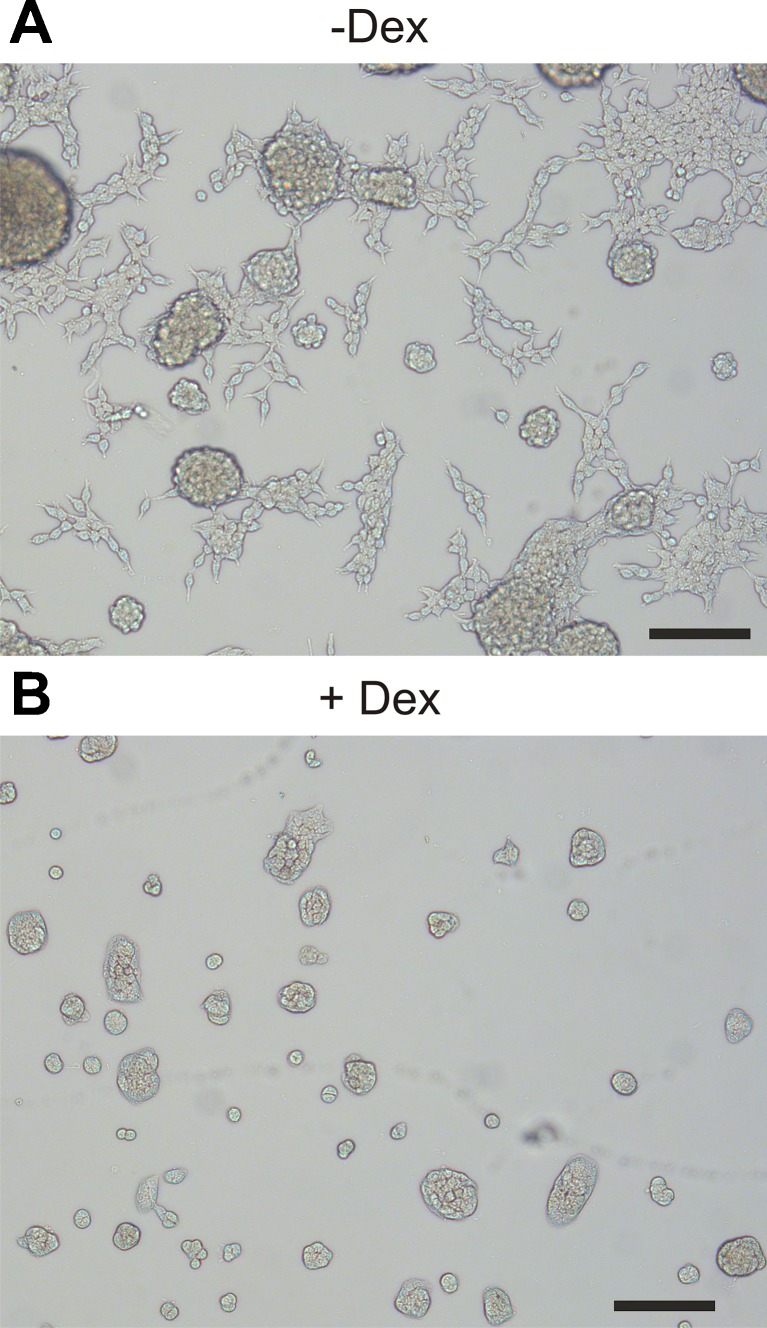
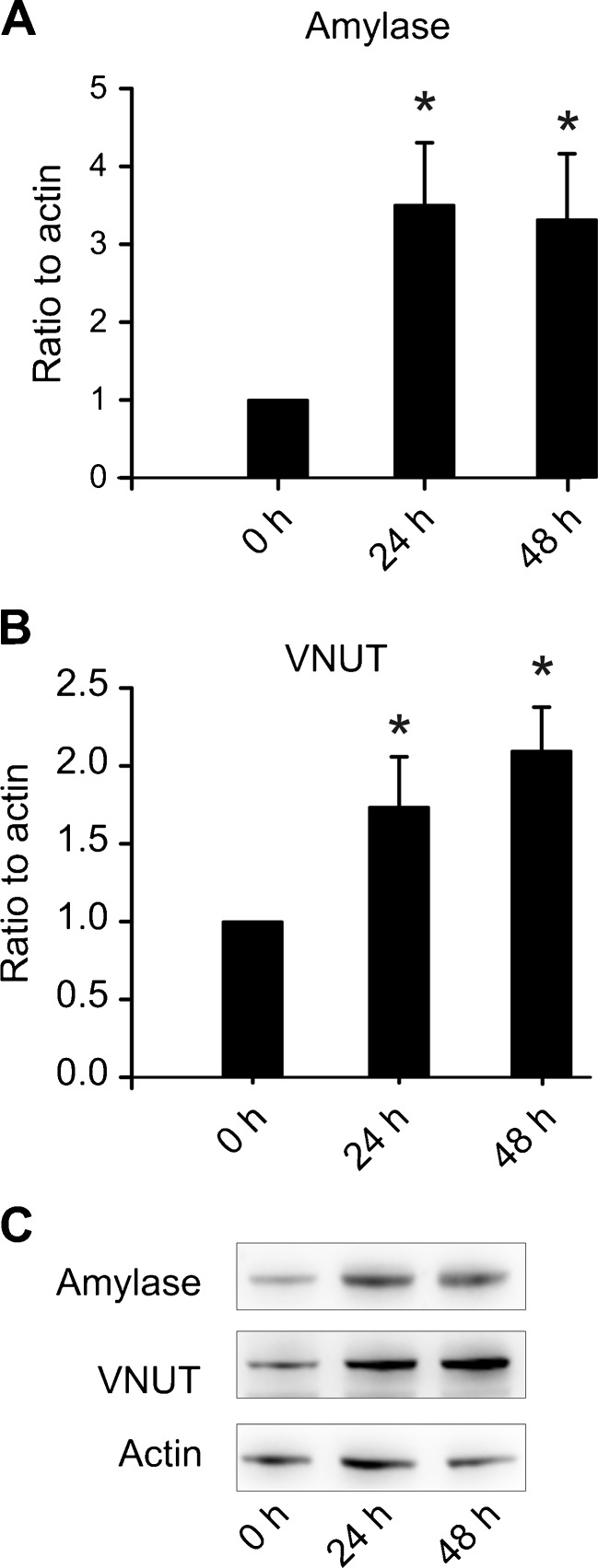
AR42J cells are chemically induced carcinoma of the rat acinar pancreas [22]. The acinar phenotype is closer to primary cells, if cells are preincubated with dexamethasone [18]. This glucocorticoid was omitted or added at 50 nM concentrations at 24 or 48 h before the experiments were conducted. Figure 3 clearly shows changes in cell morphology towards cell cluster/acinar phenotype with dexamethasone treatment. Further, we used western blot analysis to confirm the changes in protein quantities of acinar secretory markers. The major digestive enzyme amylase was upregulated 3.5 ± 0.8 fold already after 24 h and did not increase further (Fig. 4a) (n = 4). Interestingly, VNUT was also upregulated in parallel 1.7 ± 0.3 fold and further to 2.1 ± 0.3 fold after 48 h (Fig. 4b) (n = 4). A representative western blot is shown in Fig. 4c.
Fig. 3.
Effects of dexamethasone on AR42J morphology. AR42J cells were cultured a without or b with 50 nM of dexamethasone for 48 h. Cells stopped growing with dexamethasone, as the same number of cells was added in each dish. Scale bar is 100 μm. Representative picture is shown; similar morphology was also observed in 96 well plates
Fig. 4.
VNUT and amylase are upregulated by dexamethasone. a Amylase is upregulated in response to dexamethasone, relative to actin (n = 4). b VNUT is also upregulated relative to actin (n = 4). c Representative western blot for bar graphs shown in a and b
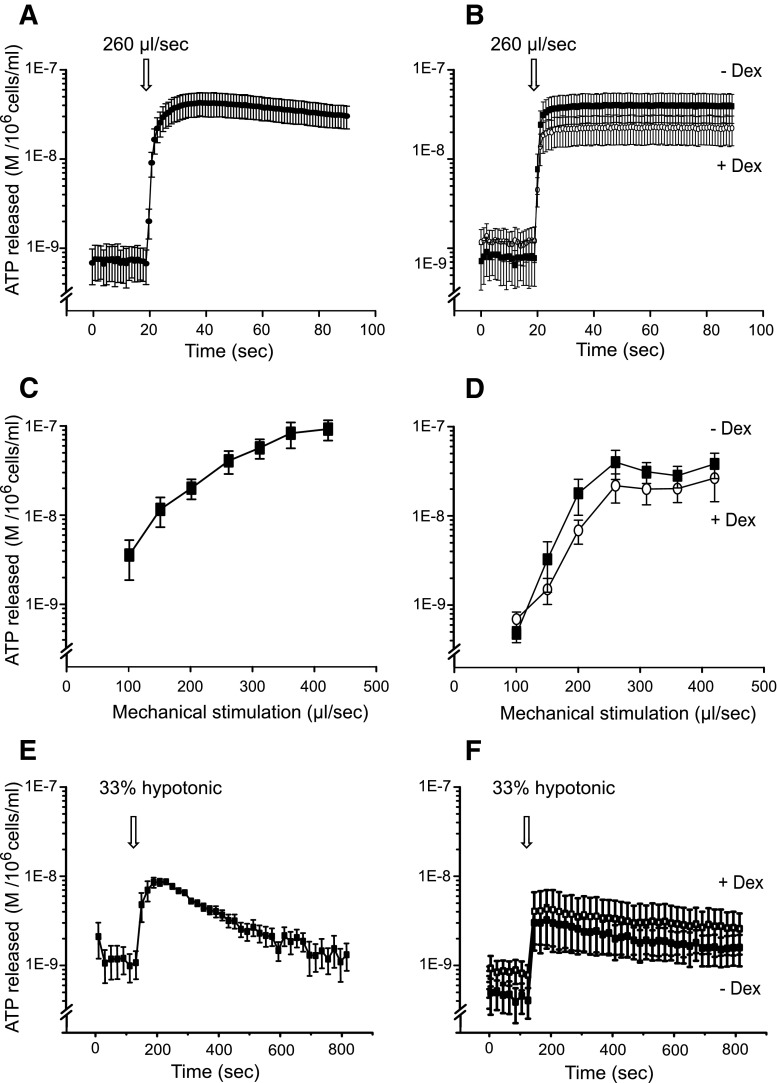
Mechanical and hypotonic stimuli induce ATP release
Since VNUT was upregulated with dexamethasone in AR42J cells, we wanted to determine if also ATP release was upregulated in differentiated AR42J cells and whether this depended on stimulus. In parallel experiments, we investigated ATP release in isolated mouse acini for comparison. Since some of the first findings on epithelial cells [23], mechanical stimulus has become one of the most common stimuli for ATP release in many cells, and here, we use it as a “control” stimulant. Figure 5a, b shows that mechanical stimulus, in this case injection of control solution at 260 μl/s by an injection pump, caused rapid and apparently sustained ATP release in both acinar cell types. Relatively high ATP concentrations were also detected in AR42J cells with protocols lasting up to 800 s (results not shown), indicating no return to basal levels within this time frame. Furthermore, there was a correlation between pump speed and ATP release (Fig. 5d, e), suggesting that extent of mechanical stress and ATP release was indeed a regulated process. Interestingly, AR42J cells had a steeper response curve to mechanical stimulus, though reached lower maximum ATP release at lower pump speeds than isolated acini. The maximum release was seen with a pump speed of 420 μl/s when acinar cells released 96 ± 18 nM ATP (per 106 cells/ml) and AR42J released 27 ± 18 nM ATP (n = 4, 5).
Fig. 5.
Effect of mechanical and hypotonic stimuli on ATP release from freshly isolated acinar cells and AR42J cells with/without dexamethasone. a and b ATP release in response to a mechanical stimulus induced by a pump injection of 50 μl of physiological saline solution at 260 μl/s into 100 μl of acinar (a) or AR42J (b) cell suspensions (n = 4–5). c and d Effect of pump injection speed on ATP release from pancreatic acini (c) or AR42J (d) (n = 4–5). e and f ATP release in response to addition of MilliQ water (33 % dilution) in acini (e) and AR42J cells (f)
Another common mechanical stimulus is a membrane distension caused by cell swelling induced by a hypotonic shock. Figure 5e, f shows that hypotonic solution (33 % dilution, from 310 to 205 mOsm/kg) caused a transient release of ATP, which was at maximum 8.5 ± 0.3 nM ATP in acini and 3.0 ± 1.4 nM ATP in dexamethasone-treated AR42J cells (n = 3, 5). Notably, in response to both types of mechanical stress that would disturb plasma membranes (sheer stress or stretch), AR42J cells behaved similarly irrespective of dexamethasone treatment (n = 4–5).
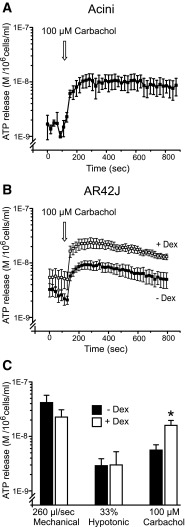
Carbachol induces VNUT-dependent ATP release
Our previous studies showed that various agonists/transmitters cause ATP release from pancreatic acini and that pancreas also contains various nucleotide/side-converting enzymes (see Introduction). The present study was aimed to elucidate the nature of agonist-stimulated ATP release mechanism. First of all, we show that carbachol (100 μM) induced ATP release from mouse acini and AR42J cells. It should be noted that in order to detect agonist-induced ATP release into media, it was necessary to protect luciferase from co-released digestive enzymes and ATP from CD39-mediated hydrolysis and to work with maximal concentrations of the agonist. This was particularly important for freshly isolated acini. Therefore, the acinar medium contained 0.1 % trypsin inhibitor, and the CD39 inhibitor ARL67156 was added together with carbachol. Isolated acini had also significant basal ATP release, which was subtracted from the stimulated values. Notably, it was not necessary to add the CD39 inhibitor to AR42J cells, suggesting that there is a difference in ecto-nucleotidase activity in mouse and rat acinar cells or between freshly isolated and cultured cells. The graphs in Fig. 6a, b show that carbachol induced characteristic ATP release curve in both mouse acini and AR42J cells. Importantly, Fig. 6b shows that the carbachol-induced ATP release was indeed increased in dexamethasone-differentiated AR42J cells. The bar graph (Fig. 6c) summarizes the ATP release in AR42J cells incubated with or without dexamethasone in different experimental conditions—two types of mechanical stress as compared to agonist stimulation. Notably, dexamethasone pre-treatment significantly increased only the ATP release with carbachol, and the increase was 2.83 ± 0.65 fold higher compared to the control (n = 4).
Fig. 6.
ATP release in response to carbachol in acini and AR42J cells with/without dexamethasone. a ATP release in response to carbachol in freshly isolated acini (n = 3). b ATP release in AR42J +/− dexamethasone (n = 5). c Bar graph presentation of ATP release for all different stimuli from AR42J ± dexamethasone (n = 5). The values presented are the stimulus-evoked changes in ATP concentrations ± SEM; that is, the baseline values for each experiment were subtracted from the means peak values induced with mechanical, hypotonic, and carbachol stimulations. * = P < 0.05
Basal ATP release (recorded as average values before introducing various stimuli) for AR42J cells treated with dexamethasone appeared slightly higher compared to untreated cells. However, these values were not significantly different for any pair of experiments; summarized all together, for cells without dexamethasone, ATP release was 1.61 ± 0.3 nM 1.3±0.4 nM, and for cells treated with dexamethasone, it was 2.19 ± 0.9 nM 2.5±0.8 nM, average for all 15 experiments (P=0.23).
Discussion
The most important finding in the present study is that recombinant VNUT co-localizes with fluorescent markers of ATP stores in secretory vesicles/zymogen granules of pancreatic acinar cells. Furthermore, dexamethasone treatment of AR42J cells increases VNUT and amylase expression, as well as ATP release induced by cholinergic stimulation. Mechanical stimulation also causes significant ATP release, presumably by mechanisms that are not associated with zymogen granules. We speculate on possible physiological and pathophysiological implications of autocrine and paracrine signalling in pancreas.
The observation that VNUT-mCherry localizes together with fluorescent markers of ATP stores, such as Bodipy FL ATP and quinacrine into vesicles (Figs. 1 and 2), confirms our early studies and validity of using such fluorescent markers [4]. VNUT and vesicular ATP release have now been demonstrated in a number of cells including T cells, biliary epithelial cells, lung cancer cells, airway epithelial cells, hippocampal neurons, and microglia [10–15]. In freshly isolated pancreatic acini, secretory/zymogen granule marker Rab3D co-localizes with VNUT (Fig. 1), suggesting that VNUT is expressed in ZG membrane, which supports our earlier findings showing functional VNUT on ZG membranes [9]. ZG are most obvious in freshly isolated acini, where they fill apical poles of the acinar cells. AR42J cells are not as polarized as native acini, and some VNUT/ATP-containing vesicles are also detected on cell periphery. We cannot exclude that VNUT also marks other secretory granules or vesicles.
Nevertheless, in AR42J cells, dexamethasone treatment promotes acini phenotype (Fig. 3) and 2- to 3-fold increase in VNUT and amylase expression (Fig. 4). Correspondingly, in dexamethasone-treated cells, carbachol induced about 3-fold higher ATP release compared to non-treated cells (Fig. 6). These experiments show that VNUT is important for ATP loading into ZG and eventual ATP release, presumably with amylase and other digestive enzymes.
Accompanied with fluid secretion originating in acini, this ATP/enzyme mixture is first in contact with luminal membranes of pancreatic ducts, which express various P2 receptors that can initiate and potentiate fluid secretion by pancreatic ducts [1, 24, 25]. This acini-to-duct purinergic signalling is well established, and the concentration of ATP released into the lumen, estimated to be in high micromolar range by imaging methods, would be sufficient to activate these receptors [4, 9].
Mechanical/shear stress induces ATP release from many types of cells that would normally be exposed to such stresses in physiological context, e.g., erythrocytes, endothelial cells, neuronal and glial cells, bone cells, and tubular structures like airway surface epithelia, bile ducts, bladder, etc. Here, we show that mechanical stimuli, e.g., shear/pressure stress, induced fast and large ATP release from both primary mouse acini and rat AR42J cells (Fig. 5). There was proportionate increase in ATP release, which reached a maximum, suggesting that the ATP release mechanisms were saturated or that the ATP pool for this release pathway was exhausted. Our study shows that this mechanically induced ATP release was independent of inducible, zymogen granule-associated VNUT pool and other releasing mechanism needs to be considered. For example, in endothelial cells that are subjected to shear stress, ATP is released from caveoli [26]. However, in esophageal keratinocytes, mechano-/chemo-sensing TRPV4 causes ATP release that involves exocytosis [27]. Also in biliary epithelial cells, hypotonic stress caused ATP release by VNUT-dependent exocytosis [11]. Nevertheless, there are different forms of mechanical stress, and most cells tested react with ATP release, and there are probably several paths for ATP release, and some may depend on intracellular Ca2+ stores [2, 3]. In any case, we show that mechanical release and basal release are not associated with the inducible zymogen granule and VNUT expression and therefore ZG exocytosis towards lumen (Fig. 5). However, we cannot exclude that acini have other sub-membrane vesicles that may also express VNUT (or transporters), which not necessarily point towards lumen but are close to the basolateral membranes, and therefore mediate fast and easily detected ATP release compared to across the luminal ZG release. This may explain why mechanically induced ATP release is relatively large compared to, for example, agonist-induced release. Moreover, mechanically induced ATP release seems to be persistent and/or shows slow recovery. However, one should recall that we monitor ATP concentrations that depend on a balance between ATP release and ATP hydrolysis. In pancreatic acini, agonists and hormones also stimulate release/activation of ecto-nucleotidases (CD39 and CD73) that contribute to rapid breakdown of ATP [5, 7]. Whether mechanical stimuli bypass these processes in acini is not clear.
Nevertheless, it is pertinent to ask whether the pressure stimulus could be relevant in pancreas. Pancreatic ducts can be subjected to obstruction caused by pancreatic stones or gallstones, and pancreatic acini would in turn experience increased back pressure, and this stimulus could result in ATP release. Luckily, adult pancreatic acini so far studied do not express many functional purinergic P2 receptors [17, 28], and thus, we propose that autocrine activation and autolysis of pancreatic acini are not imminent. However, the neighboring cells, such as pancreatic stellate cells, are stimulated by ATP and express P2 receptors [16, 29], where at least the P2X7 receptor can lead to proliferation and fibrosis in moderate ATP concentrations cells. Though, at high ATP concentrations, PSCs die possibly by necrosis, and release of cytokines would lead to stimulation of inflammatory cells, nerve endings, and other cells [30].
One of the most commonly used experimental protocols for ATP release is the hypotonic stress. Our experiments show that hypotonic shock caused significant (but relatively transient) ATP release (Fig. 5), and this is in agreement with other studies on epithelial cells [31, 32]. The implication of ATP release in pancreatic acinar cells is not clear, as cell swelling occurs in many situations, including epithelial transport (recovery after secretion), proliferation processes, and cell cycle progression [33]. Again, as with the mechanical/shear stress, the paracrine signalling to other cells may be of relevance.
Apart from transiently induced ATP release (e.g., by agonists and hormones), cells have basal release of ATP and other nucleotides, which can involve a number of releasing mechanisms and ecto-enzymatic modifications, and serve auto/paracrine modulation of cellular functions [34, 35]. Our experiments indicate that there were no significant differences in basal extracellular ATP between cells with/without dexamethasone, which would suggest that this was independent of VNUT expression. However, more dedicated experimental protocols would be needed to answer this question.
In conclusion, this study shows that mechanical stimuli and cholinergic stimulation induce ATP release in pancreatic acini, although the mechanism of release appears different. The cholinergically induced ATP release clearly involves exocytosis of ZG. Our previous study showed that VNUT contributes to ATP loading of ZG, and here, we show that VNUT is also involved in exocytotic release. We propose that ATP release towards lumen could act on the ductal cells to regulate secretion, and towards interstitium on the surrounding stromal cell, e.g., PSCs to induce proliferation and protective behavior. The latter events may be associated with pathophysiological processes in pancreas, such as in acute and chronic pancreatitis.
Acknowledgments
Imaging was performed at the Center for Advanced Bioimaging (CAB) Denmark, University of Copenhagen, and support from N.M. Christensen is greatly appreciated. The technical assistance of Pernille Roshof and David Sørensen is greatly acknowledged.
This project was supported by The Danish Council for Independent Research | Natural Sciences. Part of instrumentation was supported by The Lundbeck Foundation, The Novo Nordisk Foundation, and The Carlsberg Foundation.
Conflict of interest
There are no conflicts of interest, financial or otherwise.
Abbreviations
- ZG
zymogen granule
- VNUT
vesicular nucleotide transporter
References
- 1.Burnstock G, Novak I. Purinergic signalling in the pancreas in health and disease. J Endocrinol. 2012;213:123–141. doi: 10.1530/JOE-11-0434. [DOI] [PubMed] [Google Scholar]
- 2.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baroja-Mazo A, Barbera-Cremades M, Pelegrin P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta. 2013;1828:79–93. doi: 10.1016/j.bbamem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem. 2001;276:329525–329532. doi: 10.1074/jbc.M103313200. [DOI] [PubMed] [Google Scholar]
- 5.Yegutkin GG, Samburski SS, Jalkalen S, Novak I. ATP-consuming and ATP-generating enzymes secreted by pancreas. J Biol Chem. 2006;281:29441–29447. doi: 10.1074/jbc.M602480200. [DOI] [PubMed] [Google Scholar]
- 6.Novak I, Jans IM, Wohlfahrt L. Effect of P2X7 receptor knockout on exocrine secretion of pancreas, salivary glands and lacrimal glands. J Physiol Lond. 2010;588(18):3615–3627. doi: 10.1113/jphysiol.2010.190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sørensen CE, Amstrup J, Rasmussen HN, Ankorina-Stark I, Novak I. Rat pancreas secretes particulate ecto-nucleotidase CD39. J Physiol Lond. 2003;551(3):881–892. doi: 10.1113/jphysiol.2003.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haanes KA, Novak I. ATP storage and uptake by isolated pancreatic zymogen granules. Biochem J. 2010;429:303–311. doi: 10.1042/BJ20091337. [DOI] [PubMed] [Google Scholar]
- 10.Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem. 2010;285:17406–17416. doi: 10.1074/jbc.M110.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathe MN, Woo K, Kresge C, Bugde A, Luby-Phelps K, Lewis MA, Feranchak AP. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J Biol Chem. 2011;286:25363–25376. doi: 10.1074/jbc.M111.232868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takai E, Tsukimoto M, Harada H, Sawada K, Moriyama Y, Kojima S. Autocrine regulation of TGF-beta1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J Cell Sci. 2012;125:5051–5060. doi: 10.1242/jcs.104976. [DOI] [PubMed] [Google Scholar]
- 13.Larsson M, Sawada K, Morland C, Hiasa M, Ormel L, Moriyama Y, Gundersen V. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb Cortex. 2012;22:1203–1214. doi: 10.1093/cercor/bhr203. [DOI] [PubMed] [Google Scholar]
- 14.Imura Y, Morizawa Y, Komatsu R, Shibata K, Shinozaki Y, Kasai H, Moriishi K, Moriyama Y, Koizumi S. Microglia release ATP by exocytosis. Glia. 2013;61:1320–1330. doi: 10.1002/glia.22517. [DOI] [PubMed] [Google Scholar]
- 15.Sesma JI, Kreda SM, Okada SF, van Heusden C, Moussa L, Jones LC, O’Neal WK, Togawa N, Hiasa M, Moriyama Y, Lazarowski ER. Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules. Am J Physiol Cell Physiol. 2013;304:C976–C984. doi: 10.1152/ajpcell.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haanes KA, Schwab A, Novak I. The P2X7 receptor supports both life and death in fibrogenic pancreatic stellate cells. PLoS ONE. 2012;7:e51164. doi: 10.1371/journal.pone.0051164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak I, Nitschke R, Amstrup J. Purinergic receptors have different effects in rat exocrine pancreas. Calcium signals monitored by Fura-2 using confocal microscopy. Cell Physiol Biochem. 2002;12:83–92. doi: 10.1159/000063784. [DOI] [PubMed] [Google Scholar]
- 18.Logsdon CD, Moessner J, Williams JA, Goldfine ID. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol. 1985;100:1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen PA, Rasmussen JH, Joorgensen PL. Expression in high yield of pig alpha 1 beta 1 Na, K-ATPase and inactive mutants D369N and D807N in Saccharomyces cerevisiae. J Biol Chem. 1996;271:2514–2522. doi: 10.1074/jbc.271.5.2514. [DOI] [PubMed] [Google Scholar]
- 20.Cesarini G, Murray J. Plasmid vectors carrying the replication origin of filamentous single-stranded phages. In: Setlow JK, Hollaender A, editors. Genetic engineering, principles and methods. New York: Plenum; 1987. pp. 135–154. [Google Scholar]
- 21.Chen X, Ernst SA, Williams JA. Dominant negative Rab3D mutants reduce GTP-bound endogenous Rab3D in pancreatic acini. J Biol Chem. 2003;278:50053–50060. doi: 10.1074/jbc.M309910200. [DOI] [PubMed] [Google Scholar]
- 22.Jessop NW and Hay RJ (1980) Characteristics of two rat pancreatic exocrine cell lines derived from transplantable tumors. In Vitro 16:212
- 23.Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- 24.Novak I. ATP as a signaling molecule - the exocrine focus. News Physiol Sci. 2003;18:12–17. doi: 10.1152/nips.01409.2002. [DOI] [PubMed] [Google Scholar]
- 25.Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 2008;4:237–253. doi: 10.1007/s11302-007-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto K, Furuya K, Nakamura M, Kobatake E, Sokabe M, Ando J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci. 2011;124:3477–3483. doi: 10.1242/jcs.087221. [DOI] [PubMed] [Google Scholar]
- 27.Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol. 2011;589:3471–3482. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park HS, Betzenhauser MJ, Won JH, Chen J, Yule DI. The type 2 inositol (1,4,5)-trisphosphate (InsP3) receptor determines the sensitivity of InsP3-induced Ca2+ release to ATP in pancreatic acinar cells. J Biol Chem. 2008;283:26081–26088. doi: 10.1074/jbc.M804184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Won JH, Zhang Y, Ji B, Logsdon CD, Yule DI. Phenotypic changes in mouse pancreatic stellate cell Ca2+ signaling events following activation in culture and in a disease model of pancreatitis. Mol. Biol Cell. 2011;22:421–436. doi: 10.1091/mbc.E10-10-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apte MV, Wilson JS. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol. 2012;27(Suppl 2):69–74. doi: 10.1111/j.1440-1746.2011.07000.x. [DOI] [PubMed] [Google Scholar]
- 31.Akopova I, Tatur S, Grygorczyk M, Luchowski R, Gryczynski I, Gryczynski Z, Borejdo J, Grygorczyk R. Imaging exocytosis of ATP-containing vesicles with TIRF microscopy in lung epithelial A549 cells. Purinergic Signal. 2012;8:59–70. doi: 10.1007/s11302-011-9259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novak I. Purinergic signalling in epithelial ion transport: regulation of secretion and absorption. Acta Physiol (Oxf) 2011;202:501–522. doi: 10.1111/j.1748-1716.2010.02225.x. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen SF, Hoffmann EK, Novak I. Cell volume regulation in epithelial physiology and cancer. Front Physiol. 2013;4:233. doi: 10.3389/fphys.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010;3:re1. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helenius M, Jalkanen S, Yegutkin G. Enzyme-coupled assays for simultaneous detection of nanomolar ATP, ADP, AMP, adenosine, inosine and pyrophosphate concentrations in extracellular fluids. Biochim Biophys Acta. 2012;1823:1967–1975. doi: 10.1016/j.bbamcr.2012.08.001. [DOI] [PubMed] [Google Scholar]