Abstract
Ohmic heating (OH) which is among to electro-thermal methods and helps to inactivate microorganisms and enzymes was used in this study as thermal treatment on orange juice production for pectin methylesterase (PME) inactivation. Response surface methodology (RSM) was used for optimization of OH conditions. The effects of voltage gradient and temperature (independent variables) were investigated on PME activity (response) of orange juice. After optimization orange juice was produced and compared with untreated control juices and conventional thermally heated juices on the aspect of PME inactivation and some quality characteristics. Reduction of PME activities was found approximately 96 % in OH groups where conventional thermally heated juice has 88.3 % reduction value. Total pectin content was increased 1.72–2 % after OH applications. Ascorbic acid contents of OH samples were found between 43.08–45.20 mg/100 mL where conventional thermally heated juice has 42.9 mg/100 mL. As a result, it was determined that OH can be applied as a thermal treatment on orange juice production in moderate temperatures for PME inactivation and may improve functional properties of orange juice.
Keywords: Orange juice, Ohmic heating, Optimization, Pectin methylesterase, Quality
Introduction
Orange juice is highly consumed in many countries and a common problem associated with orange juice (fresh squeezed, concentrated and preserved) is the loss of cloudiness (Basak and Ramaswamy 1996). Cloud stability is a critical orange juice quality parameter imparting characteristic flavor, color and mouthfeel. Cloud stability is affected by the impact of pectic enzymes, particularly pectin methylesterase (PME) (Espachs-Barroso et al. 2006). PME, a ubiquitous enzyme in plants, de-esterifies the methoxylated pectin in the plant cell wall. PME (EC 3.1.1.11) is also referred as pectin demethoxylase, pectin methoxylase, pectase, pectinoesterase and pectinesterase, released into juice during extraction (Rouse and Atkins 1952). PME de-esterifies the methyl groups on the galacturonic acid backbone of pectin, creating charged regions which complex with Ca2+, forming Ca2+ pectate gels which precipitate and clarify the juice (Baker and Bruemmer 1972). It has been reported that a degree of esterification of the pectin backbone of <36 % is necessary to cause cloud loss in orange juice (Baker 1979; Krop and Pilnik 1974). The design for thermal pasteurization of orange juice is based on the thermal destruction characteristics of PME which is thermally stable than many vegetative microorganisms (Chen and Wu 1998; Lee et al. 2003).
Conventional thermal processing is the most common method to inactivate PME and to prevent juice cloud precipitation; additionally to control microbial growth in orange juice production. Pasteurization at 90–95 °C for 60–90 s is used to inactivate the most tolerant PME isoenzyme. However it can be reduce freshness, affecting sensory and nutritional characteristics of orange juice. This conventional treatment in elevated temperature causes adverse effects on the final products such as color alterations, flavor damages, vitamin and other nutritional losses. As consumers are highly demanding minimally processed and fresh-like food products, the use of novel technologies is gaining popularity. The food industry is interested in novel electrical thermal method which inactivates enzymes and microorganisms without significant adverse effects on flavor and nutrients (Demirdöven and Baysal 2009a). One of the novel electro-thermal techniques is ohmic heating. Ohmic heating (OH) is an electrical thermal method that also known as electrical resistance heating, joule heating and electro-heating. In recent years, all over the world food industry has focused increasing demand on ohmic heating of food products. It is a highly attractive technique for food processing. Ohmic heating is based on the passage of an electrical current through a food product, which serves as an electrical resistance (Knirsch et al. 2010). Heat is generated instantly inside of the food. The amount of heat generated is directly related to the current induced by the voltage gradient in the field and to the electrical conductivity (Sastry and Li 1996; Jha et al. 2011). The applicability of ohmic heating is dependents on the electrical conductivity of the product. Most food preparations contain a moderate percentage of free water with dissolved ionic salts and therefore conduct sufficiently well for the ohmic effect to be applied (Parrott 1992). Several applications for ohmic heating in the food manufacturing industry include: blanching, thawing, starch gelatinization, sterilization, peeling of fruits; dehydration and extraction (Ramaswamy et al. 2005; Demirdöven and Baysal 2009b; Sagar and Kumar 2010).
Enzymes have negative effects on food quality such as production of off-odors and tastes and altering textural properties. Therefore, control of enzymatic activity is required in many food processing steps (Demirdöven and Baysal 2009b). There have been limited researches on the effect of ohmic heating on enzymes. Electrical fields, applied during ohmic heating of lipoxygenase and polyphenoloxidase caused faster inactivation than during conventional heating (Castro et al. 2004). Peroxidase in pea puree was also inactivated in a shorter processing time by ohmic heating than by conventional heating (Icier et al. 2006). Moreover, ohmic heating caused less browning. The effects of voltage gradient, temperature and holding time on the polyphenoloxidase activity were investigated for ohmic heating of grape juice (Icier et al. 2008). The inactivation kinetics of this enzyme was described by several mathematical models but simple first-order kinetics was found to be the best. Similarly, ohmic heating was found to be more efficient for the required PME inactivation due to a shorter residence time when released flavor compounds were not degraded as quickly as during conventional pasteurization. PME activity is presented as percentage of PME activity in fresh orange juice. Generally, the residual PME activity decreases by applying higher temperatures or times, as the impact of thermal treatments during ohmic heating increases. During ohmic heating, PME activity showed a reduction of 90–98 % compared to its activity in fresh orange juice where under conventional pasteurization conditions, the residual PME activity was reduced to 5 % (Leizerson and Shimoni 2005a; Leizerson and Shimoni 2005b).
Therefore the objective of this study was to investigate the effect of ohmic heating on the inactivation of PME on orange juice and to optimize the moderate temperature conditions for electrical field application with response surface methodology (RSM) and to compare it with the conventional thermal treatment. Then effects of OH treatment at optimized conditions on the PME activity and some quality characteristics were determined by physical and chemical analyses.
Materials and methods
Material
Oranges (Citrus sinensis Osb.) of Navel variety were used as raw material in this study. The oranges were purchased from Zumdieck Frozen and Canned Food Company (Salihli-Manisa, TURKEY). They were stored in a refrigerator at 7 °C and 80–90 % humidity for maximum of 48 hours before processing.
Processing methods
OH application
Ohmic heating experiments were conducted in laboratory scale ohmic heating system consisting of a power supply, an isolating transformer, a variable transformer and a microprocessor board. The detailed technical information about the system used was provided by Icier and Ilicali (2005). A teflon coated electronic temperature sensor (Omega Eng. Inc., Stanford, CT) with a compression fitting was used to measure temperature at the different sections of the sample in the test cell (4 × 4 cm). The microprocessor board monitored the temperatures, of current and voltage applied and transmitted this information to the microcomputer at constant time intervals (1 s). The distance between two electrodes was 0.04 m and the diameter of the electrodes was 0.025 m. After the system was sealed, the orange juices were ohmically heated up to different temperatures (34–76 °C) at 50 Hz frequency using different voltages (20–60 V/cm) to obtain different voltage gradients. Voltage, current and temperature data were logged at 1 s time intervals during heating. The temperature of each sample was assumed uniform in the cell, since the maximum difference among the measured temperatures at different locations was approximately 0.5 °C. The experiments were replicated three times. The average temperature of the replicated heating experiments was accepted or used as the measured temperature values.
Orange juice production
After washing and peeling applications oranges were processed to orange juice by using a juice extractor (Moulinex, JU5000–800 W). Then oranges were divided into three groups; (i) control group (without any treatment) and (ii) OH application group to determine optimum PME inactivation and quality effects; (iii) Conventional thermal treatment group (CH). Orange juices in OH group were processed and conditions (voltage gradient and temperature) were optimized by using RSM. Conventional thermal treatment was realized in a water bath (DKZ Series). Shelf stable orange juices, processing times for thermal pasteurization are equivalent to 90–95 °C for 60–90 s (Eagerman and Rouse 1976; Sharma et al. 2012). As given in literature; 200 mL bottled orange juice were heated until 95 °C and kept at the same temperature for 60 s. Then orange juice was processed at optimized conditions to compare quality characteristics. After OH production of orange juices, all samples were cooled +4 °C in an ice bath and analyzed.
After heating treatments pasteurization unit (PU) and decimal reduction times of PME (D values) were calculated for OH optimum points and conventional heating treatments. The PU values were calculated with 60 °C as the reference temperature, on the basis of z = 7 °C (Glevitzky et al. 2007; Anon 2011).
Methods of analysis
Response measurement techniques
RSM was used for optimization of OH conditions (Cochran and Cox 1957; Myers and Montgomery 1995). The effects of voltage gradient and temperature (independent variables) were investigated on PME activity (response) of orange juice. A central composite rotatable design was used in designing the OH treatment of two variables at five levels (Design Expert 7.0.0 STAT-EASE, 2005). In each experiment 20 mL orange juice were processed by OH. PME activities were determined after OH applications.
The model adequacies were checked by R2, adjusted-R2, predicted-R2 and prediction error sum of squares (PRESS) (Myers and Montgomery 1995). A good model will have a large predicted R2, and a low PRESS. After model fitting was performed, residual analysis was conducted to validate the assumptions used in the ANOVA (results are not shown). This analysis included calculating case statistics to identify outliers and examining diagnostic plots such as normal probability plots and residual plots. Maximization and minimization of the polynomials thus fitted was performed by desirability function method and mapping of the fitted responses was achieved using Design Expert Version 7.0.0 software.
Chemical and physical analysis
PME activity and quality characteristics of orange juices were investigated and samples were analyzed to determine the following:
PME activity was measured by continuous recording of the titration of carboxyl groups released from a pectin solution using a pH meter (WTW InoLab, Weilheim, Germany) and 0.01 M NaOH. Routine assays were performed with a 0.5 % pectin (Sigma-Aldrich Corp., St. Louis, Mo., U.S.A.) solution (25 mL) containing 0.117 M NaCl (pH 7.0) at 30 °C. An activity unit (U) of PME is defined as the amount of enzyme required to release 1 μmol of carboxyl groups per minute (Yemenicioglu and Cemeroglu 1998; Rayman and Baysal 2011).
Pectin content was investigated according to Anon (1968). The method is based on the extraction of pectin with ethanol after centrifugation (4000 rpm, 15 min, 20 °C) (CFC free Universal Hettich Zentrifugen, Tuttlingen, Germany); the precipitated part was treated with 5 mL NaOH and completed to 100 mL with deionized water. After filtration, samples were prepared with 0.5 mL carbazol (Merck, Darmstadt, Germany) and 0.5 mL ethanol. Sulfuric acid (Merck) (6 mL) was added to both samples, then they were placed in a water bath at 85 °C for 5 min. Absorbance values were taken at 525 nm with a Varian Cary 50 Scan (Sydney, Australia) spectrophotometer and the pectin content was calculated with the calibration curve that was made by using gallacturonic acid anhydrate standards (Sigma-Aldrich Corp.).
Ascorbic acid content was detected according to the spectrophotometric method for using Varian Cary 50 Scan (Sydney, Australia) spectrophotometer at a wavelength of 518 nm as described by Hışıl (2004). Determination of ascorbic acid based on its reaction with 2,6-dichlorophenolindophenol and the ascorbic acid content was calculated with the calibration curve that was made by using different concentration of L(+) ascorbic acid standard (Carlo Erba Reagenti SpA).
Total soluble matter (ºBrix) of juices was measured with a refractometer at 20 °C (RFM 330; Bellingham + Stanley Limited, Tunbridge Wells, Kent, U.K.) (Assoc. of Official Analytical Chemists [AOAC] 1995). The pH values of orange juices were measured with pH meter-WTW InoLab at 20 °C (Anon 1995).
The color (L*, a*, b*) values of orange juice were measured with a HunterLab Colorflex colorimeter (Hunter Associates Laboratory, Reston, Va., U.S.A.) and total color differences (ΔE) and chroma (ΔC) values were calculated according to control group. Orange juices were placed on the light port using a 5 cm diameter glass dish with cover. Color parameters were recorded as L* (lightness), a* (redness) and b* (yellowness) and total color differences (ΔE) and chroma values (ΔC) were calculated according to Eqs. 1 and 2.
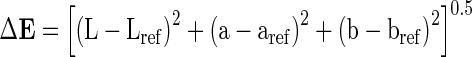 |
1 |
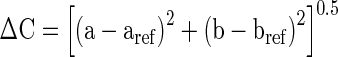 |
2 |
Statistical methods
Results were statistically analyzed by analysis of variance (ANOVA) using the software SPSS 13 (SPSS Inc., Chicago, IL, USA) with the Duncan test to evaluate differences between treatments at levels of significance P ≤ 0.05. Each experiment was repeated at least three times; means and standard deviations of results were calculated.
Results and discussion
Working conditions of OH application were determined by pretesting. By the aim of choosing the suitable electric voltage gradient interval and temperature interval some experiments were made between a voltage range of 20–60 V/cm. Above 50 V/cm application, adverse color changes were seen on orange juices. L* value of orange juices measured under 40 above 50 V/cm OH application whereas control group has a 55 L* value. Temperature was limited at 70 °C because of color changes and there weren’t any additional PME inactivation was evaluated. The treatment time is 60 s for all OH applications because of color change so all OH samples were kept each voltage gradient and temperature for 60 s. So independent variables (voltage gradient and temperature) interval were chosen as 30–50 V/cm; 40–70 °C for RSM. By the ANOVA, voltage gradient and temperature were found significantly important on the PME inactivation of orange juice at 95 % confidence interval. Model was tested for lack of fit. Table 1 shows the experimental design and responses.
Table 1.
Central composite rotatable design with experimental values* of response variables
| Run # | Voltage gradient (V/cm) (x 1 ) | Temperature (°C) (x 2 ) | PME activity** (μmol/min/ml) | ||
|---|---|---|---|---|---|
| 1 | 40(0) | 34(−√2) | 5.086 ± 0.3 | ||
| 2 | 40(0) | 76(+√2) | 0.282 ± 0.1 | ||
| 3 | 40(0) | 55(0) | 1.307 ± 0.2 | ||
| 4 | 40(0) | 55(0) | 1.257 ± 0.2 | ||
| 5 | 30(−1) | 70(+1) | 0.487 ± 0.1 | ||
| 6 | 50(+1) | 70(+1) | 0.244 ± 0.1 | ||
| 7 | 40(0) | 55(0) | 1.307 ± 0.2 | ||
| 8 | 50(+1) | 40(−1) | 3.257 ± 0.1 | ||
| 9 | 26(−√2) | 55(0) | 2.860 ± 0.2 | ||
| 10 | 40(0) | 55(0) | 1.121 ± 0.1 | ||
| 11 | 54(+√2) | 55(0) | 0.980 ± 0.1 | ||
| 12 | 40(0) | 55(0) | 1.121 ± 0.1 | ||
| 13 | 30(−1) | 40(−1) | 4.875 ± 0.1 | ||
| * Experimental values | −√2 | −1 | 0 | +1 | +√2 |
| Voltage gradient (x 1 ) | 26 | 30 | 40 | 50 | 54 |
| Temperature (x 2 ) | 34 | 40 | 55 | 70 | 76 |
** Data are means ± S.D. (n = 3)
Table 2 shows the analysis of variance for fitting the second order polynomial models to experimental data. It can be seen that all the regression models were found to be statistically significant at 99 % confidence level. Statistical significance of all main effects, linear, quadratic, and interaction of effects calculated for each response can also be shown in Table 2. The effects that are not significant (p > 0.05) were stepped down from the models without damaging the model hierarchy. The ANOVA also showed that lack of fit was not significant for all response surface models at 95 % confidence level. On the other hand, R2, adj-R2 and coefficient of variation (CV) was calculated to check the model adequacy. A high proportion of variability (R2 > 0.99) in the response model can be explained successfully by the models (Table 2). However, a large value of R2 does not always imply that the regression model is better. Adding a variable to the model will always increase R2, regardless of whether the additional variable is statistically significant or not. Thus, it is preferred to use an adj-R2 to evaluate the model adequacy and should be over 90 %. Table 2 shows that R2 and adj-R2 values for the models did not differ dramatically indicating non-significant terms have not been included in the model. The coefficient of variation (CV), which indicates the relative dispersion of the experimental points from the predictions of the model, was found to be 8.53 % PME activity. Model adequacy checking may be carried out stepping down the effects that are not significant (p > 0.05) and then considering the PRESS and predicted R2. A low PRESS and predicted-R2 comparable to fitted R2 implies that the model as fitted is adequate to predicting. Predicted-R2 measures the amount of variation in new data explained by the model. Generally, a number closer to one is preferred and the predicted residual sum of squares (PRESS) is a measure of how well the model fits each point in the design. Multiple regression equations relating juice yield to coded levels of the variables developed are as in Eq. 3:
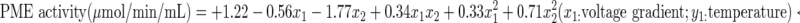 |
3 |
Table 2.
ANOVA table showing the variables as quadratic terms on response variable and coefficient for the prediction model
| Source | DFa | Coefficient | Sum of squares | p-value* |
|---|---|---|---|---|
| Model | 5 | 1.22 | 32.10 | <0.0001 |
| x 1 | 1 | −0.56 | 2.55 | <0.0001 |
| x 2 | 1 | −1.77 | 25.19 | <0.0001 |
| x 1 x 2 | 1 | 0.34 | 0.47 | 0.0035 |
| x 1 2 | 1 | 0.33 | 0.74 | 0.0010 |
| x 2 2 | 1 | 0.71 | 3.5 | <0.0001 |
| Residual | 7 | 0.18 | ||
| Lack of fit | 3 | 0.14 | 0.0730 | |
| Pure error | 4 | 0.036 | ||
| Total | 12 | 32.28 | ||
| R2 | 0.9945 | |||
| Adj-R2 | 0.9906 | |||
| Pred-R2 | 0.9673 | |||
| PRESS | 1.05 | |||
| CV | 8.53 |
a DF Degress of freedom
*p-value < 0.05 is significant at a = 0.05. Lack of fit is not significant at p-value > 0.05
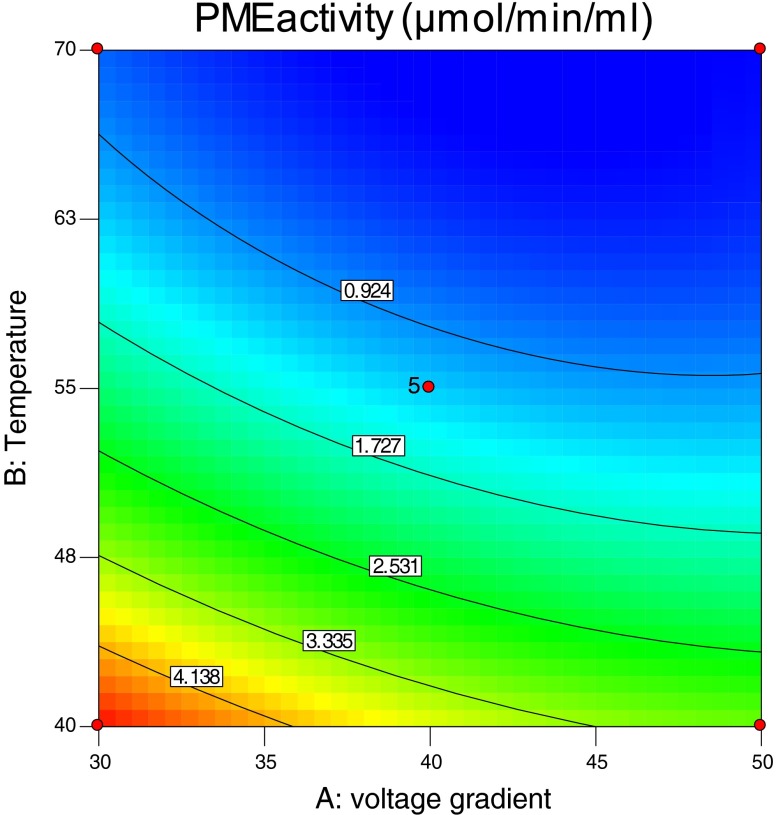
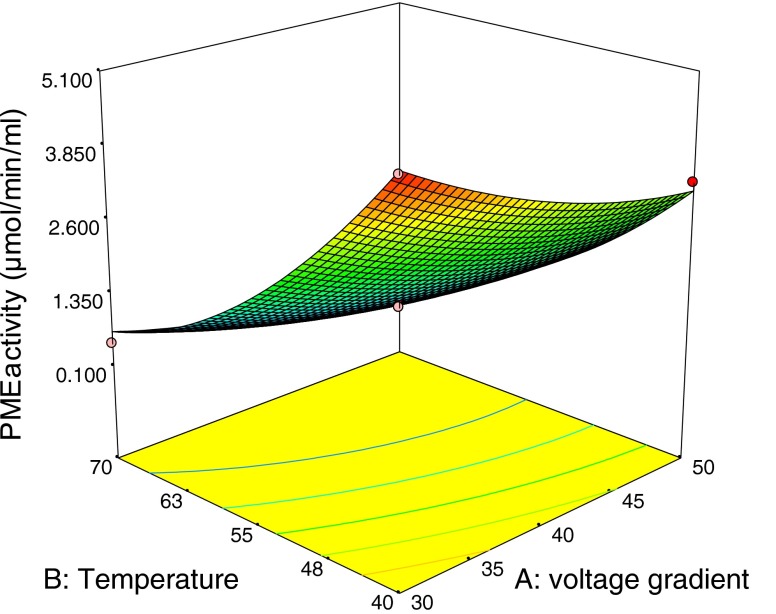
Optimum condition for OH of orange juices was determined to obtain minimum PME activity. Second order polynomial models obtained in this study were utilized for response in order to determine the specified optimum conditions. These regression models are valid only in the selected experimental domain. In this study, voltage gradient and temperature were selected in the range of 30–50 V/cm, 40–70 °C respectively. Figure 1 shows the effect of voltage gradient and temperature on PME inactivation of the orange juice at OH application and Fig. 2 shows the response surface and contour plots for PME activity (μmol/min/mL). By applying desirability function method, two solutions were obtained for the optimum covering the criteria. The first one is 42 V/cm for voltage gradient and 69 °C for temperature. The second one is 44 V/cm for voltage gradient and 70 °C for temperature. The results indicate that the high temperatures on moderate electric fields can decreases PME activities and both solutions gave same desirability values. So, the factor level combinations obtained both solutions were selected as the optimum. Production of orange juices was made at two optimum point for determining PME activities and quality properties. The experimental errors for voltage gradient and temperature were 0.1 V/cm and 0.5 °C respectively. Predicted value by RSM was suitable with the observed value of PME activities as shown in Table 2.
Fig. 1.
The effect of voltage gradient and temperature on pectin methylesterase inactivation of the orange juice at ohmic heating applications
Fig. 2.
Response surface and contour plots for pectin methylesterase activity (μmol/min/mL)
PME activities of orange juices were found as 0.137 μmol/min/mL (OH; 42 V/cm–69 °C) and 0.128 μmol/min/mL (OH; 44 V/cm–70 °C) whereas control group has 3.250 μmol/min/mL PME activities. In CH (95 °C–60 s) group PME activities of orange juices were found as 0.380 μmol/min/mL. It was found statistically significant (P ≤0.05). Reduction of PME activities were found as 96 %, 95.5 %, 88.30 % for OH (42 V/cm–69 °C), OH (44 V/cm–70 °C), CH (95 °C–60 s) respectively (Table 3).
Table 3.
Predicted and observed responses at optimum level of variables
| Sample | Predicted value of response, pectin methylesterase activities (μmol/min/mL) | Observed response, pectin methylesterase activities* (μmol/min/mL) | Reduction of pectin methylesterase activities (%)a |
|---|---|---|---|
| Ohmic heating (42 V/cm–69 °C) | 0.137 | 0.130 ± 0.05a | 96.0 |
| Ohmic heating (44 V/cm–70 °C) | 0.128 | 0.146 ± 0.08b | 95.5 |
| Conventional heating (95 °C–60 s) | – | 0.380 ± 0.09c | 88.3 |
| Control | – | 3.250 ± 0.1d | – |
aReduction of PME activities (%) were calculated according to control PME activitie
*Statistically significant difference shown levels a, b (P ≤ 0.05); data are means ± S.D. (n = 3)
Results were in agreement with Leizerson and Shimoni (2005a,b). They found 90–98 % reduction of PME activity during ohmic heating (50 kW) compared to its activity in fresh orange juice. In the literature, there is only one study was found about OH heating of orange juice for PME inactivation but there are some other studies on PME inactivation for orange juice by using high pressure, PEF etc. Polydera et al. (2004) were evaluated higher PME inactivation rates as processing temperature increased by high pressure processing of orange juices. Yeom et al. (2002) found a 90 % orange PME activity reduction with 125 pulses of 2-μs-pulse-width at 25 kV/cm and Elez-Martınez et al. (2003) reached an 80 % activity reduction of orange PME after 375 pulses of 4-μs-pulse-width at 35 kV/cm. Moreover, Rodrigo et al. (2003) achieved an 81.3 % PME reduction in orange-carrot juice after a 350-μs HIPEF treatment of 35 kV/cm. Giner et al. (2005) also observed an 86.8 % PME inactivation in a commercial pectolytic enzyme preparation. However, orange PME suspended in distilled water or in orange juice as media showed less than 10 % of activity reduction after 1000 pulses of 1-μs-pulse- width at 35 kV/cm (Van Loey et al. 2002). HIPEF treatments are more effective in reducing PME activity when the HIPEF treatments are carried out at moderate temperatures (55–65 °C) than at room temperature. Rayman and Baysal (2011) evaluated that PME was inactivated completely in carrot juice after microwave heating (a flow rate of 90 mL/min; at a power of 900 W) whereas after the traditional pasteurization 1.98 μmol/min/mL, PME activity remained compared to the control sample.
The reason of this difference is the result of thermostable PME ratio of total-PME. The relative ratio of the thermostable PME to total-PME can vary between 0 and 33 % depending on the citrus cultivars. In the case of oranges, the percentage of PME fractions depends on the variety of the oranges (Rombouts et al. 1982; Snir et al. 1996; Van den Broeck et al. 1999), geographic location, growth practice, post-harvest handling, seasonal differences (Snir et al. 1996), fruit tissues (Cameron et al. 1997), and experimental changes in protocol (Wicker 1992). A 5 % thermostable PME fraction was observed for Valencia oranges (Van den Broeck et al. 1999). In Navel oranges, Van den Broeck et al. (2000) and Versteeg et al. (1980) also found a 5 % heat-stable PME fraction. In addition, according to Rombouts et al. (1982), the thermostable PME represented 6 % of the total activity in Navel oranges, 11 % in Salustiana oranges and 7 % in Shamouti oranges.
Thermal heating histories, PU and D values of ohmic heating (optimum points) and conventional thermally heated orange juices were shown in Table 4. PU values were found quite different for OH and CH heated orange juices. The aim of this study was to investigate the effect of ohmic heating on the inactivation of PME on orange juice at moderate temperature conditions for electrical field applications. In this respect applied different heating time and temperatures resulted different PU values. And D values were calculated for two optimum conditions of OH and CH treatments and found as 0.715 min for OH (42 V/cm–69 °C), 0.742 min for OH (44 V/cm–70 °C) and 1.07 min for CH (95 °C–1 min). D-value defined as the heating time required to result in 90 % inactivation of initial activity. As seen from the results, to inactivate 90 % of PME at OH conditions need shorter time than CH. And it shows the additional effects of electrical field and tempreture combinations of OH.
Table 4.
Termal heating histories of ohmic and conventional thermally heated orange juices
| Heating treatments | E (V/cm) | Tin (°C) | th: (sec) | Tout (°C) | tt (sec) | Tc-out (°C) | PU* | D (min) |
|---|---|---|---|---|---|---|---|---|
| Ohmic | 42 | 18 | 35 | 69 | 95 | +4 | 225 × 101 | 0.715 |
| Ohmic | 44 | 17 | 35 | 70 | 95 | +4 | 232 × 101 | 0.742 |
| Conventional heating | – | 18 | 60 | 95 | 660 | +4 | 240 × 103 | 1.07 |
E electric field strength; T in inlet temperature; T out outlet temperature after heating treatments; T c-out outlet temperature after cooling
t h total heating time; t t total treatment time; D Decimal reduction times of PME
*The PU values were calculated with 60 °C as the reference temperature, on the basis of z = 7 °C (Glevitzky et al. 2007)
Results of chemical and physical analyses of orange juice which produced at optimum conditions were shown in Table 5. Pectin content is important for cloudiness of orange juice. Total pectin content was increased 2 % and 1.72 % for OH (42 V/cm–69 °C) and OH (44 V/cm–70 °C) respectively. The difference between pectin contents of OH and control group; CH group were found statistically significant (P ≤0.05). Yıldız and Baysal (2006); investigated the effects of alternative current on pectin content in tomato samples and found 3.23 % pectin content at 68 V/cm for 23 s application (78 °C) and 3.15 % pectin content at 48 V/cm for 40 s at (82 °C). Rayman et al. (2011) investigated the effect of electroplasmolysis on carrot juice and the total pectin content was increased 14.78 % after electroplasmolysis applications. Demirdöven and Baysal (2009a,b) found 18.4 % increase in pectin content of orange juices after electroplasmolysis application at 27 V/cm.
Table 5.
Results of chemical analyses of ohmic and conventionally heated orange juices
| Sample | Total pectin (GA-AH mg ⁄ L)* | Ascorbic acid (mg/100 mL)* | ºBrix* | pH* |
|---|---|---|---|---|
| Ohmic heating (42 V/cm–69 °C) | 418.2 ± 1.1a | 45.2 ± 0.3a | 12.4 ± 0a | 3.8 ± 0.1a |
| Ohmic heating (44 V/cm–70 °C) | 417.3 ± 1.2a | 43.1 ± 0.1b | 12.4 ± 0a | 3.8 ± 0.1a |
| Conventional heating (95 °C–60 s) | 411.2 ± 0.5b | 42.9 ± 0.0c | 12.2 ± 0a | 3.9 ± 0.2a |
| Control | 410.3 ± 1.1b | 48.6 ± 0.4d | 12.2 ± 0a | 3.8 ± 0.2a |
*Statistically significant difference shown levels a, b compared with same column (P ≤ 0.05); data are means ± S.D. (n = 3)
Ascorbic acid was found in control sample as 48.6 mg/100 mL where, it was observed 42.9 mL/100 mL in CH group. Ascorbic acid contents of OH samples were found as 45.2 mg/100 mL and 43.1 mL/100 mL for OH (42 V/cm–69 °C) and OH (44 V/cm–70 °C), respectively. High ascorbic acid content of OH samples can be explained by moderate temperature applications. And also it can be explained by increasing in cell permeability and components can be transferred to orange juice easily. The difference between ascorbic acid content of samples was also found statistically significant (P ≤ 0.05). The content of vitamin C in fresh orange juice has been widely studied and the results obtained in the present work are in the range of those published in the literature, which varied from 25 mg/100 mL to 68 mg/100 mL (Farnworth et al. 2001; Fernandez-Garcia et al. 2001; Kabasakalis et al. 2000; Lee and Coates 1999; Rapisarda et al. 2001; Sanchez-Moreno et al. 2003). Vikram et al. (2005) investigated the effects of different electro-heating method on vitamin C degradation. They evaluated that the destruction of vitamin C was influenced by the method of heating and the temperature of processing as found in the present study. They found highest vitamin C degradation during microwave heating due to uncontrolled temperature generated during processing and ohmic heating gave the best result facilitating better vitamin retention at all temperatures. Lima et al. (1999), examined ascorbic acid degradation in pasteurized orange juice during conventional and ohmic heating. They performed matching time-temperature histories in both conventional and ohmic heating batch treatments. They also found that the type of heating had no significant effect on vitamin C degradation. They measured a decrease of 21–23 % in ascorbic acid during thermal treatments at 90 °C for 30 min.
There are no significant differences between the samples for water soluble matters and pH values (P > 0.05). Effects of PEF application on ºbrix were investigated by some researchers. Rivas et al. (2006) applied thermal pasteurization and PEF to the blended orange–carrot juice and found brix values: 9.5 and 10.4 for control and pasteurized samples. Torregrosa et al. (2006) who studied PEF determined comparable brix values for the pasteurized juice and juice treated by PEF. Like the mentioned previous study Cserhalmi et al. (2006) reported that in citrus juices PEF treatment (50 pulses at 28 kV/cm) did not change the brix value significantly as we found.
The color values (L*, a* and b*), the total color difference (ΔE) and chroma values (ΔC) of samples in CIE Lab system are summarized in Table 6. The difference between L* values of samples was found as statistically significant (P ≤ 0.05). The difference between a* and b* values of samples and the color differences of all samples were found as statistically important (P ≤ 0.05) and the highest chroma value is observed in OH (44 V/cm–70 °C) applied samples. There are no significant differences evaluated between CH and OH (42 V/cm–69 °C) samples for chroma values (P > 0.05). By OH and CH treatments a* and b* values decreased compared to control group, this can be explained by color of samples became lighter after thermal applications. Same effects were observed after electrical application in apple juices (McLellan et al. 1991).
Table 6.
Colour values of ohmic and conventionally heated orange juices
| Sample | L* | a* | b* | ΔE | ΔC |
|---|---|---|---|---|---|
| Ohmic heating (42 V/cm–69 °C) | 52.7 ± 0.03a | 0.3 ± 0.05a | 46.4 ± 0.08a | 8.2 ± 0.1a | 7.6 ± 0.1 a |
| Ohmic heating (44 V/cm–70 °C) | 51.9 ± 0.10b | −0.5 ± 0.01b | 43.1 ± 0.1b | 11.2 ± 0.2b | 10.7 ± 0.2 b |
| Conventional heating (95 °C–60 s) | 52.8 ± 0.01c | 0.1 ± 0.01c | 46.1 ± 0.1c | 8.5 ± 0.1c | 7.9 ± 0.1 a |
| Control | 55.9 ± 0.2d | 5.3 ± 0.09d | 52.1 ± 0.1d | – | – |
*Statistically significant difference shown levels a, b compared with same column (P ≤ 0.05), data are means ± S.D. (n = 3)
In another research, Demirdöven and Baysal (2009a) found statistically significant decrease in L*, a* and b* values of orange juices after electroplasmolysis application. ΔE value was determined as 6.93 in electroplasmolysis (68 V/cm, 1.5 s) baked tomato puree by Yıldız and Baysal (2007). In a previous study after treatment of PEF in citrus juices ΔE values were determined as 0.45 for grape fruit; 0.59 for lemon; 0.47 for orange and 2.44 for tangerine juices. L* value of PEF treated (50 pulses at 28 kV/cm) tangerine juice was found as 20.76 where control has 22.16 L* value (Cserhalmi et al. 2006). Rivas et al. (2006) in blended orange–carrot juice investigated the effects of HTST (98 °C, 21 s) and PEF (25 kV/cm, 280 μs) treatments and found L* values for control, pasteurized and PEF treated; 62.80 ± 0.03; 62.65 ± 0.20; 63.08 ±0.09, respectively.
Conclusion
In this study; the effect of ohmic heating on the inactivation of PME in orange juice and to optimize the moderate temperature conditions for electrical field application with response surface methodology (RSM) were investigated. An antagonistic effect of electric current and temperature on orange juice PME inactivation was found under ohmic heating processing conditions. The PME inactivation rate was described satisfactorily as a function of ohmic heating conditions. The PME can be inactivating in moderate temperatures by ohmic heating. The electric current and temperature combinations necessary to inactivate the labile fraction of PME can be estimated allowing selection of optimal process conditions that should also provide sensorial quality. Reduction of PME activities was found approximately 96 % in OH groups where conventional thermally heated juice has 88.3 % reduction value. Total pectin content was increased 1.72–2 % after OH applications. And the lost of ascorbic acid content of OH (42 V/cm–69 °C) sample was lower than other applications. Due to these results, there was an important improvement in functional properties of orange juice. And it was determined that OH can be applied as a thermal treatment on orange juice production in moderate temperatures for PME inactivation and improving functional properties of orange juice. There will be further studies about OH effects during storage conditions and combinations with other electro methods.
Acknowledgments
Financial support for this research (scientific research project) was provided by Gaziosmanpaşa University (Tokat-TURKEY) and MEYED (Meyve Suyu Endüstrisi Derneği), Ankara/TURKEY. The authors wish to thank to Assoc Prof. Filiz İçier and Hayriye Bozkurt (MS) for their help during study.
Contributor Information
Aslıhan Demirdöven, Phone: +90-356-2521616, FAX: +90-356-2521729, Email: ademirdoven@hotmail.com.
Taner Baysal, Email: taner.baysal@ege.edu.tr.
References
- Methods of analyses. Method-26. Paris: Intl. Federation of Fruit Juice Producers; 1968. [Google Scholar]
- Assoc. of official analytical chemists. Official methods of analysis of AOAC International. 16. Arlington: Assoc. of Official Analytical Chemists; 1995. [Google Scholar]
- Anon (2011) Pasteurization & Sterilization Application Note, www.madgetech.com. Accessed 02/03/2012.
- Baker RA. Clarifying properties of pectin fractions separated by ester content. J Agric Food Chem. 1979;27(6):1387–1389. doi: 10.1021/jf60226a056. [DOI] [Google Scholar]
- Baker RA, Bruemmer JH. Pectinase stabilization of orange juice cloud. J Agric Food Chem. 1972;20(6):1169–1172. doi: 10.1021/jf60184a011. [DOI] [Google Scholar]
- Basak S, Ramaswamy HS. Ultra high pressure treatment of orange juice: a kinetic study on inactivation of pectin methyl esterase. Food Res Int. 1996;29(7):601–607. doi: 10.1016/S0963-9969(96)00068-3. [DOI] [Google Scholar]
- Cameron RG, Baker RA, Grohman K. Citrus tissue extracts affect juice cloud stability. J Food Sci. 1997;62(2):242–245. doi: 10.1111/j.1365-2621.1997.tb03976.x. [DOI] [Google Scholar]
- Castro I, Macedo B, Teixeira JA, Vicente AA. The effect of electric field on important food-processing enzymes: comparison of inactivation kinetics under conventional and ohmic heating. J Food Sci. 2004;69(9):696–701. doi: 10.1111/j.1365-2621.2004.tb09918.x. [DOI] [Google Scholar]
- Chen CS, Wu MC. Kinetic models for thermal inactivation of multiple pectinesterases in citrus juices. J Food Sci. 1998;63(5):747–750. doi: 10.1111/j.1365-2621.1998.tb17891.x. [DOI] [Google Scholar]
- Cochran WG, Cox GM. Experimental designs. 2. Wiley: New York; 1957. p. 335. [Google Scholar]
- Cserhalmi Z, Sass-Kiss A, Toth-Markus M, Lechner N. Study of pulsed electric field treated citrus juices. Innovat Food Sci Emerg Tech. 2006;7(1–2):49–54. doi: 10.1016/j.ifset.2005.07.001. [DOI] [Google Scholar]
- Demirdöven A, Baysal T (2009a) Combined effects of electrical methods on orange juice production. Proceedings of the International Conference on Bio and Food Electrotechnologies, Compiegne, France, 81–87 (http://www.applphys.org.ua/files/Prof_stuff/Personal_Data/Lebovka/Content_book4.pdf Accessed 02/08/2011).
- Demirdöven A, Baysal T (2009b) Ohmic heating applications on fruit and vegetable products. International Conference on Bio and Food Electrotechnologies 22–23 October 2009, Compiègne, France, 294–300. (http://www.applphys.org.ua/files/Prof_stuff/Personal_Data/Lebovka/Content_book4.pdf Accessed 02/08/2011).
- Eagerman BA, Rouse AH. Heat inactivation temperature–time relationships for pectinesterase inactivation in citrus juices. J Food Sci. 1976;41(6):1396–1397. doi: 10.1111/j.1365-2621.1976.tb01180.x. [DOI] [Google Scholar]
- Elez-Martınez P, Suarez-Recio M, Espachs-Barroso A, Barbosa-Canovas GV, Martin-Belloso O (2003) High Intensity pulsed electric field inactivation of pectin methylesterase in orange juice, 12th World Congress of Food Science and Technology, Chicago, extended abstracts, 7E-9.
- Espachs-Barroso A, Loey AN, Hendrickx M, Martín-Belloso O. Inactivationof plant pectin methylesterase by thermal or high intensity pulsed electric field treatments. Innovat Food Sci Emerg Tech. 2006;7(1–2):40–48. doi: 10.1016/j.ifset.2005.07.002. [DOI] [Google Scholar]
- Farnworth ER, Lagace M, Couture R, Yaylayan V, Stewart B. Thermal processing, storage conditions, and the composition and physical properties of orange juice. Food Res Int. 2001;34(1):25–30. doi: 10.1016/S0963-9969(00)00124-1. [DOI] [Google Scholar]
- Fernandez-Garcia A, Butz P, Bognar A, Tauscher B. Antioxidative capacity, nutrient content and sensory quality of orange juice and an orange-lemon–carrot juice product after high pressure treatment and storage in different packaging. Eur Food Res Technol. 2001;213(4–5):290–296. doi: 10.1007/s002170100332. [DOI] [Google Scholar]
- Giner J, Grouberman P, Gimeno V, Martin O. Reduction of pectinesterase activity in a commercial enzyme preparation by pulsed electric fields: Comparison of inactivation kinetics models. J Sci Food Agric. 2005;85(10):1613–1621. doi: 10.1002/jsfa.2154. [DOI] [Google Scholar]
- Glevitzky M, Bogdan I, Brusturean GA, Silaghi-Perju D (2007) Use of pasteurization units or equivalent for the quality estimation of fruit juices submitted to different thermal treatments. Chem Bull “POLITEHNICA” Univ. (Timişoara) 52(66): 18–20.
- Hışıl Y (2004) Instrumental analysis of Foods, Ege University. Engineering Faculty Edition Number: 45., Izmir-TURKEY. pp 205–235
- Icıer F, Yıldız H, Baysal T. Peroxidase ınactivation and color changes during ohmic blanching of pea puree. J Food Eng. 2006;74(3):424–429. doi: 10.1016/j.jfoodeng.2005.03.032. [DOI] [Google Scholar]
- Icıer F, Yıldız H, Baysal T. Polyphenoloxidase deactivation kinetics during ohmic heating of grape juice. J Food Eng. 2008;85(3):410–417. doi: 10.1016/j.jfoodeng.2007.08.002. [DOI] [Google Scholar]
- Icier F, Ilicali C. The use of tylose as a food analog in ohmic heating studies. J Food Eng. 2005;69(1):67–77. doi: 10.1016/j.jfoodeng.2004.07.011. [DOI] [Google Scholar]
- Jha SN, Narsaiah K, Basediya AL, Sharma R, Jaiswal P, Kumar R, Bhardwaj R. Measurement techniques and application of electrical properties for nondestructive quality evaluation of foods—a review. J Food Sci Tech. 2011;48(4):387–411. doi: 10.1007/s13197-011-0263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabasakalis V, Siopidou D, Moshatou E. Ascorbic acid content of commercial fruit juices and its rate of loss upon storage. Food Chem. 2000;70(3):325–328. doi: 10.1016/S0308-8146(00)00093-5. [DOI] [Google Scholar]
- Knirsch MC, Santosa CA, Vicente AA, Penna TCV. Ohmic heating-a review. Trends Food Sci Technol. 2010;21(9):436–441. doi: 10.1016/j.tifs.2010.06.003. [DOI] [Google Scholar]
- Krop JJP, Pilnik W. Effect of pectic acid and bivalent cations on cloud loss of citrus juices. Lebensm Wiss Technol. 1974;7(1):62–63. [Google Scholar]
- Lee JY, Lin YS, Chang HM, Chen W, Wu MC. Temperature time relationships for thermal inactivation of pectinesterases in orange juice. J Sci Food Agric. 2003;83(7):681–684. doi: 10.1002/jsfa.1360. [DOI] [Google Scholar]
- Lee HS, Coates GA. Vitamin C in frozen fresh squeezed, unpasteurized, polyethylene-bottled orange juice: a storage study. Food Chem. 1999;65(2):165–168. doi: 10.1016/S0308-8146(98)00180-0. [DOI] [Google Scholar]
- Leizerson S, Shimoni E. Effect of ultrahigh-temperature continuous ohmic heating treatment on fresh orange juice. J Agric Food Chem. 2005;53(9):3519–3524. doi: 10.1021/jf0481204. [DOI] [PubMed] [Google Scholar]
- Leizerson S, Shimoni E. Stability and sensory shelf life of orange juice pasteurized by continuous ohmic heating. J Agric Food Chem. 2005;53(10):4012–4018. doi: 10.1021/jf047857q. [DOI] [PubMed] [Google Scholar]
- Lima M, Heskitt BF, Burianek LL, Nokes SE, Sastry SK. Ascorbic acid degradation kinetics during conventional and ohmic heating. J Food Process Eng. 1999;23(5):421–434. doi: 10.1111/j.1745-4549.1999.tb00395.x. [DOI] [Google Scholar]
- McLellan MR, Kime RL, Lind LR. Electroplasmolysis and other treatment to improveapple juice yield. J Sci Food Agric. 1991;57(2):303–306. doi: 10.1002/jsfa.2740570214. [DOI] [Google Scholar]
- Myers RH, Montgomery DC. Response surface methodology, process and product optimization using designed experiments. 2. New York: Wiley; 1995. [Google Scholar]
- Parrott D. Use of ohmic heating for aseptic processing of food particulates. Food Tech. 1992;46(12):68–72. [Google Scholar]
- Polydera AC, Galanou E, Stoforos NG, Taoukis PS. Inactivation kinetics of pectin methylesterase of greek Navel orange juice as a function of high hydrostatic pressure and temperature process conditions. J Food Eng. 2004;62(3):291–298. doi: 10.1016/S0260-8774(03)00242-5. [DOI] [Google Scholar]
- Ramaswamy R, Balasubramanıam VM, Sastry SK (2005) Ohmic heating of foods-fact sheet for food processors. Ohio State University (OSU). http://ohioline.osu.edu/fsefact/0004.html Accessed 22/04/2009).
- Rapisarda P, Bellomo SE, Intelisano S. Storage temperature effects on blood orange fruit quality. J Agric Food Chem. 2001;49(7):3230–3235. doi: 10.1021/jf010032l. [DOI] [PubMed] [Google Scholar]
- Rayman A, Baysal T. Yield and quality effects of electroplasmolysis and microwave applications on carrot juice production and storage. J Food Sci. 2011;76(4):598–605. doi: 10.1111/j.1750-3841.2011.02156.x. [DOI] [PubMed] [Google Scholar]
- Rayman A, Baysal T, Demirdöven A. Optimisation of electroplasmolysis application for increased juiceyield in carrot juice production. Int J Food Sci Technol. 2011;46(4):781–786. doi: 10.1111/j.1365-2621.2011.02561.x. [DOI] [Google Scholar]
- Rivas A, Rodrigo D, Martinez A, Barbosa-Canovas GV. Effect of PEF and heat pasteurization on the physical–chemical characteristics of blended orange and carrot juice. LWT-Food Sci Tech. 2006;39(10):1163–1170. doi: 10.1016/j.lwt.2005.07.002. [DOI] [Google Scholar]
- Rodrigo D, Barbosa-Cánovas GV, Martinez A, Rodrigo M. Pectin methylesterase and natural micro flora of fresh mixed orange and carrot juice treated with pulsed electric fields. J Food Protect. 2003;66(12):2336–2342. doi: 10.4315/0362-028x-66.12.2336. [DOI] [PubMed] [Google Scholar]
- Rombouts FM, Versteeg C, Karman AH, Pilnik W. Pectinesterase in components parts of citrus fruits related to problems of cloud loss and gelation in citrus products. In: Dupuy P, editor. Use of enzymes in food technology. Paris: Technique et documentation Lavoisier; 1982. pp. 483–487. [Google Scholar]
- Rouse AH, Atkins CD. Heat inactivation of pectinesterase in citrus juices. Food Tech. 1952;6(8):291–294. [Google Scholar]
- Sagar VR, Kumar SP. Recent advances in drying and dehydration of fruits and vegetables: a review. J Food Sci Tech. 2010;47(1):15–26. doi: 10.1007/s13197-010-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Moreno C, Plaza L, De Ancos B, Cano MP. Quantitative bioactive compounds assessment and their relative contribution to the antioxidant capacity of commercial orange juices. J Sci Food Agric. 2003;83(5):430–439. doi: 10.1002/jsfa.1392. [DOI] [Google Scholar]
- Sastry SK, Li Q. Modeling the ohmic heating of foods. Food Tech. 1996;50(5):246–248. [Google Scholar]
- Sharma SK, Juyal S, Rao VK, Yadav VK, Dixit AK (2012) Reduction of non-enzymatic browning of orange juice and semi-concentrates by removal of reaction substrate. J Food Sci Tech. doi:10.1007/s13197-012-0632-0 [DOI] [PMC free article] [PubMed]
- Snir R, Koehler PE, Sims KA, Wicker L. Total and thermostable pectinesterase in citrus juices. J Food Sci. 1996;61(2):379–382. doi: 10.1111/j.1365-2621.1996.tb14198.x. [DOI] [Google Scholar]
- Torregrosa F, Esteve MJ, Frigola A, Cortes C. Ascorbic acid stability during refrigerated storage of orange–carrot juice treated by high pulsed electric field and comparison with pasteurized juice. J Food Eng. 2006;73(4):339–345. doi: 10.1016/j.jfoodeng.2005.01.034. [DOI] [Google Scholar]
- Vikram VB, Ramesh MN, Prapulla SG. Thermal degradation kinetics of nutrients in orange juice heated by electromagnetic and conventional methods. J Food Eng. 2005;69(1):31–40. doi: 10.1016/j.jfoodeng.2004.07.013. [DOI] [Google Scholar]
- Van den Broeck I, Ludikhuyze LR, Van Loey AM, Weemaes CA, Hendrickx ME. Thermal and combined pressure– temperature inactivation of orange pectinesterase: Influence of pH and additives. J Agric Food Chem. 1999;47(7):2950–2958. doi: 10.1021/jf981169n. [DOI] [PubMed] [Google Scholar]
- Van den Broeck I, Ludikhuyze LR, Van Loey A, Hendrickx M. Inactivation of orange pectinesterase by combined highpressure and temperature treatments: A kinetic study. J Agric Food Chem. 2000;48(5):1960–1970. doi: 10.1021/jf990659s. [DOI] [PubMed] [Google Scholar]
- Van Loey A, Verachtert B, Hendrickx M. Effects of high electric field pulses on enzymes. Trends Food Sci Technol. 2002;12(3/4):94–102. [Google Scholar]
- Versteeg C, Rombouts FM, Spaansen CH, Pilnik W. Thermostability and orange juice cloud destabilization properties of multiple pectinesterase from orange. J Food Sci. 1980;45(4):969–998. doi: 10.1111/j.1365-2621.1980.tb07489.x. [DOI] [Google Scholar]
- Wicker L. Selective extraction of thermostable pectinesterase. J Food Sci. 1992;57(2):534–535. doi: 10.1111/j.1365-2621.1992.tb05536.x. [DOI] [Google Scholar]
- Yemenicioglu A, Cemeroglu B. The effects of activation and regeneration of enzymes on food quality. Food. 1998;23(6):415–23. [Google Scholar]
- Yeom HW, Zhang QH, Chism GW. Inactivation of pectin methyl esterase in orange juice by pulsed electric fields. J Food Sci. 2002;67(6):2154–2159. doi: 10.1111/j.1365-2621.2002.tb09519.x. [DOI] [Google Scholar]
- Yıldız H, Baysal T. Effects of alternative current heating treatment on Aspergillus niger, pectin methylesterase and pectin content in tomato. J Food Eng. 2006;75(3):327–332. doi: 10.1016/j.jfoodeng.2005.04.020. [DOI] [Google Scholar]
- Yıldız H, Baysal T. Color and lycopene content of tomato puree affected by electroplasmolysis. Int J Food Properties. 2007;10(3):489–495. doi: 10.1080/10942910600909063. [DOI] [Google Scholar]