Abstract
According to the World Health Organization, bladder cancer is the seventh most common cancer among men in the world. The current treatments for this malignancy are not efficient to prevent the recurrence and progression of tumors. Then, researches continue looking for better therapeutic targets which can end up in new and more efficient treatments. One of the recent findings was the identification that the purinergic system was involved in bladder tumorigenesis. The ectonucleotidases, mainly ecto-5′-nucleotidase/CD73 have been revealed as new players in cancer progression and malignity. In this work, we investigated the NTPDase3 and ecto-5′-nucleotidase/CD73 expression in cancer progression in vivo. Bladder tumor was induced in mice by the addition of 0.05 % of N-butyl-N-(hydroxybutyl)-nitrosamine (BBN) in the drinking water for 4, 8, 12, 18, and 24 weeks. After this period, mice bladders were removed for histopathology analysis and immunofluorescence assays. The bladder of animals which has received BBN had alterations, mainly inflammation, in initial times of tumor induction. After 18 weeks, mice’s bladder has developed histological alterations similar to human transitional cell carcinoma. The cancerous urothelium, from mice that received BBN for 18 and 24 weeks, presented a weak immunostaining to NTPDase3, in contrast to an increased expression of ecto-5′-nucleotidase/CD73. The altered expression of NTPDase3 and ecto-5′-nucleotidase/CD73 presented herein adds further evidence to support the idea that alterations in ectonucleotidases are involved in bladder tumorigenesis and reinforce the ecto-5′-nucleotidase/CD73 as a future biomarker and/or a target for pharmacological therapy of bladder cancer.
Keywords: Bladder cancer, BBN, Purinergic signaling, NTPDase3, Ecto-5′-nucleotidase/CD73
Introduction
Bladder cancer is the second most prevalent tumor in the genitourinary tract [1, 2]. It is the seventh most common cancer worldwide with about 336,000 new cases per year [3]. The main risk factors are smoking, which increase the risk up to six times, and occupational and environmental exposure to carcinogens [4, 5]. About 90 % of bladder cancer corresponds to transitional cell carcinoma (TCC) [1, 6] due to the fact that urothelium is constantly exposed to potential carcinogens [7]. The tumor invasiveness of TCC defines the patient’s prognosis. For example, 70–80 % of patients present superficial non-muscle-invasive TCCs which are generally not life threatening; while 20–30 % of individuals have muscle-invasive TCCs with increased risk of metastasis and death [8, 9]. Superficial cancers are treated by transurethral resection (TUR), followed by chemo/immunotherapy. However, nearly 70 % of patients present tumor recurrence, where 30 % of the recurrent tumors progress to muscle-invasive disease within 5 years of TUR [8, 10]. Given the high recurrence rates and the need for frequent monitoring, this disease is one of the most expensive cancers to treat on a per patient basis from diagnosis until death [11], and hence, poses a tremendous burden on health systems worldwide [4]. Therefore, new therapeutic targets, which end up in more efficient treatments, are necessary to prevent bladder cancer recurrence and progression.
Accordingly, recent researches have focused in the potential involvement of purinergic system in bladder tumors [1, 12–14]. Nucleosides and nucleotides mediate a variety of biological functions in both short- and long-term signaling functions (development, regeneration, differentiation, proliferation, and cell death) [1, 12]. These events are mediated by the activation of P1 (for adenosine) or P2 (for ATP, ADP, UDP, and UTP) receptors and are controlled by the action of ectonucleotidases [15]. The ecto-nucleoside triphosphate diphosphohydrolases refer to a family of cell-surface enzymes that hydrolyze extracellular ATP and ADP to AMP. Studies have demonstrated the involvement of these enzymes in cancer progression [16–18]. The final dephosphorylation of nucleotides, conversion of nucleoside monophosphates (e.g., AMP) to their respective nucleosides (e.g., adenosine), is catalyzed by ecto-5′-nucleotidase/CD73 (ecto-5′-NT/CD73). This enzyme is highly expressed in a variety of solid tumors [19–22] and has both its enzymatic activity and its adhesion protein function associated with cancer progression [23, 24]. Besides, ecto-5′-NT/CD73 was found to be involved in cancer cell growth, maturation, differentiation, adhesion, migration, invasiveness, metastasis, immune escape, and drug resistance [19, 21–27].
A previous study with mouse bladders showed that mouse healthy urothelium expresses only NTPDase3, not expressing the ecto-5′-NT/CD73 [28]. This is in agreement with a previous work from our group, where we showed a differential pattern of ectonucleotidases expression in two malignant bladder cancer cells. We showed that a less malignant lineage from a TCC grade 1 of malignancy (RT4) expresses NTPDase3 and ecto-5′-NT/CD73, while a more malignant lineage, from a TCC grade 4 of malignancy (T24) only expresses ecto-5′-NT/CD73 [14]. Although little is known about the role of NTPDase3 in cancer, these findings prompted us to suspect that the loss of NTPDase3 expression and the parallel ecto-5′-NT/CD73 expression might be involved in the bladder cancer progression.
Therefore, herein we investigate the NTPDase3 and ecto-5′-NT/CD73 expression in a model of mouse bladder cancer induced by N-butyl-N-(hydroxybutyl)-nitrosamine (BBN).
Materials and methods
Reagents
BBN was purchased from Sigma (Sigma Chemical Company, St. Louis, MO, USA). Rabbit antibodies anti-rat ecto-5′-nucleotidase (rNu-9l) and anti-rat NTPDase3 (rN3-1l) were obtained from http://ectonucleotidases-ab.com. Alexa fluor 568 goat anti-rabbit IgG and Alexa fluor 488 phalloidin were purchased from Invitrogen (Invitrogen Co., Carlsbad, CA, USA). Optimum cutting temperature (OCT) freezing medium (Tissue-Tek; Sakura Finetek, Torrance, CA, USA). All other chemicals and solvents used were of analytical grade.
Animals
Male mice from the Balb-c lineage were used at the age of 10 weeks. Animals were obtained and maintained in the Unidade de Experimentação Animal do Hospital de Clínicas de Porto Alegre (HCPA) under a standard dark–light cycle (lights on between 7:00 a.m. and 7:00 p.m.) at room-controlled temperature (22 ± 2 °C). The mice had free access to standard laboratory chow and water. Mice were euthanatized by isoflurane inhalation. After euthanasia, the bladders were rapidly excised and processed as described below. All animal studies were carried out in strict accordance with the recommendations in the Brazilian national law number 11.794, from October 8, 2008, which determines the procedures to scientific use of animals. The protocol (protocol 10-0104) was approved by the Research Ethics Committee at group of research and graduation of HCPA. All efforts were made to minimize animal suffering.
Bladder cancer induction
The animal model of bladder cancer induction by BBN have been used in many studies [29–33] mainly because BBN induced alterations in the bladder of rodents that are correspondent to histopathological and molecular features of human transitional cell carcinoma [34–36]. In this work, bladder cancer was induced by the addition of 0.05 % of BBN in the drinking water for 4, 8, 12, 18, and 24 weeks with a respective control group for each induction time. The number of mice per group was 8 for control groups of 4, 8, and 12 weeks and 9, 10, and 10 for BBN groups of 4, 8, and 12 weeks, respectively; and for the cancer induction times of 18 and 24 weeks, it was 3 for control groups and 5 and 4 for groups that received BBN, respectively. The animals were weighed in an analytical balance, as well as the excised bladders. Then, the bladder wet weight was expressed as milligrams of bladder per 100 g of animal as an additional measure of edema [37].
Bladder processing
A Y-shaped cut was made in the excised bladders for further tissue processing. Thereby, bladders were disposed as a monolayer and were divided in two parts: apex and base. The tissues were embedded in OCT freezing medium and snap frozen in isopentane in dry ice and stored at −80 °C until use. In this study, we analyzed only the base of bladders due to its major and constant contact with urine, which ends up in the major probability of cancer. For this, the frozen base of bladders was sliced in cryostat to achieve histological slides (5 μm) that were used for histopathological analysis or for immunofluorescence.
Histopathological analysis
Histological slides were stained with hematoxylin and eosin (HE) for histopathological analysis. The lesions induced by BBN were classified in three groups: degeneration and inflammation, pre-neoplastic lesion, and cancer in accordance with the histological characteristics of each one. The analyses were done by a pathologist, blinded for the experimental data.
Immunofluorescence analysis of NTPDase3 and ecto-5′-NT/CD73 expression
Frozen cryostat sections (5 μm) from mouse bladder tissues were fixed in 10 % phosphate-buffered formalin mixed with cold acetone and washed three times for 5 min in Tris-buffered saline (TBS). Tissue sections were then incubated in 5 % fetal bovine serum prepared in TBS containing 0.25 % Triton X-100 for 30 min at room temperature. These sections were incubated 120 min at room temperature with the following primary antibodies: rabbit rN3-1l [38]; rabbit rNu-9l [39, 40]; each diluted in 5 % fetal bovine serum prepared in TBS containing 0.25 % Triton X-100. They were then incubated with Alexa 568-conjugated goat anti-rabbit IgG secondary antibody and Alexa 488 phalloindin (1:40) for 120 min at room temperature. Sections were counterstained with 4′, 6-diamidino-2-phenylindole, dihydrochloride (DAPI) blue (1:10,000) for 5 min at room temperature. All immunofluorescent localization data shown are representative images of staining performed on at least three individual bladders.
Scanning laser confocal analysis of fluorescently labeled cells
Imaging was performed on a Olympus FluoView™ 1,000 confocal microscope equipped with solid state lasers of 405, 473, 559, 635 nm (Centro de Microscopia Eletrônica da Universidade Federal do Rio Grande do Sul). Images were acquired by sequential scanning with Olympus UPLSAPO ×40 N.A 0.9 objective and the appropriate filter combinations. All images were acquired with the same power of lasers that resulted in images with the same size (512 × 512 pixels). The images quantification was made in MacBiophotonics ImageJ software. Only the red channel of the enzyme (ecto-5′-NT/CD73 or NTPDase3) immunostaining was chosen, and then background subtraction was done. Following, the whole area of urothelium (with or without cancer) was selected with a region of interest (ROI) and the mean of fluorescence from ROI was acquired in number. With the mean of fluorescences, statistical analysis comparing the different times of bladder cancer induction with control was performed. The images were quantified and saved as TIFF files in MacBiophotonics ImageJ software, and finally imported in CorelDRAW X6 software.
Statistical analysis
All results are presented as mean ± SD. Bladder weight data were analyzed by two-way ANOVA (between-group factor: treatments; within-group factor: weeks of treatment), followed by Bonferroni post test. Quantified immunofluorescence data were analyzed by one-way ANOVA, followed by Tukey post hoc test. Differences between mean values were considered significant at p < 0.05.
Results
Mouse bladder cancer induction by BBN exposition
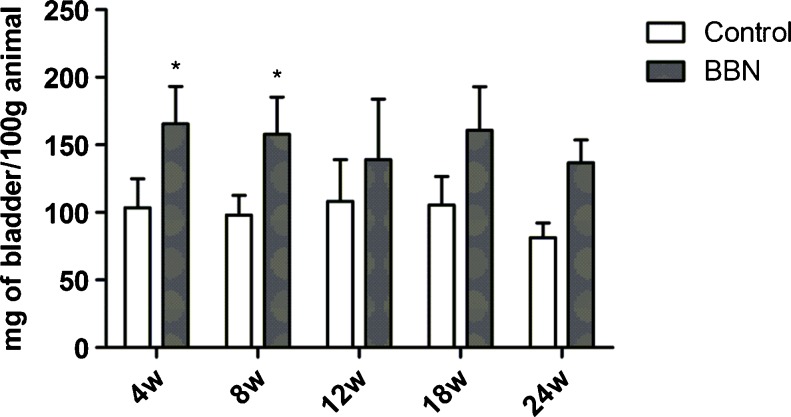
Over the time of bladder cancer induction, the animals did not exhibit unusual or altered behavior. Importantly, BBN toxicity was limited to bladder tissue and the visual analysis of other organs, including liver, kidney, lung, and heart showed no morphological alterations between BBN-treated and control mice. Macroscopically, the bladders of animals that received BBN had thicker walls than that of control animals. Figure 1 shows the bladder wet weight in different times of bladder cancer induction where animals that received BBN for 4 and 8 weeks had a significant increase in bladder weight in comparison with their respective control groups [F(1,57) = 44.96, p < 0.0001] without difference between different times of bladder cancer induction [F(4,57) = 1.361, p = 0.26]. For example, the bladder weight of control (healthy) animals killed at 4 weeks, was 103 ± 22 mg of bladder/100 g animal, while the bladder weight of animals which received BBN for 4 weeks was 166 ± 28 mg of bladder/100 g of animal.
Fig. 1.
Bladder wet weight expressed as mg of bladder/100 g animal to groups which received BBN in different times of cancer induction: 4, 8, 12, 18, and 24 weeks and their respective control groups. Values represent mean ± standard deviation and were analyzed through two-way ANOVA. *Differences between mean values of control and BBN, with p < 0.0001
Pathological analysis
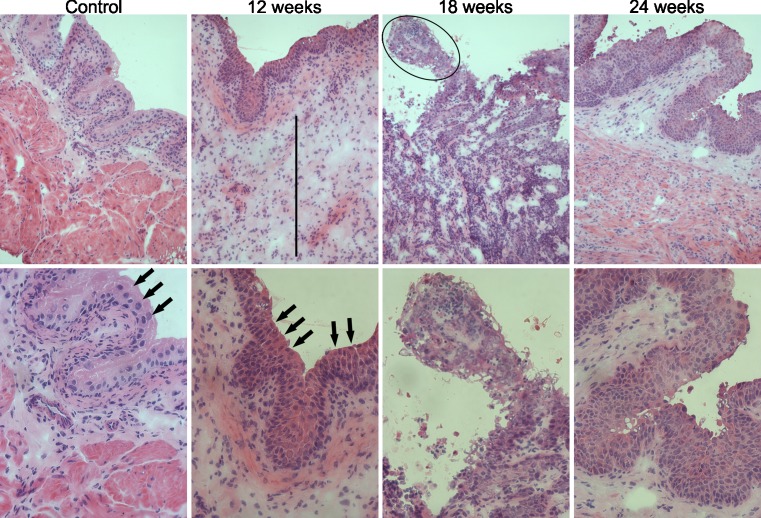
To characterize the tumor induced by BBN, pathological analysis was performed. As shown in Table 1, after the initial weeks of tumor induction (4, 8, and 12 weeks) BBN-treated mice developed bladder inflammation, which was eventually followed by cell degenerative alterations and pre-neoplastic transformation. In addition to promote inflammatory features, BBN exposition for 18 weeks also induced pathological alterations related to bladder cancer in 80 % of treated mice (Table 1). The BBN exposition for 24 weeks was effective to induce bladder cancer in 100 % of treated mice, as observed by the presence of pathological bladder alterations that resemble the human bladder tumors, including higher cell number and cell crowding, increased mitotic index, presence of giant cells, loss of umbrella cells, chromatin deposition, and other features as shown in Table 1. Figure 2 illustrate the features described on Table 1, showing HE staining from bladder of control mouse and from mouse that received BBN for 12, 18, and 24 weeks.
Table 1.
Incidence (%) of pathological features which comprises the different pathological conditions observed in HE staining of bladders from animals, which received BBN 0.05 % for 4 (n = 9), 8 (n = 10), 12 (n = 10), 18 (n = 5), and 24 (n = 4) weeks
| Pathological condition | Pathological features | Positive animals (%) of each cancer induction time | |||||
|---|---|---|---|---|---|---|---|
| 4 weeks | 8 weeks | 12 weeks | 18 weeks | 24 weeks | |||
| Inflammation | Cytoplasmic eosinophilia | 100 | 100 | 90 | – | – | |
| Bulkier nuclei | 56 | 90 | 90 | – | – | ||
| Atypical apoptosis | 33 | 80 | 70 | – | – | ||
| Degenerative changes | 56 | 100 | 90 | 20 | – | ||
| Surface erosion | 89 | 50 | 60 | 20 | – | ||
| Endothelial proliferation | 100 | 90 | 70 | 20 | – | ||
| Submucosal edema | 100 | 100 | 70 | 40 | – | ||
| Submucosal Inflammatory infiltrate | Mild | 22 | 50 | 40 | 100 | – | |
| Moderate | 11 | 40 | 30 | – | – | ||
| Strong | 56 | 10 | 30 | – | – | ||
| Muscle inflammatory infiltrate | Mild | 78 | 70 | 70 | 100 | – | |
| Strong | – | 30 | 30 | – | – | ||
| Pre-neoplastic lesion | Chromatin deposition | 33 | 70 | 80 | 100 | 100 | |
| Loss of umbrella cells | 11 | – | 40 | 80 | 100 | ||
| Dysplasia | 22 | – | 70 | – | – | ||
| Loss of intercellular cohesion | 22 | 40 | 90 | 80 | 100 | ||
| Hyperplasia | Focal | 44 | 20 | 10 | – | – | |
| Diffuse | – | – | 70 | – | – | ||
| Cancer | Papillary | – | – | – | 20 | 25 | |
| In situ | – | – | – | – | – | ||
| Invasive | Until lamina propria | – | – | – | – | – | |
| Until muscle | – | – | – | 80 | 100 | ||
| Lesion degree | Low | – | – | – | 40 | 50 | |
| High | – | – | – | 60 | 50 | ||
| Higher number of cells and cell crowding | – | – | – | 80 | 100 | ||
| Pleomorphism | – | – | – | 80 | 100 | ||
| Nuclear hyperchromia | – | – | – | 80 | 100 | ||
| Giant cells | – | – | – | – | 25 | ||
| Increased mitotic index | – | – | – | 80 | 100 | ||
| Inflammatory infiltrate | Peritumoral | – | – | – | – | 75 | |
| Intratumoral | – | – | – | – | 50 | ||
Fig. 2.
The images correspond to hematoxylin and eosin staining of bladders from control animal and bladders from animals which received BBN for 12, 18, and 24 weeks, correspondent to each column respectively. Images of the top row were taken with ×200 of increase and the images of the second row with ×400. Black arrows indicate examples of umbrella cells, specific from urothelium. It could be observed that the bladder from animals that received BBN for 12 weeks present features of inflammation, mainly edema (black line indicates the length of edema area). Cancer features as a papillary carcinoma (indicated by black circle) and loss of umbrella cells, increase of number of cells and cell crowding, could be observed in bladders from 18 to 24 weeks
Immunofluorescence analysis of NTPDase3 and ecto-5′-NT/CD73 in bladder after induction of cancer
Taking in account that the results presented in Table 1 demonstrate that the bladders from animals receiving BBN after 4, 8, and 12 weeks showed pathological features similar to each other typical of inflammation, and after 18 and 24 weeks typical features of cancer, although we have been done the immunofluorescence analysis of all times of treatment, here, we are showing only the results of control, and 12 and 24 weeks, which are representative of all groups.
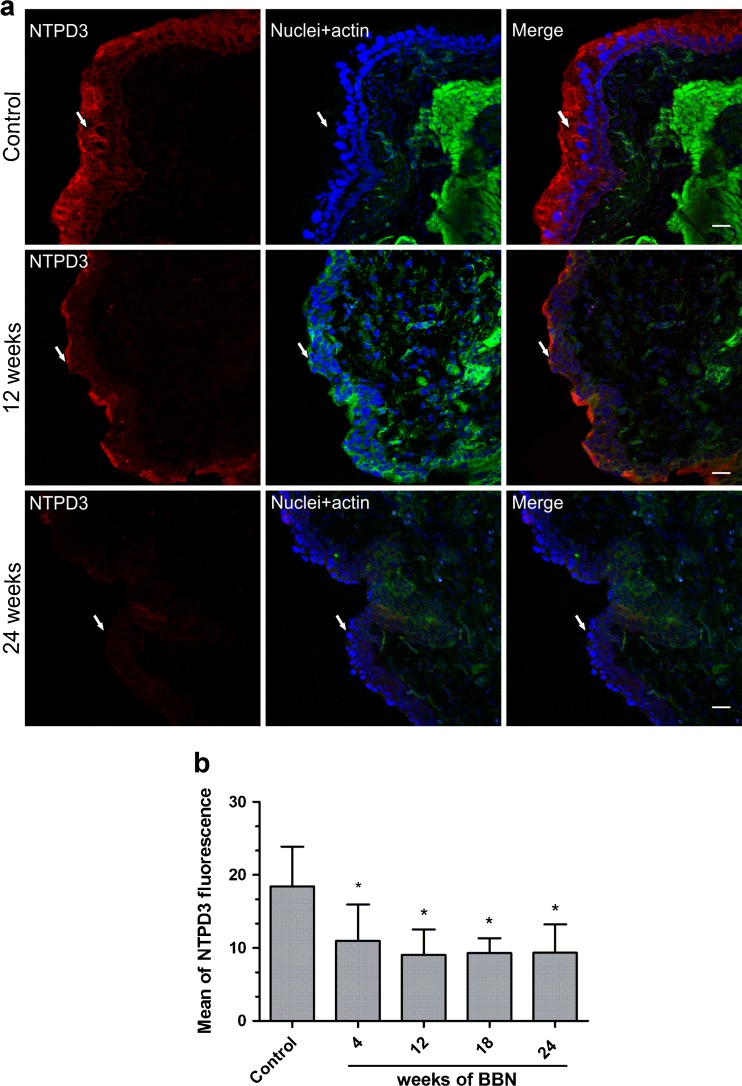
Previously, we have reported that the expression of NTPDase3 is absent in the T24 cell line, a representative in vitro model of invasive and metastatic bladder cancer [14]. To better investigate the involvement of NTPDases in an in vivo bladder cancer model, cryosections of bladder tissues from control or BBN-treated mice were labeled with antibody against NTPDase3. Accordingly, to the results previously published by Yu et al. [28], NTPDase3 was expressed only in the urothelium of bladder tissue from control mice while a decrease of NTPDase3 expression was observed over the time of BBN exposition (Fig. 3a). The decrease of NTPDase3 expression along the development of malignant alterations of bladder tissue was confirmed by quantitative analysis showed in the Fig. 3b. Importantly, the weaker NTPDase3 expression during the induction of bladder cancer suggests the participation of this enzyme in in vivo bladder cancer progression, which is in agreement to our previously published data [14].
Fig. 3.
a The images correspond to immunofluorescence of NTPDase3 in bladder urothelium at different times of bladder cancer induction. Cryosections of mouse bladders were labeled with antibody to NTPDase3 (red), Alexa 488 phalloidin to label actin cytoskeleton (green), and DAPI to label nuclei (blue). Color-merged panels are shown on the right. Pictures correspond to increase of ×400, whose focus was in urothelium (white arrows). White scale bars = 25 μm. b Quantification of NTPDase3 staining of bladder urothelium from mice which received BBN for 4, 8, 12, 18, and 24 weeks. The immunofluorescent images of NTPDase3 were quantified in MacBiophotonics ImageJ software as described in Materials and methods. Bars represent mean ± standard deviation of at least 30 mean of fluorescence of Z project acquired from three different animals. The data were analyzed for statistical significance by one-way ANOVA, followed by Tukey post hoc. Differences between mean values were significant at p < 0.001. * Significantly different compared to control
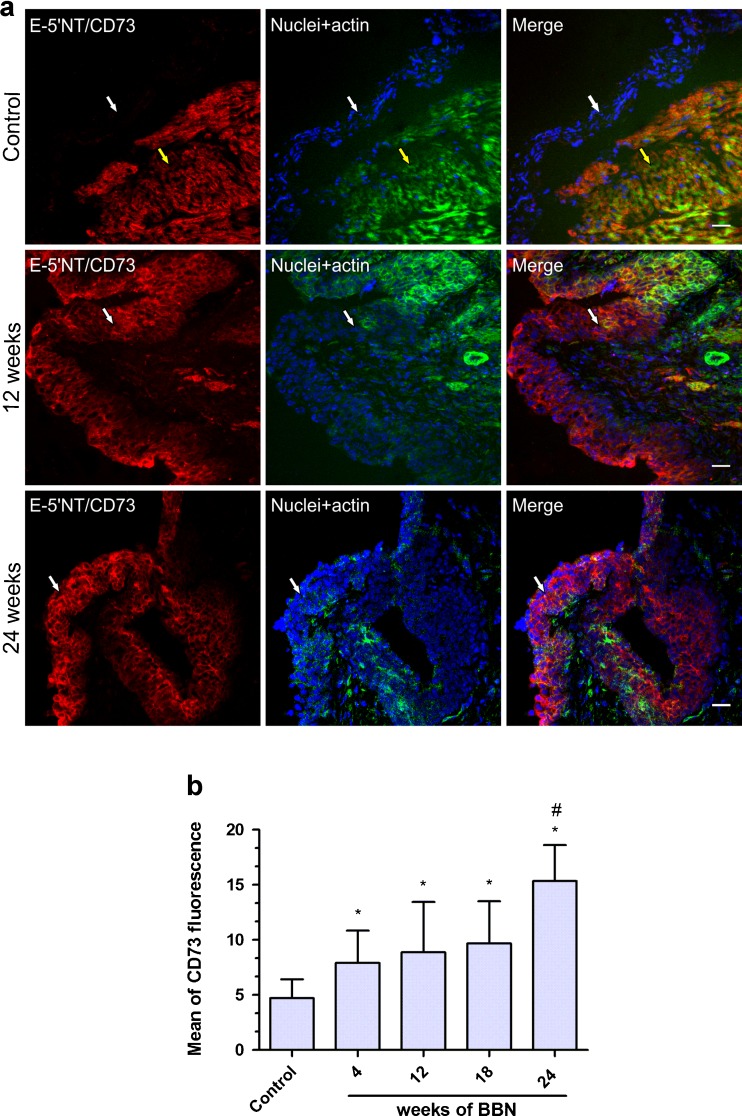
Next, we evaluate whether the ecto-5′-NT/CD73 could also be associated to bladder cancer progression in our model. As shown in Fig. 4a, the ecto-5′-NT/CD73 expression in bladder of control mice was absent in the urothelium cell layer, being restricted to detrusor smooth muscle cells as also previously shown by Yu et al. [28]. Notably, and in contrast to NTPDase3, ecto-5′-NT/CD73 expression increased dramatically in the urothelium of bladder of BBN-treated mice (Fig. 4a). This result was confirmed by quantitative analysis of the ecto-5′-NT/CD73 immunofluorescence, which increased in a time-response profile of BBN exposition (4, 8, 12, 18, and 24 weeks). Moreover, it was observed that an increase in the areas of increased cell proliferation and decreased presence of umbrella cells are important pathological characteristics of a malignant bladder process. These features can be better observed in the cancer urothelium from mice following 24 weeks of BBN exposition (Fig. 4a), which had an increase of ecto-5′-NT/CD73 significantly different of all other groups (Fig. 4b). Taken together, these results suggest that the increasing in the ecto-5′-NT/CD73 expression correlates to bladder cancer progression.
Fig. 4.
a The images correspond to immunofluorescent staining of ecto-5′-NT/CD73 in different times of bladder cancer induction. Cryosections of mouse bladders were labeled with antibody to ecto-5′-NT/CD73 (red), Alexa 488 phalloidin to label actin cytoskeleton (green), and DAPI to label nuclei (blue). Color-merged panels are shown on the right. Pictures correspond to increase of ×400 whose focus was in urothelium (white arrows), but in the control lamina propria and muscle (yellow arrows) can also be observed. White scale bars = 25 μm. b Quantification of ecto-5′-NT/CD73 staining of bladder urothelium from mice which received BBN for 4, 8, 12, 18, and 24 weeks. The immunofluorescent images of ecto-5′-NT/CD73 were quantified in MacBiophotonics ImageJ software as described in Materials and methods. Bars represent mean ± standard deviation of at least 30 mean of fluorescence of Z project acquired from three different animals. The data were analyzed for statistical significance by one-way ANOVA, followed by Tukey post hoc. Differences between mean values were significant at p < 0.001. * Significantly different compared to control. # Significantly different to all other groups
Discussion
Bladder cancer remains a tremendous burden for health systems worldwide [4] mainly due to the high rate of tumor recurrence and progression. Wherefore, the molecular biology of this tumor needs to be better understood in an attempt to find new therapeutic targets, which end up in more efficient treatments.
The alterations induced by BBN exposition for 4 and 8 weeks have shown histopathological features typical of bladder inflammation, with hard edema in submucosal and few pre-neoplastic lesions. After 12 weeks and more clearly after 18 weeks, progressive decrease of inflammation and increase of malignant features transformations could be observed up to 24 weeks, when all mouse bladder presented features of bladder transitional cell carcinoma (Table 1 and Fig. 2). This sequence of alterations are in agreement with data from literature, where long-time administration of BBN and other carcinogenic compounds results in a series of proliferative changes in bladder mucosa before the invasive bladder cancer [29–31]. Initially, hyperplasia and papillary or nodular hyperplasia occurs, followed by invasive carcinoma [31]. In addition, the bladder wet weight was significantly increased in animals which received BBN for 4 and 8 weeks (Fig. 1), according to the strong edema observed in their bladders by HE staining (Table 1 and Fig. 2).
As described, for healthy urothelium [28] and bladder cancer cell lines (T24 and RT4) [14], our results showed that, in fact, there is a decrease in NTPDase 3 expression (Fig. 3) and the outset followed to increase of ecto-5′-NT/CD73 expression (Fig. 4) during bladder cancer progression in vivo.
The enzymatic action of NTPDase3 and ecto-5′-NT/CD73 results to ATP hydrolysis and generation of immunosuppressive adenosine, respectively. Inflammation acts in all stages of tumorigenesis, at early stages, creates a favorable microenvironment which favors mutations, genomic instability, and epigenetic modifications and in cancer progression stimulating angiogenesis, immune escape, and tumor growth [41, 42]. In addition, ATP and adenosine participates in inflammatory signaling [43]. ATP has been described as a pro-inflammatory molecule acting as a chemotactic signal to immune phagocytes [44, 45] while adenosine has an immunosuppressive role suppressing innate and adaptative immune responses [44]. The participation of altered ectonucleotidases expression in the modulation of immune cells to contribute to cancer progress have been described to gliomas [46], that have a similar ATP/ADP/AMPase activity pattern of bladder cancer. This profile of nucleotide metabolism may favor extracellular ATP and adenosine accumulation within the tumor interstice, so while ATP could induce the dead of healthy cells, tumor proliferation, and recruitment of immune cells, adenosine is responsible for angiogenesis and immunosuppression [46]. Although, the BCG antimutor activity is due to stimulation of the local and acute immune response and the recruitment of polymorphonuclear neutrophil granulocytes [33], the presence of ATP and adenosine may be important to create chronic inflammatory conditions observed in tumor microenvironment, which suppress the immune response. This hypothesis is reinforced by the increasing density of immune cells in the bladder tissues during the course of BBN treatment. Moreover, the massive presence of tumor-associated macrophages in late clinical staging of patients with bladder cancer [47] and the association between tumor infiltrating lymphocytes and the recurrence of non-muscle-invasive bladder cancer [48] further suggest the chronic inflammatory process and cancer progression association.
Moreover, both, ATP and adenosine have been extensively described to participate in bladder signaling [28, 49–53]. Urothelium is a source of ATP release [28, 50, 51] and it is also an important site of adenosine biosynthesis. Importantly, both ATP and adenosine have functions in exocytosis of umbrella cell layer [52, 53], which is the mechanism to increase the luminal surface area when the bladder fills (cytoplasmatic discoidal/fusiform vesicles fuse with the apical plasma membrane) [54]. The ATP released by urothelium is responsible for micturition reflex, through P2X3 from subepithelial nerve fibers [55]. Then, although it needs to be better elucidated, the changes in NTPDase3 and ecto-5′-NT/CD73 expression with malignant transformation of urothelial cells described in these work, would be expected to perturb nucleotide signaling in the bladder and thus affect some bladder functions.
Although little is known about the role of NTPDase3 in cancer, an important finding of the present study was the absence of ecto-5′-NT/CD73 in healthy urothelium [28] followed by increase in ecto-5′-NT/CD73 expression with the increase of features of malignancy in mice that received BBN (Fig. 4). These findings indicate that this enzyme is involved in bladder cancer progression, and makes it a pro-missory therapeutic target to local treatments by instillation of inhibitors of this enzyme. This result is in agreement with the literature that shows the increase of ecto-5′-NT/CD73 expression in many other cancers such as breast cancer, glioma, and melanoma [19, 23, 56]. Furthermore, ecto-5′-NT/CD73 overexpression promotes invasion, migration, adhesion, and metastasis of human breast cancer cells [20, 57], indicating higher invasiveness and metastatic capability to melanomas [56, 58] and poor prognosis in human colorectal cancer [22]. Ecto-5′-NT/CD73 expression in cancer cells has been also linked with drug resistance [26, 59] and immune escape [44, 60]. Moreover, researchers have already targeted ecto-5′-NT/CD73 in an attempt to develop promising therapies, for instance, ecto-5′-NT/CD73 inhibitors have shown antiproliferative effects in bladder cancer cell lines and gliomas [61, 62], and decrease in ovarian cancer progression [63]. The treatment of mice with anti-CD73 antibody inhibited breast tumor growth and metastasis [20], and the use of RNA interference of ecto-5′-NT/CD73 inhibited cell growth and invasion in human breast cancer cells [25].
In conclusion, the altered expression of NTPDase3 and ecto-5′-NT/CD73 presented herein add further evidence to support the idea that alterations in the activity and expression of ectonucleotidases are involved in bladder tumorigenesis. Although additional studies are needed to determine whether the alterations in the ectonucleotidases expression are the cause or consequence of malignant transformation of urothelium, we bring the ecto-5′-NT/CD73 as a future biomarker and/or a target for pharmacological therapy.
Acknowledgments
This work was supported by Fundo de Incentivo a Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA protocol 10-0104), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) e Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS). L. Rockenbach and F. Dietrich were recipients of CAPES fellowships and F. Figueiró was a recipient of CNPq fellowship. JS was supported by grants from the Canadian Institutes of Health Research (CIHR) and was the recipient of a Senior Scholarship from the “Fonds de la Recherche en Santé du Québec”. We thank L.R. Blazina, M.S. Quevedo, H.B. Biehl, L.A. Martins, and the team of Unidade de Experimentação Animal of HCPA for their excellent technical assistance.
Contributor Information
Liliana Rockenbach, Phone: +55-51-33085553, FAX: +55-51-33085535, Email: lilarockk@gmail.com.
Ana Maria Oliveira Battastini, Email: abattastini@gmail.com.
References
- 1.Burnstock G. Therapeutic potential of purinergic signaling for diseases of the urinary tract. BJU Int. 2001;107:192–204. doi: 10.1111/j.1464-410X.2010.09926.x. [DOI] [PubMed] [Google Scholar]
- 2.Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G. Purinergic receptor-mediated effects of ATP in high-grade bladder cancer. BJU Int. 2007;101:106–112. doi: 10.1111/j.1464-410X.2007.07286.x. [DOI] [PubMed] [Google Scholar]
- 3.Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. (2004) Pathology and genetics of tumours of the urinary system and male genital organs. In: Kleihues P, Sobin LH editors. World Health Organization Classification of Tumours. IARCPress. pp. 89–157. Available http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb7/bb7-chap2.pdf. Accessed 10 January 2013.
- 4.Bachir BG, Kassouf W. Cause–effect? Understanding the risk factors associated with bladder cancer. Expert Rev Anticancer Ther. 2012;12:1499–1502. doi: 10.1586/era.12.140. [DOI] [PubMed] [Google Scholar]
- 5.van Roekel EH, Cheng KK, James ND, Wallace DM, Billingham LJ, Murray PG, Bryan RT, Zeegers MP. Smoking is associated with lower age, higher grade, higher stage, and larger size of malignant bladder tumours at diagnosis. Int J Cancer. 2013 doi: 10.1002/ijc.28017. [DOI] [PubMed] [Google Scholar]
- 6.Rivas A, Burzio V, Landerer E, Borgna V, Gatica S, Ávila R, López C, Villota C, de la Fuente R, Echenique J, Burzio LO, Villegas J. Determination of the differential expression of mitochondrial long non-coding RNAs as noninvasive diagnosis of bladder cancer. BMC Urol. 2012;12:37. doi: 10.1186/1471-2490-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 8.Ariel I, Ayesh S, Gofrit O, Ayesh B, Abdul-ghani R, Pizov G, Smith Y, Sidi AA, Birman T, Schneider T, de Groot N, Hochberg A. Gene expression in the bladder carcinoma rat model. Mol Carcinog. 2004;41:69–76. doi: 10.1002/mc.20046. [DOI] [PubMed] [Google Scholar]
- 9.Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, Wu R, Chen C, Li X, Zhou L, He M, Li Z, Sun X, Jia W, Chen J, Yang S, Zhou F, Zhao X, Wan S, Ye R, Liang C, Liu Z, Huang P, Liu C, Jiang H, Wang Y, Zheng H, Sun L, Liu X, Jiang Z, Feng D, Chen J, Wu S, Zou J, Zhang Z, Yang R, Zhao J, Xu C, Yin W, Guan Z, Ye J, Zhang H, Li J, Kristiansen K, Nickerson ML, Theodorescu D, Li Y, Zhang X, Li S, Wang J, Yang H, Wang J, Cai Z. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011;43:875–878. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenk-Braat EA, Bangma CH. Immunotherapy for superficial bladder cancer. Cancer Immunol Immunother. 2005;54:414–423. doi: 10.1007/s00262-004-0621-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avritscher EB, Cooksley CD, Grossman HB, Sabichi AL, Hamblin L, Dinney CP, Elting LS. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68:549–553. doi: 10.1016/j.urology.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G. Potential therapeutic targets in the rapidly expanding field of purinergic signalling. Clin Med. 2002;2:45–53. doi: 10.7861/clinmedicine.2-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shabbir M, Burnstock G. Purinergic receptor-mediated effects of adenosine-5′-triphosphate in urological malignant deseases. Int J Urol. 2009;16:143–150. doi: 10.1111/j.1442-2042.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 14.Stella J, Bavaresco L, Braganhol E, Rockenbach L, Farias PF, Wink MR, Azambuja AA, Barrios CH, Morrone FB, Oliveira Battastini AM. Differential ectonucleotidase expression in human bladder cancer cell lines. Urol Oncol. 2010;28:260–267. doi: 10.1016/j.urolonc.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Dev Res. 2001;52:44–56. doi: 10.1002/ddr.1097. [DOI] [Google Scholar]
- 16.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia. 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson SW, Hoshi T, Wu Y, Sun X, Enjyoji K, Cszimadia E, Sundberg C, Robson SC. Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice. Am J Pathol. 2007;171:1395–1404. doi: 10.2353/ajpath.2007.070190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braganhol E, Morrone FB, Bernardi A, Huppes D, Meurer L, Edelweiss MI, Lenz G, Wink MR, Robson SC, Battastini AM. Selective NTPDase2 expression modulates in vivo rat glioma growth. Cancer Sci. 2009;100:1434–1442. doi: 10.1111/j.1349-7006.2009.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spychala J. Tumor-promoting functions of adenosine. Pharmacol Ther. 2000;87:161–173. doi: 10.1016/S0163-7258(00)00053-X. [DOI] [PubMed] [Google Scholar]
- 20.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B. CD73 promotes tumor growth and metastasis. Oncoimmunology. 2012;1:67–70. doi: 10.4161/onci.1.1.18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu XR, He XS, Chen YF, Yuan RX, Zeng Y, Lian L, Zou YF, Lan N, Wu XJ, Lan P. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol. 2012;106:130–137. doi: 10.1002/jso.23056. [DOI] [PubMed] [Google Scholar]
- 23.Bavaresco L, Bernardi A, Braganhol E, Cappellari AR, Rockenbach L, Farias PF, Wink MR, Delgado-Cañedo A, Battastini AM. The role of ecto-5′-nucleotidase/CD73 in glioma cell line proliferation. Mol Cell Biochem. 2008;319:61–68. doi: 10.1007/s11010-008-9877-3. [DOI] [PubMed] [Google Scholar]
- 24.Cappellari AR, Vasques GJ, Bavaresco L, Braganhol E, Battastini AM. Involvement of ecto-5′-nucleotidase/CD73 in U138MG glioma cell adhesion. Mol Cell Biochem. 2012;359:315–322. doi: 10.1007/s11010-011-1025-9. [DOI] [PubMed] [Google Scholar]
- 25.Zhi X, Chen S, Zhou P, Shao Z, Wang L, Ou Z, Yin L. RNA interference of ecto-5′-nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis. 2007;24:439–448. doi: 10.1007/s10585-007-9081-y. [DOI] [PubMed] [Google Scholar]
- 26.Quezada C, Garrido W, Oyarzún C, Fernández K, Segura R, Melo R, Casanello P, Sobrevia L, San Martín R. 5′-ectonucleotidase mediates multiple-drug resistance in glioblastoma multiforme cells. J cell Physiol. 2012;228:602–608. doi: 10.1002/jcp.24168. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Zhou X, Zhou T, Ma D, Chen S, Zhi X, Yin L, Shao Z, Ou Z, Zhou P. Ecto-5′-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol. 2008;134:365–372. doi: 10.1007/s00432-007-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu W, Robson SC, Hill WG. Expression and distribution of ectonucleotidases in mouse urinary bladder. Plos One. 2011;6:e18704. doi: 10.1371/journal.pone.0018704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen SM, Jacobs JB, Arai M, Johansson S, Friedell GH. Early lesions in experimental bladder cancer: experimental design and light microscopic findings. Cancer Res. 1976;36:2508–2511. [PubMed] [Google Scholar]
- 30.Ito N. Early changes caused by N-Butyl-N-(4-hydroxybutyl)nitrosamine in the bladder epithelium of different animal species. Cancer Res. 1976;36:2528–2531. [PubMed] [Google Scholar]
- 31.Oyasu R, Iwasaki T, Matsumoto M, Hirao Y, Tabuchi Y. Induction of tumors in heterotopic bladder by topical application of N-methyl-N-nitrosourea and N-butyl-N-(3-carboxypropyl) nitrosamine. Cancer Res. 1978;38:3019–3025. [PubMed] [Google Scholar]
- 32.Chihara Y, Fujimoto K, Miyake M, Hiasa Y, Hirao Y. Anti-tumor effect of cimetidine via inhibiting angiogenesis factors in N-butyl-N-(4-hydroxybutyl) nitrosamine-induced mouse and rat bladder carcinogenesis. Oncol Rep. 2009;22:23–28. doi: 10.3892/or_00000401. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, Cho YH, Park K, Kim EJ, Kang BS, Jung KH, Kim CH, Kim WJ, Moon SK. Inhibitory effects of the aqueous extract of Magnolia officinalis on the responses of human urinary bladder cancer 5637cells in vitro and mouse urinary bladder tumors induced by N-Butyl-N-(4-hydroxybutyl) nitrosamine in vivo. Phytother Res. 2009;23:20–27. doi: 10.1002/ptr.2413. [DOI] [PubMed] [Google Scholar]
- 34.Hirose M, Fukushima S, Hananouchi M, Shirai T, Ogiso T. Different susceptibilities of the urinary bladder epithelium of animal species to three nitroso compounds. Gann. 1976;67:175–189. [PubMed] [Google Scholar]
- 35.Chen G, Chan FL, Zhang X, Chan PS. Identification of differently expressed genes in chemical carcinogen-induced rat bladder cancers. Med Sci. 2009;29:220–226. doi: 10.1007/s11596-009-0217-y. [DOI] [PubMed] [Google Scholar]
- 36.Williams PD, Lee JK, Theodorescu D. Molecular credentialing of rodent bladder carcinogenesis models. Neoplasia. 2008;10:838–846. doi: 10.1593/neo.08432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batista CK, Brito GA, Souza ML, Leitão BT, Cunha FQ, Ribeiro RA. A model of hemorrhagic cystitis induced with acrolein in mice. Braz J Med Biol Res. 2006;39:1475–1481. doi: 10.1590/S0100-879X2006001100011. [DOI] [PubMed] [Google Scholar]
- 38.Vekaria RM, Shirley DG, Sévigny J, Unwin RJ. Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol. 2006;290:F550–F560. doi: 10.1152/ajprenal.00151.2005. [DOI] [PubMed] [Google Scholar]
- 39.Fausther M, Lecka J, Soliman E, Kauffenstein G, Pelletier J, Sheung N, Dranoff JA, Sévigny J. Co-expression of ecto-5′-nucleotidase/CD73 with specific NTPDases differentially regulates adenosine formation in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2011;302:G447–G459. doi: 10.1152/ajpgi.00165.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koszalka P, Özüyaman B, Huo Y, Zernecke A, Flögel U, Braun N, Buchheiser A, Decking UK, Smith ML, Sévigny J, Gear A, Weber AA, Molojavyi A, Ding Z, Weber C, Ley K, Zimmermann H, Gödecke A, Schrader J. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res. 2004;95:814–821. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 41.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and in inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Stagg J, Smyth MJ. Extracellular adenosine triphophate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 45.Säve S, Persson K. Extracellular ATP and P2Y receptor activation induce a proinflammatory host response in the human urinary tract. Infect Immun. 2010;78:3609–3615. doi: 10.1128/IAI.00074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergamin LS, Braganhol E, Zanin RF, Edelweiss MI, Battastini AM. Ectonucleotidases in tumor cells and tumor-associated immune cells: an overview. J Biomed Biotechnol. 2012;2012:959848. doi: 10.1155/2012/959848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ (2012) Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. 7: e50946 [DOI] [PMC free article] [PubMed]
- 48.Krpina K, Babarović E, Dorđević G, Fuckar Z, Jonjić N. The association between the recurrence of solitary non-muscle invasive bladder cancer and tumor infiltrating lymphocytes. Croat Med J. 2012;53:598–604. doi: 10.3325/cmj.2012.53.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burnstock G. Purinergic signalling in the lower urinary tract. Acta Physiol (Oxf) 2013;207:40–52. doi: 10.1111/apha.12012. [DOI] [PubMed] [Google Scholar]
- 50.Kumar V, Chapple CC, Chess-Williams R. Characteristics of adenosine triphosphate [corrected] release from porcine and human normal bladder. J Urol. 2004;172:744–747. doi: 10.1097/01.ju.0000131244.67160.f4ABSTRACT. [DOI] [PubMed] [Google Scholar]
- 51.Munoz A, Gangitano DA, Smith CP, Boone TB, Somogyi GT. Removal of urothelium affects bladder contractility and release of ATP but not release of NO in rat urinary bladder. BMC Urol. 2010;10:10. doi: 10.1186/1471-2490-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–2422. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu W, Zacharia LC, Jackson EK, Apodaca G. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol. 2006;291:C254–C265. doi: 10.1152/ajpcell.00025.2006. [DOI] [PubMed] [Google Scholar]
- 54.Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell. 2002;13:830–846. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ford AP, Cockayne DA. ATP and P2X purinoceptors in urinary tract disorders. Handb Exp Pharmacol. 2011;202:485–526. doi: 10.1007/978-3-642-16499-6_22. [DOI] [PubMed] [Google Scholar]
- 56.Sadej R, Spychala J, Skladanowski AC. Expression of ecto-5′-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res. 2006;16:213–222. doi: 10.1097/01.cmr.0000215030.69823.11. [DOI] [PubMed] [Google Scholar]
- 57.Zhou P, Zhi X, Zhou T, Chen S, Li X, Wang L, Yin L, Shao Z, Ou Z. Overexpression of ecto-5′-nucleotidase (CD73) promotes T-47D human breast cancer cells invasion and adhesion to extracellular matrix. Cancer Biol Ther. 2007;6:426–431. doi: 10.4161/cbt.6.3.3762. [DOI] [PubMed] [Google Scholar]
- 58.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71:2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 59.Ujházy P, Berleth ES, Pietkiewicz JM, Kitano H, Skaar JR, Ehrke MJ, Mihich E. Evidence for the involvement of ecto-5′-nucleotidase (CD73) in drug resistance. Int J Cancer. 1996;68:493–500. doi: 10.1002/(SICI)1097-0215(19961115)68:4<493::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 60.Zhang B. Opportunities and challenges for anti-CD73 cancer therapy. Immunotherapy. 2012;4:861–865. doi: 10.2217/imt.12.83. [DOI] [PubMed] [Google Scholar]
- 61.Rockenbach L, Bavaresco L, Fernandes Farias P, Cappellari AR, Barrios CH, Bueno Morrone F, Oliveira Battastini AM. Alterations in the extracellular catabolism of nucleotides are involved in the antiproliferative effect of quercetin in human bladder cancer T24 cells. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Braganhol E, Tamajusuku AS, Bernardi A, Wink MR, Battastini AM. Ecto-5′-nucleotidase/CD73 inhibition by quercetin in the human U138MG glioma cell line. Biochim Biophys Acta. 2007;1770:1352–1359. doi: 10.1016/j.bbagen.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]