Abstract
Phenolic compounds of fruits have been shown to maintain human health. However, the relative amounts of phenolic compounds and the variation in the types of phenolics are still poorly understood. The purpose of this study was to investigate the most effective solvent for extracting the potent antioxidant compounds, especially phenolics from pomegranate aril. Pomegranate aril was subjected to extraction using different solvents viz., water, ethanol, acetone and diethyl ether either alone or in combination, and the extraction yield, total phenolic contents, and antioxidant activity were investigated. The extracts derived from various solvents were also analysed using high performance liquid chromatography (HPLC) for quantification of major polyphenols (punicalagins, ellagic acid and gallic acid) of pomegranate. Amongst the tested solvents, combination of ethanol, diethyl ether and water (8:1:1) extract exhibited the highest 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging power (IC50 = 10.12 μg mL-1). Further, HPLC analysis of different extracts revealed that ethanol, diethyl ether and water (8:1:1) mixture contained significantly higher (p < 0.05) amounts of punicalagin A (1.06 μg mg-1 extract), punicalagin B (2.07 ± 0.03 μg mg-1 extract), ellagic acid (34.5 μg mg-1 extract) and gallic acid (3.37 μg mg-1 extract) in comparison to the other solvents used for extraction. The results demonstrate that pomegranate aril is a good source of phenolic compounds with high antioxidant activity and the antioxidant activity is dependent on the type of solvent system that extracts different phenolic compounds with varying polarity. The solvent extracts that showed effective antioxidants activities have the potential for application in suitable food products.
Keywords: Antioxidant, Extraction, Phenolics, Pomegranate
Introduction
Punica granatum L., commonly known as pomegranate, of Punicaceae family, holds an important place in Indian and global scenario owing to its nutritional and medicinal benefits. It is a deciduous shrub or small tree and widely distributed in tropical and sub-tropical countries. In folk medicine, pomegranate has been used to treat various ailments such as cuts, sore throats, tapeworms, dysentery, and gum disease. It also possesses antibacterial, antioxidant, anti-atherosclerotic, anti-inflammatory and anti-allergic properties (Perez-Vicente et al. 2002; Yu et al. 2005; Mertens-Talcott et al. 2006; Panichayupakaranant et al. 2010) Apart from this, recent research has focused on its potential use in treatments of cardiovascular diseases, diabetes, and various forms of cancer (Julie Jurenka 2008). The broad-spectrum properties of pomegranate can be ascribed to its numerous secondary metabolites. Amongst all, ellagic acid, gallic acid and punicalagin are the most important and major polyphenolic compounds (Seeram et al. 2005; Qua et al. 2012).
The solvent extraction has been widely used to extract phenolic compounds from fruits and vegetables. Among all the investigated variables (pre-treatment of the sample, solvent/sample ration, type of solvent, time and temperature of extraction) to ensure the efficiency of extraction, type of solvent has been the most studied factor. Polarity of solvents play a vital role in extraction process since with change in solvent polarity its ability to dissolve especial group of antioxidant compounds alters and influences the antioxidant activity estimation. It is impossible to develop a universal solvent that is suitable for the all kinds of antioxidant compounds extraction from plants because plant materials have diverse chemical profile. Thus, screening process is important to justify the best solvent in antioxidant compounds extraction so that the maximum antioxidant activity for a certain sample could be identified.
During the past decade, considerable efforts have been made to extract and identify pomegranate bioactive compounds (Gil et al. 2000; Kulkarni and Aradhya 2005; Mousavinejad et al. 2009; Qua et al. 2010; Zhang et al. 2011; Ҫama and Hișil 2011), however so far, work on the screening and selection of best solvent and/or solvent combination to obtain highest antioxidant activity and phenolic compound is lacking. Moreover, there is no report on the analysis of different extracts of pomegranate for ellagic acid, gallic acid, punicalagin A and B content. Beside this, despite the multifaceted uses of pomegranate, no perceptible advances have been made for this fruit to exploit or enhance its utility for use in developing functional food products.
The present study is therefore conducted with the objective to investigate the most effective solvent for extracting the potent antioxidant compounds, especially phenolics from pomegranate. In order to compare the antioxidant property of different extracts, DPPH. (2, 2-diphenyl-1-picrylhydrazyl) and ABTS (2, 2-azinobis-3-ethyl-benzothiazoline-6-sulfonicacid) methods were followed. Furthermore, high performance liquid chromatography (HPLC) was performed to quantify the amount of ellagic acid, gallic acid, punicalagin A and B present in different extracts.
Materials and methods
Materials
Mature fresh fruits of pomegranate were purchased from the local market of Varanasi, India, and brought to the Centre of Food Science and Technology, Banaras Hindu University, Varanasi, India. Fruits of uniform size and colour were chosen and washed in water. Thereafter, pomegranate arils were manually separated from the seed and used for extraction.
Chemicals
All the analytical grade organic solvents (viz., ethanol, acetone and diethyl ether) used for extraction and HPLC grade solvents (acetonitrile, methanol, water and formic acid) used for chemical analysis were procured from Qualigens, India. Tannic acid, gallic acid, DPPH. and ABTS were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ellagic acid and punicalagin standards were obtained from Alfa Aesar Organics (Ward Hill, MA, USA) and Chromadex (Santa Ana, CA, USA), respectively. For total phenolic determination, Folin-Ciocalteu reagent was procured from Merck (Mumbai, India).
Preparation of samples
To prepare samples, 50 g fresh arils were separately soaked in 100 mL of different solvents (ethanol, acetone, diethyl ether and water either alone or in combination) and placed in incubator shaker at 150 rpm for 12 h. The supernatant was transferred into a new tube and the residue was re-extracted thrice with 100 mL solvent. Thereafter, the residue was discarded and the supernatants were pooled, filtered and evaporated to dryness in a rotary evaporator (Perfit, Chennai, India) at 40 °C. The water extracts were lyophilized in a Martin Christ Alpha 1–2 freeze dryer (Osterode, Germany) and used for further studies. The percentage yield of extracts was calculated relative to the weight of fresh tissue.
DPPH. radical scavenging activity
Stock solutions (50 mg mL−1 each) of the extracts were prepared in ethanol. About 80 μg mL−1 solution of 2, 2-diphenyl-1-picrylhydrazyl (DPPH.) in ethanol was prepared and 1.0 mL of this solution was added to 200 μL of extract solution. Thirty minutes later, absorbance of the solution was recorded on an ultraviolet (UV)-1800 spectrophotometer (Shimadzu, Kyoto, Japan) at 517 nm using a blank containing the same concentration of DPPH. radicals. A lower absorbance of the reaction mixture indicated a higher free radical scavenging activity. The inhibition of the DPPH. radical by the sample was calculated according to the following formula:
 |
Where A blank is the absorbance value of the control reaction (containing all reagents except the extract) and A sample is the absorbance values of the extract.
ABTS radical scavenging assay
The 2,2-azinobis-3-ethyl-benzothiazoline-6 sulfonicacid (ABTS) radical scavenging assay was done according to Re et al. (1999) with slight modifications. The ABTS radical was generated by the oxidation of ABTS with ammonium persulphate. The ABTS radical cation solution was obtained as follows: 5 mL of ABTS (7 mM) was mixed with 88 μL of ammonium persulphate (140 mM) and incubated in dark at room temperature (25 °C) for 12–16 h. The working solution was prepared by diluting the previous solution with phosphate buffered saline (PBS; pH 7.2) until the absorbance at 734 nm was 0.70 ± 0.02. After which, 1 mL of each sample was mixed with 3 mL of the ABTS working solution, shaken vigorously, and left to stand for 10 min at room temperature. The absorbance of the reaction mixture was determined at 734 nm. The ABTS radical scavenging capacity of the sample was calculated using the following formula:
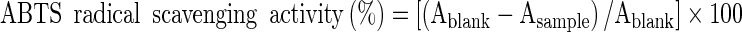 |
Where A blank is the absorbance value of the control reaction (containing all reagents except the extract) and A sample is the absorbance value of the extract.
Assay for total phenolics
Total phenolic constituents of extracts were determined using Folin-Ciocalteu reagent and tannic acid standard. Solutions of each extract (500 μL; 5 mg mL−1) were taken individually in test tubes. To this solution, 2.5 mL of 10-fold diluted Folin-Ciocalteu reagent was added, and the flasks were thoroughly shaken. After 1 min, 2.0 mL of 7.5 % Na2CO3 solution was added and the mixtures were allowed to stand for 30 min at room temperature (25 °C) with intermittent shaking. Absorbance was taken at 760 nm. The same procedure was repeated for all the standard tannic acid solutions, and a standard curve was obtained. Total phenols of the extract as tannic acid equivalents, were determined by using the absorbance of the extract measured at 760 nm as input to the standard equation. All tests were carried out in triplicate, and phenolic contents as tannic acid equivalents were reported.
Preparation of standard solution
Stock solutions (1,000 μg mL−1) of ellagic acid, gallic acid and punicalagins (A and B isomers) were prepared by dissolving 5 mg of the compound in 5 mL of HPLC grade methanol. The solutions were then stored at −20 °C. Quantification was carried out using 5 levels of external standards obtained by serial dilutions of stock solutions at a concentration range of 50 to 0.4 μg mL−1. Each concentration of standard was filtered through a 0.2 μm membrane filter (Axiva, Delhi, India) before HPLC analysis.
High performance liquid chromatography (HPLC)
Detection and quantification of ellagic acid, gallic acid and punicalagin A and B isomers were carried out using Shimadzu 20 AD, HPLC system (Shimadzu, Japan) consisted of Ultraviolet (UV) detector, a binary pump, a 20 μL injection loop, and RP-18 column of dimensions 4.6 × 250 mm.
The mobile phase used for ellagic acid was 30 % A (water + 1 % formic acid) and 70 % B (methanol + 1 % formic acid) with a flow rate of 1.0 mL min−1. The eluted samples were detected by UV detector at 254 nm.
For gallic acid, mobile phase used was 90 % A (water + 1 % formic acid) and 10 % B (acetonitrile + 1 % formic acid) with a flow rate of 1.0 mL min−1. The eluted samples were detected by UV detector at 280 nm.
For analysis of punicalagin A and B isomers, a linear gradient elution programme was applied, and elution was carried out with solvent A (water + 1 % formic acid) and solvent B (methanol + 1 % formic acid) as mobile phase. During HPLC analysis, the solvent gradient was programmed from 10 to 45 % B in A in 30 min with a flow rate of 1.0 mL min−1. UV detection was carried out at 254 nm with attenuation of 0.1 absorbance units at full scales (AUF).
Calibration curve was constructed by plotting the peak area (y) against concentration in μg mL−1 of standard solutions (x). The standard equation obtained from the curve was used for quantification of phenolic compounds in the unknown samples. Ellagic acid, gallic acid, punicalagin A and B content were reported as μg mg−1 extract of sample. Precision of developed assay was evaluated by running the same concentration of standard compounds at least thrice on the same day (intraday) and twice at 1 day intervals (interday).
Statistical analyses
Data were reported as mean ± standard deviation for at least triplicate analyses of the same extract. All statistical analyses were carried out using the SPSS software package (version 16). Multiple mean comparisons within the sample set were carried out at the 5 % significance level using Duncan’s multiple range test. Statistical significance was considered for p < 0.05.
Results and discussion
Extraction yield
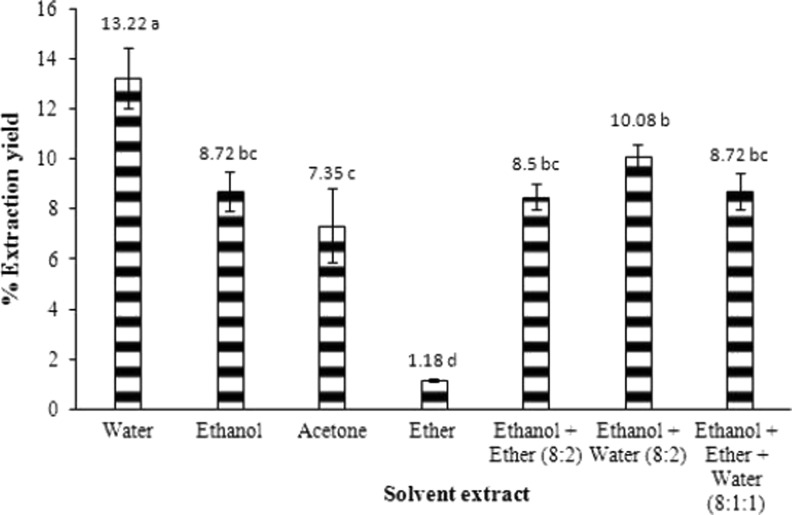
Selection of solvent is an important step for obtaining extracts with acceptable yields and strong antioxidant activity. Yield of the extracts with different solvents is in the order: water > ethanol + water > ethanol = ethanol + ether + water > ethanol + ether > acetone > ether (Fig. 1). The highest (13.22 %) and the lowest yields (1.18 %) were obtained in water and ether extracts (p < 0.05), respectively (Fig. 1). Zielinski and Kozłowska (2000) also obtained the highest yield in water extract. However, higher extraction yield does not necessarily imply that it will also have high antioxidant activity because the antioxidant activity depends on the active antioxidant compounds present in the extract.
Fig. 1.
Percentage yield of pomegranate extract in different solvent systems. Values are mean ± standard deviation of triplicates. Mean values sharing the same letter do not differ significantly (p < 0.05) according to Duncan’s multiple range test
Total phenolic content
Several studies have revealed that phenolic contents in plants are associated with antioxidant activities probably due to their redox properties that allow them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers (Chang et al. 2001) Therefore, the content of total phenolic compounds in the pomegranate extracts was determined through a linear tannic acid standard curve, y = 0.007x + 0.024; R2 = 0.991, and the results are presented in Fig. 2. The total phenolic content varied from 6.44 to 28.13 μg tannic acid equivalents mg−1 pomegranate extract. The highest value of total phenolic compounds was detected in the ethanol extract of pomegranate, whereas the lowest content was in the acetone extract (p < 0.05). These findings clearly demonstrate the influence of the solvents on the extractability of phenolics. Findings of this study are in agreement with previous reports which suggested that the nature of solvent exerts a great influence on phenolic extraction capacity of the plant (Akowuah et al. 2005; Turkmen et al. 2006).
Fig. 2.
Total phenolic content of different pomegranate extracts. Values are mean ± standard deviation of triplicates. Mean values sharing the same letter do not differ significantly (p < 0.05) according to Duncan’s multiple range test
DPPH. radical scavenging activity of various extracts
The DPPH. radical scavenging assay is a widely used method to evaluate the antioxidant capacity of extracts from different plant materials. The essence of DPPH. radical assay is that the antioxidant reacts with the stable free radical 1,1-Diphenyl-2-picrylhydrazyl (deep violet color) and reduces it to 1,1-Diphenyl-2-picrylhydrazine with a yellow colour. The degree of discoloration indicates the scavenging potential of the sample antioxidant resulting in a decrease in absorbance at 517 nm.
In this study, different extracts of pomegranate aril showed a significant variability in their inhibitory activity against DPPH. radical. A noticeable effect of the extract on radical scavenging activity was observed and the effect was dose dependent. Amongst tested solvents, the highest radical scavenging activity was detected in ethanol + ether + water extract, followed by ethanol + ether extract. The water extract of pomegranate showed very low radical scavenging activity with DPPH..
Variations in the antioxidant capacity of different extracts may be attributed to differences in their phenolic content and the type of phenolics which in turn depends on the solvent used for the extraction. It can be concluded that the extract obtained using combination of ethanol, ether and water was considerably more radical scavenger than the absolute solvents, indicating that the mixture of solvents, with change polarity, has ability to dissolve selected group of bioactive compounds. The determined antioxidant activity (DPPH.) of extracts correlated well with the total phenolics contents in pomegranate extracts. Our result was in agreement with several previous reports that higher phenolic content in extracts had greater antioxidant activity (Barros et al. 2008).
ABTS radical scavenging activity of various extracts
The ABTS radical cation decolourisation test is another technique usually used to investigate antioxidant activity. In the present study, all the extracts reduced the absorbance at 734 nm, and the concentration of the extracts was directly proportional to the reduction. Ethanol extract exhibited the highest ABTS radical scavenging activity. The ABTS radical scavenging activities of the pomegranate extracts are in the order: ethanol > ether > ethanol + ether + water > acetone > ethanol + ether > water > ethanol + water.
IC50 of DPPH and ABTS
The IC50 value is defined as the concentration of the sample necessary to cause 50 % inhibition, which is obtained by interpolation from linear regression analysis. A lower IC50 value is associated with higher radical scavenging activity. It was found that ethanol + ether + water extract had the strongest DPPH. radical scavenging activity, while ethanol extract showed the strongest ABTS radical scavenging activity (Table 1). The observed differential scavenging activities of the pomegranate extracts against DPPH. and ABTS systems may be due to the different mechanisms of the radical antioxidant reactions. Hagerman et al. (1998) have reported that the high molecular weight phenolics (tannins) have more abilities to quench free radicals (ABTS•+) and their efficiency depends on the molecular weight, the number of aromatic rings and nature of hydroxyl group’s substitution than the specific functional groups.
Table 1.
Free radical-scavenging activity (FRSA) of pomegranate extracts measured by DPPH and ABTS methods in terms of IC50 value (μg mL−1 of extract)
| Sample | IC50 value (μg mL−1) | |
|---|---|---|
| DPPH | ABTS | |
| Ethanol | 42.73 ± 2.8c | 81.31 ± 3.0e |
| Water | 112.32 ± 3.6a | 218.72 ± 6.5b |
| Ether | 30.53 ± 1.7d | 101.19 ± 4.3d |
| Acetone | 50.25 ± 1.8b | 112.87 ± 5.8d |
| Ethanol + Water (8:2) | 45.26 ± 2.2c | 315.82 ± 9.5a |
| Ethanol + Ether (8:2) | 26.29 ± 1.2e | 168.73 ± 7.8c |
| Ethanol + Water + Ether (8:1:1) | 10.12 ± 0.8f | 107.61 ± 3.2d |
Values are mean ± standard deviation of triplicate experiments
Mean values superscripting the same letter do not differ significantly (p < 0.05) according to Duncan’s multiple range test
In the past, few attempts have been made to evaluate the antioxidant activity of pomegranate extracts (Gil et al. 2000; Kulkarni and Aradhya 2005; Mousavinejad et al. 2009; Qua et al. 2010; Zhang et al. 2011). However, screening of solvents for antioxidant property was not performed in either of these studies.
Calibration curve analysis
In order to quantify the amount of ellagic acid, gallic acid and punicalagins in the different extracts of pomegranate aril, calibration curve was prepared with the standards. Standards showed high linearity at tested concentrations (50 to 0.40 μg mL−1) with correlation coefficients (R2) of 0.987, 0.998, 0.971 and 0.998 for ellagic acid, gallic acid, punicalagin A and B, respectively.
The equation, generated from the curve by external standard method was used to calculate the amount of compound present in crude samples (Table 2). The chromatographic peaks of the analytes were confirmed by comparing their retention time with those of the standards. Presence of compound was further reconfirmed with the use of internal standard by co-injecting it with the crude extract. A very distinct and clear separation of compounds could be seen in the chromatograms of ellagic acid, gallic acid and punicalagins. Under the optimized HPLC conditions, standard ellagic acid and gallic acid were separated within 10 min. Whereas, punicalagin A and B were separated by gradient elution in 20 min. Ellagic acid, gallic acid, punicalagin A and B eluted at retention time of 3.9, 3.6, 12.2 and 16.7 min, respectively (Figs. 3a–c, 4a–c, and 5a–c). The precision of the developed method was evaluated by measuring intra- and inter-day variability in terms of relative standard deviation (Table 2).
Table 2.
Standard curve analysis for ellagic acid, gallic acid and punicalagin A and B
| Compound | Retention time (min) | Standard equation | R2 | % RSD | |||
|---|---|---|---|---|---|---|---|
| Interday | Intraday | ||||||
| Area | Rt | Area | Rt | ||||
| Ellagic acid | 3.9 ± 0.01 | y = 0.29 x + 3.88 | 0.987 | 4.32 | 1.54 | 1.76 | 0.25 |
| Gallic acid | 3.6 ± 0.07 | y = 58.38 x−0.13 | 0.998 | 1.10 | 1.21 | 0.06 | 1.21 |
| Punicalagin A | 12.2 ± 0.02 | y = 14.24 x + 2.14 | 0.971 | 1.9 | 2.1 | 0.12 | 0.62 |
| Punicalagin B | 16.7 ± 0.05 | y = 25.29 x−0.55 | 0.998 | 2.8 | 1.87 | 0.89 | 1.32 |
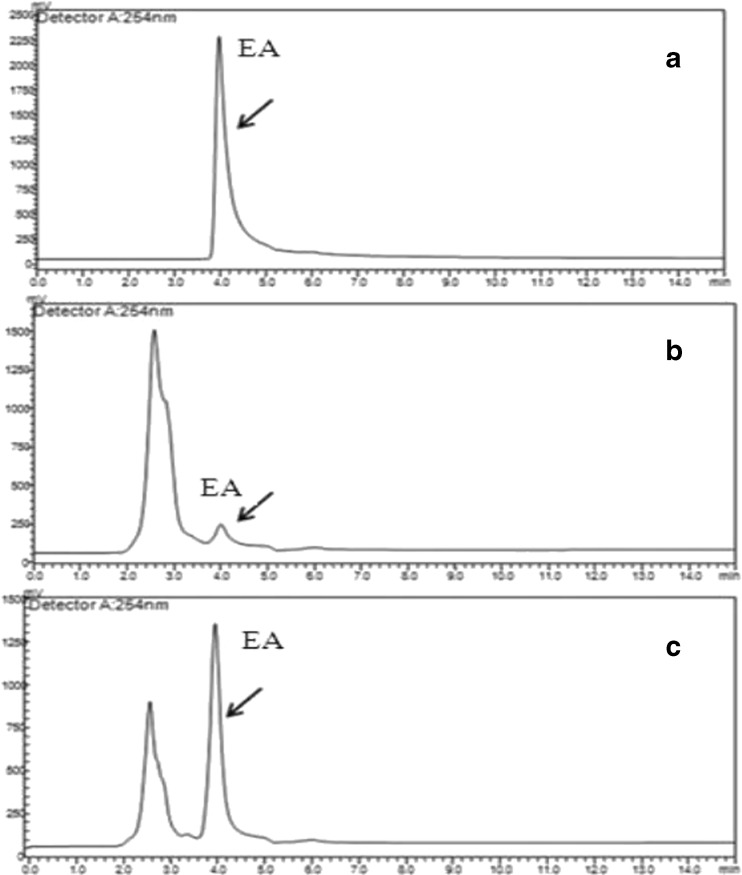
Fig. 3.
Chromatograms of ellagic acid analysis. a A HPLC chromatogram of standard ellagic acid (EA) showing single peak of ellagic acid (arrow marked). b A HPLC chromatogram of pomegranate crude extract showing presence of ellagic acid (arrow marked). c A HPLC chromatogram of pomegranate extract, spiked with standard ellagic acid. Sample ellagic acid and standard ellagic acid co-eluted at the same retention time (arrow marked)
Fig. 4.
Chromatograms of gallic acid analysis. a A HPLC chromatogram of standard gallic acid (GA) showing single peak of gallic acid (arrow marked). b A HPLC chromatogram of pomegranate crude extract showing presence of gallic acid (arrow marked). c A HPLC chromatogram of pomegranate extract, spiked with standard gallic acid. Sample gallic acid and standard gallic acid co-eluted at the same retention time (arrow marked)
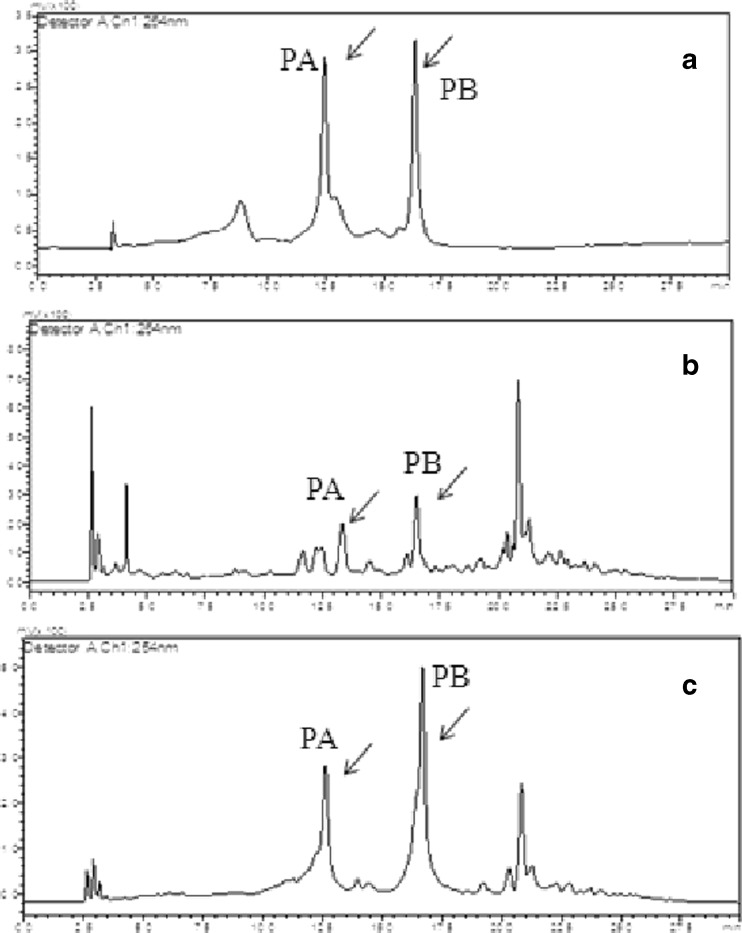
Fig. 5.
Chromatograms of punicalagins analysis. a A HPLC chromatogram of standard punicalagin showing punicalagin A (PA) and punicalagin B (PB) peaks (arrow marked). b A HPLC chromatogram of pomegranate crude extract showing presence of punicalagin A and B (arrow marked). c A HPLC chromatogram of pomegranate extract, spiked with standard punicalagin (arrow marked) showing co elution of standard and sample punicalagins
Quantification of ellagic acid, gallic acid and punicalagins
Pomegranate is reported to contain a wide array of compounds with diverse range of bioactivities (Gil et al. 2000; Kulkarni and Aradhya 2005; Mousavinejad et al. 2009; Qua et al. 2012). Of these, three main constituents viz., ellagic acid, gallic acid and punicalagin possess immense pharmacological properties (Qua et al. 2012). During the past years, a few efforts were made for identification and quantification of these major polyphenolic compounds from pomegranate (Seeram et al. 2005; Qua et al. 2012). Nevertheless, the literature has little information on the effect of solvent on extraction of ellagic acid, gallic acid and punicalagin from pomegranate aril.
Since pomegranate aril is the main reservoir of bioactive compounds, in the present work, different extracts of aril were utilized for screening and quantification of above three main constituents of pomegranate. By following the protocol as described in Materials and methods, different extracts of pomegranate were analyzed by HPLC, for the quantification of ellagic acid, gallic acid, punicalagin A and B.
The ellagic acid was detected in all the extracts, except the water extract. Amount of ellagic acid was found in the order: ethanol + ether + water > ethanol > ethanol + ether > ether > ethanol + water > acetone > water (p < 0.05). It varied from 34.5 to 0.0 μg mg−1 pomegranate aril extracts (Table 3). The highest and the lowest yields were obtained from ethanol + ether + water and water extracts (p < 0.05), respectively.
Table 3.
Ellagic acid, gallic acid and punicalagin A and B content in different extracts of pomegranate
| Sr. no. | Extract | Ellagic acid content (μg mg−1 extract) | Gallic acid content (μg mg−1 extract) | Punicalagin A content (μg mg−1 extract) | Punicalagin B content (μg mg−1 extract) |
|---|---|---|---|---|---|
| 1. | Water | 0.0 ± 0.0f | 3.0 ± 0.4b | ND | 0.48 ± 0.02f |
| 2. | Ethanol | 24.61 ± 1.07b | 2.97 ± 0.03b | ND | 1.29 ± 0.01b |
| 3. | Acetone | 0.51 ± 0.01e | 1.97 ± 0.03c | ND | 0.79 ± 0.01e |
| 4. | Ether | 8.78 ± 0.27c | 1.68 ± 0.04d | ND | 0.99 ± 0.04d |
| 5. | Ethanol + water | 6.3 ± 0.21d | 0.58 ± 0.02e | ND | 0.25 ± 0.03g |
| 6. | Ethanol + ether | 9.68 ± 1.6c | 0.81 ± 0.04e | ND | 1.06 ± 0.02c |
| 7. | Ethanol + ether + water | 34.5 ± 1.63a | 3.37 ± 0.07a | 1.06 ± 0.02 | 2.07 ± 0.03a |
Values are mean ± standard deviation of triplicate experiments
Mean values superscripting the same letter do not differ significantly (p < 0.05) according to Duncan’s multiple range test
HPLC analysis of gallic acid showed that it was present in all the extracts. Similar to ellagic acid, among different extracts, the highest content of gallic acid (3.37 μg mg−1 extract) was extracted in ethanol + ether + water extract. It was obtained in the order: ethanol + ether + water > water > ethanol > acetone > ether > ethanol + ether > ethanol + water (p < 0.05) (Table 3).
Punicalagin A and B analysis showed that punicalagin A was extracted only in ethanol + ether + water extracts (1.06 μg mg−1 extract). Apart from this, punicalagin B was detected in all pomegranate extracts. It was present in the order: ethanol + ether + water > ethanol > ethanol + ether > ether > acetone > water > ethanol + water (p < 0.05) (Table 3). Statistical analysis showed that ethanol + ether + water extract contained significantly (p < 0.05) higher amount of these bioactive polyphenolic compounds (Table 3). This might be due to the use of a mixture of solvents (ethanol + ether + water) which modulate the ethanol polarity and, thus favour the solubility of hydrolysable tannins such as punicalagin, ellagic acid and gallic acid. Hydrolysable tannins are phenolic compounds which contain a central core of glucose or another polyol esterified with gallic acid (gallotannins) or with hexahydroxydiphenic acid (ellagitannins). Punicalagin isomers are part of family ellagitannin which after hydrolysis in aqueous solution release ellagic acid (Gil et al. 2000).
Conclusions
Results of the present study revealed that pomegranate arils had high phenolic content and good antioxidant activity. HPLC analysis of different extracts for ellagic acid, gallic acid, punicalagin A and B showed that the ethanol + ether + water (8:1:1) extract contained significantly higher amount of these phenolic compounds. Based on this study, it is concluded that selective extraction of antioxidants from natural sources by appropriate solvent is very important in obtaining extracts with high antioxidant activity. Results of this study suggest that ethanol + ether + water (8:1:1) extract of pomegranate may be used as a source of health promoting antioxidant compound and/or for developing new functional foods.
References
- Akowuah GA, Ismail Z, Norhayati I, Sadikun A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005;93:311–317. doi: 10.1016/j.foodchem.2004.09.028. [DOI] [Google Scholar]
- Barros L, Falcao S, Baptista P, Freire C, Vilas-Boas M, Ferreira ICFR. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008;111:61–66. doi: 10.1016/j.foodchem.2008.03.033. [DOI] [Google Scholar]
- Ҫama M, Hișil Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2011;123:878–885. [Google Scholar]
- Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem. 2001;49:3420–3424. doi: 10.1021/jf0100907. [DOI] [PubMed] [Google Scholar]
- Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Julie Jurenka MT. Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- Kulkarni AP, Aradhya SM. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005;93:319–324. doi: 10.1016/j.foodchem.2004.09.029. [DOI] [Google Scholar]
- Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, Derendorf H. Absorption, metabolism and antioxidant effects of pomegranate (Punica granatum L.). Polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem. 2006;54:8956–8961. doi: 10.1021/jf061674h. [DOI] [PubMed] [Google Scholar]
- Mousavinejad G, Emam-Djomeh Z, Rezaei K, Khodaparast MHH. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem. 2009;115:1274–1278. doi: 10.1016/j.foodchem.2009.01.044. [DOI] [Google Scholar]
- Panichayupakaranant P, Tewtrakul S, Yuenyongsawad S. Antibacterial, anti-inflammatory and anti-allergic activities of standardised pomegranate rind extract. Food Chem. 2010;123:400–403. doi: 10.1016/j.foodchem.2010.04.054. [DOI] [Google Scholar]
- Perez-Vicente A, Izquierdo A, Garcia-Viguera C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins and vitamin c. J Agric Food Chem. 2002;50:2308–2312. doi: 10.1021/jf0113833. [DOI] [PubMed] [Google Scholar]
- Qua W, Pan Z, Ma H. Extraction modeling and activities of antioxidants from pomegranate marc. J Food Eng. 2010;99:16–23. doi: 10.1016/j.jfoodeng.2010.01.020. [DOI] [Google Scholar]
- Qua W, Breksa AP, Pan Z, Ma H. Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem. 2012;132:1585–1591. doi: 10.1016/j.foodchem.2011.11.106. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Turkmen N, Sari F, Polat G, Velioglu YS. Effects of extraction solvents on concentration and antioxidant activity of black and mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006;99:835–841. doi: 10.1016/j.foodchem.2005.08.034. [DOI] [Google Scholar]
- Yu YM, Chang WC, Wu CH, Chiang SY. Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid. J Nutr Biochem. 2005;16:675–681. doi: 10.1016/j.jnutbio.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fu Q, Zhang Y. Composition of anthocyanins in pomegranate flowers and their antioxidant activity. Food Chem. 2011;127:1444–1449. doi: 10.1016/j.foodchem.2011.01.077. [DOI] [Google Scholar]
- Zielinski H, Kozłowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem. 2000;48:2008–2016. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]