Abstract
Direct acidified cottage cheese has a limited shelf life of 10–12 days under refrigeration due to high moisture content (~75 %) and a relatively high pH (~5.0). This affects its widespread marketing and distribution. Hence, a study was undertaken to improve the shelf life of direct acidified cottage cheese using thymol, a phytophenolic natural antimicrobial agent. The effect of three different levels, i.e. 30, 40 and 50 ppm of 30 % thymol solution in butteroil on the physico-chemical, microbiological and organoleptic properties was studied at four-day interval during storage under refrigeration (4–5 °C). Promising results were obtained using 40 ppm thymol for inhibiting psychrotrophs, yeasts and molds as well as retarded the proteolysis in cottage cheese. Based on the effect on flavour of the fresh samples as well as the extent of changes in quality during storage, it was observed that addition of 40 ppm thymol enhanced the keeping quality of cottage cheese by 8 days compared to the control sample.
Keywords: Cottage cheese, Shelf life extension, Thymol, Phytophenolic compound, Natural preservative, Natural antimicrobial agent
Introduction
Owing to high moisture content (~75%) and relatively high pH (~5.0), the keeping quality of direct acidified cottage cheese is limited to 10 to 12 days under refrigeration, which affects its widespread marketing and distribution. Shelf life of commercial cottage cheese hardly exceeds 7 days, while some, where stringent quality control is practiced, may exhibit a shelf life of 15 days (Luck et al. 1977; Johnson 1979; Makhal and Kanawjia 2003a). Spoilage of cottage cheese is largely because of the microbial incursion. Of the organisms causing spoilage in cottage cheese, psychrophilic bacteria as well as yeasts and molds are considered the most serious. Several factors have been reported to be the source of contamination in cottage cheese, such as milk, wash water, coagulant, starter culture, equipment, air, packaging materials, etc. (Harmon and Smith 1956a, b; Scafer 1958; Angevine 1959; Emmons and Tuckey 1967). These microbial contaminants bring about several physico-chemical changes leading to the development of off-flavour and discolouration of the surface (Makhal and Kanawjia 2003a). Since past few decades, a number of chemical and physical treatments have been attempted to extend the shelf life of cottage cheese.
Antimicrobial activities of the extracts from several types of spices are used as seasonings in foods and beverages have been recognized for many years. Promising results have been reported using the phytochemicals of spices for inhibiting psychrotrophic bacteria, yeasts and molds in different food systems (Makhal and Kanawjia 2003b, c; Makhal et al. 2003). Since ancient time, a technique that has been practised to prevent fungal growth in foods, such as cheese, involved physically rubbing the product with certain herbs and spices or their essential oils known for antimicrobial properties (Leung 1978). Spices are also applied to the surface of some cheeses to impart flavour (USDA 1953; Robinson 1995; Wendorff and Wee 1997). It has been investigated that spice oils from thyme strongly inhibits spoilage molds (A. parasiticus, P. camemberti and P. roqueforti) in oil-coated Cheddar cheeses (Wendorff and Wee 1997). Thymol, which is a natural antimicrobial phenolic compound and the main flavour ingredient of cloves and allspice is also found largely in thyme, oregano, sage and eugenol. Vazquez et al. (2001) reported antifungal effect of thymol in three different types of Gsalician cheese, i.e. Arzua-Ulloa, Cebreiro and Simon, at a concentration of 200 μg/ml. The antimicrobial action of phenolic compounds is due to their effect on cellular membrane as it causes structural and functional damage to plasma membrane (Sikkema et al. 1995; Davidson 1997; Kokkini et al. 1997; Ultee et al. 1999).
In the present study, endeavours were made to find out the possibility of using thymol to enhance the shelf life of direct acidified cottage cheese without sacrificing the quality of the product. With an aim of optimization of levels of thymol, the effect of three different levels, i.e. 30, 40 and 50 ppm of 30 % thymol solution in butteroil on the physico-chemical, microbiological and organoleptic properties was investigated.
Materials and methods
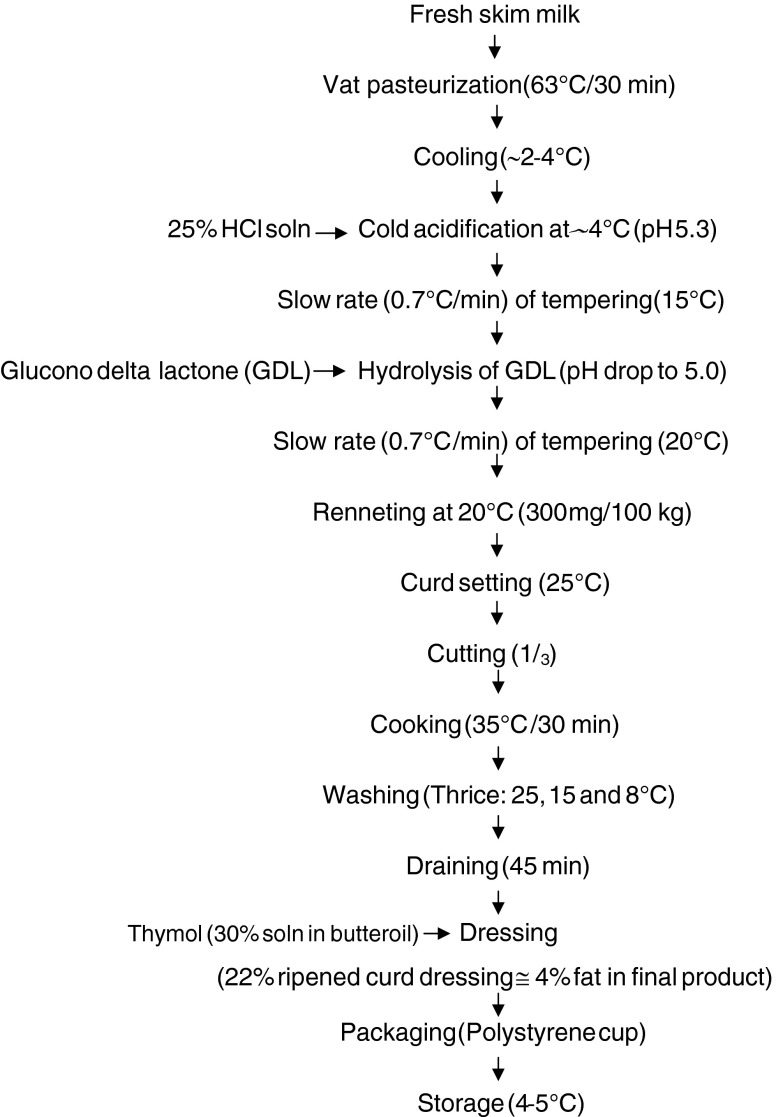
Fresh skim milk (~0.02 % fat) from the Experimental Dairy, NDRI, Karnal was transferred to a pasteurizing vat. Cottage cheese was manufactured employing dual acidification technique following the procedure (Makhal et al. 2011) as outlined in Fig. 1.
Fig. 1.
Flow diagram for manufacturing direct acidified cottage cheese employing dual acidification technique
Mixed strain multiple starter cultures (Lc. lactis subsp. lactis, Lc. lactis subsp. cremoris and Lc. lactis subsp. lactis biovar. diacetylactis) used for ripening of curd dressing were collected from the National Collection of Dairy Cultures (NCDC), NDRI, Karnal, India. Meito rennet (M/s Meito Sangyo and Co. Ltd., Tokyo, Japan) commercially produced from Mucor pusillus var. lindt was used in the experiment. Thymol (5-methyl-2-isopropylphenol) was procured from Sigma-Aldrich Inc., USA. The chemicals (AR grade) used for analytical purposes were procured from the standard firms (RANBAXY Fine Chemicals Ltd., New Delhi).
Calculated quantity of cream and skim milk were mixed to prepare curd dressing having 22 % fat. The standardized cream (40 % fat), after homogenization at 176 kg cm−2 and pasteurization at 75 °C for 15 s, was cooled to 22 °C and inoculated with the 3 % inoculum of mixed strain cultures and ripened for 14–16 h at 30 °C. The titratable acidity of the ripened curd dressing was within the range of 0.75 to 0.80 %.
Selection of the three levels of thymol was achieved based on the results of sensory evaluation of the fresh product in the preliminary trials. Calculated amount of thymol crystals was dissolved in laboratory made liquid butter oil (50 °C) to prepare a 30 % solution and appropriate quantities of this solution were mixed with the curd dressing to attain the desired concentrations of thymol in the final product.
Analysis
The samples were analyzed for sensory, biochemical as well as microbiological quality after each 4 days interval during the study.
Sensory evaluation
Sensory evaluation of cottage cheese was carried out using the standard cottage cheese score card recommended by the ADSA (2004). Descriptive sensory analysis method was performed using 10 trained panellists of the institution, constituted based on their interest, performance, motivation, compliance and availability (ISO 8586–1: 1993E). Attributes evaluated were flavour, body and texture as well as colour and appearance. The panellists received preliminary training according to the methods recommended by the ISO (ISO 8586: 1993E). The analysis was performed in Sensory Laboratory designed according to the recommendations in ISO standards (ISO 8589: 1985). Before profiling, two sessions were used to train the assessors in the definition of attributes of a standard identity cottage cheese. The panellists were allowed to use water and bland crackers for palate cleansing between the samples. All the samples (100 g), randomly coded using three digit numbers, were evaluated in each session and each panellist was given the samples (25 °C) in white 100 ml plastic cup always 2 h before and after meals. Each sample was served in duplicate. The panellists expressed their judgments about the samples using the structured numeric scale as per the guidelines of ADSA (2004).
Physico chemical analysis
Moisture content as well as titratable acidity of cottage cheese was determined as per the AOAC (2000). Moisture content in cottage cheese curd was determined by oven drying to constant weight. Titratable acidity of the samples was expressed as per cent of lactic acid (w/v). Determination of pH was carried out as per the standard method (Shakeel-Ur-Rehman et al. 2003). For this, 10 g of grated cottage cheese was mixed with 10 ml distilled water and slurry was prepared thereof. The pH of the slurry was determined using microprocessor controlled pH Analyser (Version I, Labindia, New Delhi) with combined glass electrode. In order to measure the degree of lipolysis in the samples, total free fatty acid (FFA) of the cheese sample was estimated using the standard method (Deeth and Fitzgerald 1976) and the extent of proteolysis was measured by determining soluble nitrogen following the procedure delineated by Kosikowski (1982).
Microbiological analysis
Enumeration of psychrotrophs, yeasts and molds as well as coliforms counts of cottage cheese was done in accordance with the method described by Houghtby et al. (1993). For psychrotrophic count, incubation was done at 5 °C for 7 days.
Statistical analysis
All experiments were in triplicate. The data were statistically analyzed as per the methods described by Evanston (1990) with the application of SYSTAT software, VERSION 6.01 COPYRIGHT (C) 1996, SPSS INC employing Least Squares Analysis of Variance of a two-factor design experiment. When significant (1 and 5 % levels) differences were observed, individual means were compared by using Fisher’s Least Significant Difference model. Graphical representation of the results has been made using GraphPad Prism® Software, Version 3.02, GraphPad Software Inc, 5755 Oberlin Dr #110, San Diego, CA 92121.
Results and discussion
Changes in sensory quality
Flavour
The effect of addition of thymol at different concentrations on the flavour as well as body and texture of direct acidified cottage cheese during storage is illustrated in Fig. 2. Addition of thymol imparted a distinguishing thymol flavour termed as medicinal flavour to the product as the level of addition increased. Thymol incorporated at 30 ppm (Sample T1) imparted a slight medicinal flavour, which increased with the elevation in thymol level to 40 ppm (Sample T2) and 50 ppm (Sample T3). As the consequence, initially sample T3 attained comparatively a poor flavour score as compared to the sample T2 and T1. While the control sample relatively achieved a higher score for flavour. Storage up to 8 days produced no significant variation among the samples in terms of flavour. Sensory evaluation showed that with progressing storage up to a certain period, the samples regardless of levels of thymol incorporated exhibited a slow and steady deterioration in flavour followed by a sharp change towards the end of storage.
Fig. 2.
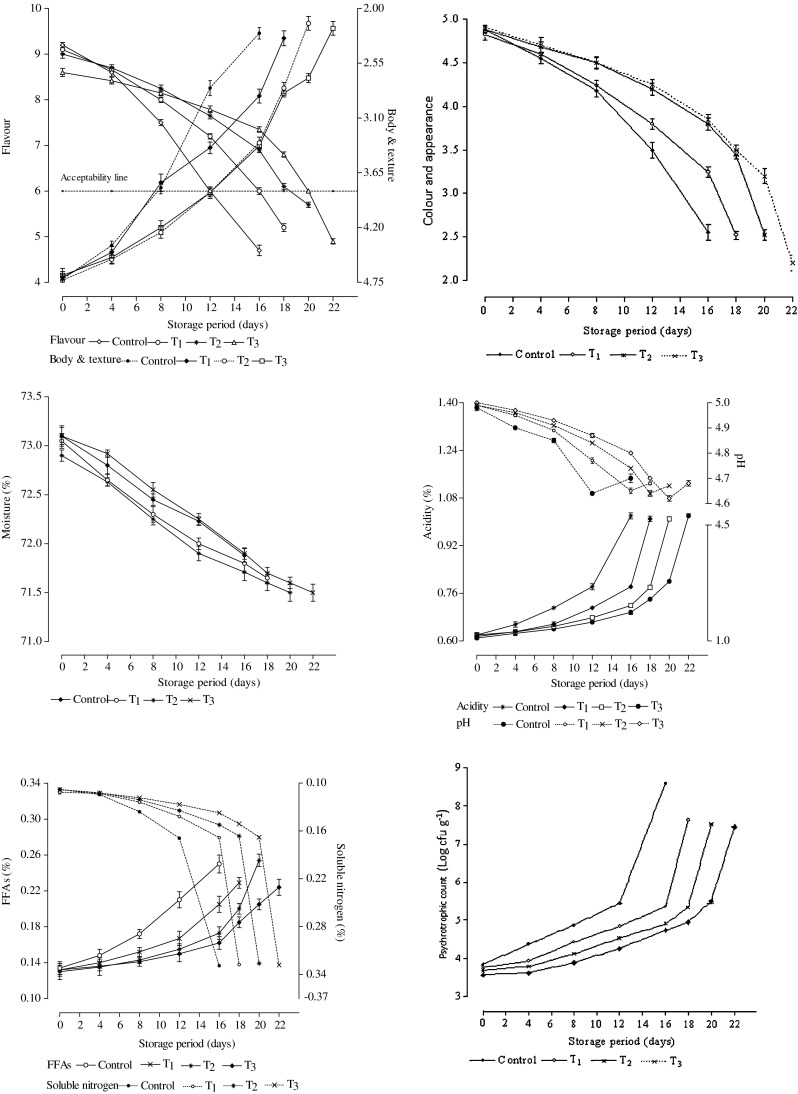
Effect of addition of different concentrations of thymol on sensory quality attributes (Flavour, Body and texture and Colour and appearance, n = 10 panelists), chemical parameters (Moisture, Acidity, pH, FFAs, Soluble nitrogen, n = 3) and Psychrotrophic count of direct acidified cottage cheese packaged in polystyrene cup and stored at 4–5 °C. T1, T2 and T3 imply samples treated with 30, 40 and 50 ppm of thymol (30 % soln in butteroil), respectively. The vertical bars indicate the errors of means
On the 12th day, flavour score of the control sample became significantly (P < 0.05) poor as compared to the treated samples added with 40 and 50 ppm thymol; however the sample T1 showed no marked difference in flavour score as compared to sample T2 and T3. It was further noticed that on the 16th day, the control sample underwent significantly (P < 0.05) faster deterioration in flavour as compared to the treated samples. The sample T1 also achieved markedly (P < 0.05) poor flavour scores after 16 days of storage in comparison to the sample T3. Nevertheless, sample T2 showed no such deterioration, although on the 20th day, it experienced a sharp decrease (P < 0.05) in flavour score compared to the T3 sample, which too exhibited the same trend of change upon storage up to 22 days.
Initially, control sample was characterized with an acidic sharpness accompanied by a pleasant note of diacetyl flavour, which was somewhat subdued by the addition of thymol depending upon its concentration. On storage for 12 days, control sample was found unacceptable and criticized for distinct off-flavour and a pronounced bitterness, and consequently the sample was judged unacceptable. Similarly, beyond 16, 20 and 20 days of storage, sample T1, T2 and T3, respectively exhibited the similar observations. It was thus found that increasing the level of thymol considerably delayed deterioration in flavour of cottage cheese during storage through protection against microbial spoilage.
Body and texture
Addition of thymol at varying concentrations initially produced no significant effect on the average body and texture score. With progressing storage period, all samples showed a gradual and steady deterioration in body and texture (Fig. 2). It was found that change in body and texture quality was quite affected by the addition of thymol during storage. On the 12th day, body and texture score of the control sample became significantly (P < 0.05) lower in comparison to the treated samples and on the 16th day, it sharply decreased (P < 0.05) and the product became fairly compact associated with a mealy and pasty texture. Following storage up to 18 and 20 days, correspondingly the sample T1 and T2 exhibited the similar observations. Upon further progress in storage period to 22 days, parallel observations were recorded for the sample T3 and body and texture of the product became unacceptable because of the distinct sign of liquefaction of curd particles and gelatinization of curd resulting in a compact, mealy and pasty body. The treated samples were observed to display a delayed deterioration in body and texture compared to the control sample (Fig. 2) because of the fact that addition of thymol likely produced antimicrobial effect against several spoilage causing microorganisms, which, in turn, might cause deterioration in body and texture by their metabolic activity (Vazquez et al. 2001).
Colour and appearance
Figure 2 shows that addition of thymol at varying levels exhibited no noticeable effect on the colour and appearance of the fresh samples. With the progress in storage period, colour and appearance was found to undergo a slow and gradual deterioration. Regardless of the treatment, colour and appearance scores showed no significant variation upon storage up to 8 days. However, after 12 days, the control sample exhibited significantly (P < 0.05) poor colour and appearance score compared to the sample T2 and T3. Storage up to 16 days produced a significant (P < 0.05) deterioration in the control sample in comparison to the treated samples. Figure 2 also shows that on the 18th day, colour and appearance score of the sample T1 sharply decreased (P < 0.05) compared to the sample T2 and T3; however upon subsequent storage up to 20 and 22 days, sample T2 and T3 correspondingly exhibited the identical observations.
Changes in physico-chemical properties
Moisture
It is clear from the Fig. 2 that moisture content of all samples declined slowly and steadily during storage. At the early stage of storage, the samples showed relatively rapid moisture loss followed by a slow and gradual decrease with further advances in storage. Moisture content of the fresh samples varied from 72.90 to 73.10 %; while at the end of storage life, it ranged from 71.50 to 71.88 % depending upon the length of storage period with a corresponding reduction in moisture content by 1.69 and 2.19 %, respectively.
It was also observed that addition of thymol exhibited no direct effect on the loss of moisture in cottage cheese during the storage, instead of that it was quite dependent upon the length of storage period. Regardless of the treatment exercised, loss of moisture content occurred relatively slowly towards the end of storage period.
Acidity
Initially acidity of the fresh cottage cheese samples ranged between 0.61 and 0.62 %. With progressing storage, development of acidity followed a slow and gradual increase. Figure 2 illustrates that cottage cheese samples irrespective of the addition of thymol exhibited an increase in acidity moderately slowly up to a certain period during storage followed by subsequent sharp rise in acidity. Upon storage for 12 days, no significant variation in acidity was noticed among the different samples; however, acidity of the control sample sharply increased (P < 0.05) after 16 days in comparison to the treated samples. Upon subsequent storage up to 18, 20 and 22 days, the sample T1, T2 and T3, respectively exhibited similar trend in respect of change in acidity. Figure 2 also portrays that increasing the level of addition of thymol caused relatively a slow acid development. It was also observed that maximum change in acidity was in control sample followed by T1, T2 and T3. Addition of thymol in cottage cheese delayed the acid development during storage, possibly because of its antimicrobial activity against the spoilage causing microorganisms.
pH
pH of the fresh samples of cottage cheese ranged from 4.98 to 5.0. Afterwards, it steadily decreased up to a certain period of storage depending upon the level of thymol added (Fig. 2). Upon storage up to 12, 16, 18 and 20 days, control as well as the sample T1, T2 and T3 exhibited a gradual decrease in pH, respectively. On subsequent storage, pH of all samples showed a tendency to go up again. After 12 days, pH of the control sample declined significantly (P < 0.05) in comparison to the treated samples. Upon storage up to 16 days, unlike treated samples, control sample experienced a small increase in pH. Following storage for 18, 20 and 22 days, sample T1, T2 and T3, respectively showed a similar trend. It was noticed that the cottage cheese added with thymol showed delayed and small change in pH compared to the control sample. With the increase in concentration of thymol added, the rate of change in pH happened slowly because of fact that thymol at high concentration exerted a strong antimicrobial activity against the lactose fermenting organisms.
The steady decline in pH during the storage was due to the slow rate development of acidity. Subsequently, the increase in pH might be attributed to the facts that microbial activity causes break down of citrate and hydrolysis of protein resulting in the formation of more protein bound residues having high pK (=logka) values and the medium with higher pK value is likely to have high pH values (Walstra and Jenness 1984). Direct acidified cottage cheese contains ~1.10 % lactose and hence, the effect of protein bound residues formed and the release of basic amino acids towards the end of storage due to higher rate of proteolysis seem to override the mere effect of lactic acid causing a decrease in the pH. Suriyarachchi and Fleet (1981) also observed that some proteolytic yeast species are able to raise the pH through their excessive casein hydrolysis capabilities.
Free fatty acids
Initially FFA content of different fresh cottage cheese samples ranged from 0.130 to 0.134 % oleic acid. With the advances in storage, FFA content of the cheese regardless of the type of samples gradually increased (Fig. 2). Storage up to 8 days produced no remarkable variation in FFA content among the samples. However, upon 12 days of storage, control sample exhibited markedly higher (P < 0.05) value for FFA content compared to the treated samples. On subsequent storage for 16 days, FFA content of the control sample sharply increased (P < 0.05) in comparison to the treated samples; however, the treated samples showed no significant variation upon storage up to 16 days. However, upon storage up to 18 and 20 days, sample T1 and T2, respectively, exhibited the similar observations compared to the T3 sample, which upon storage up to 22 days also produced a sharp increase in FFA content. Figure 2 also depicts that control sample comparatively exhibited maximum increase in FFA content compared to the treated samples. With increasing the thymol concentration, the rate of increase in FFA content decreased, which could be attributed to the restricted rate of lipolysis in cottage cheese due to the antimicrobial activity of thymol.
Soluble nitrogen
It was observed that as the storage period advanced, soluble nitrogen content, regardless of the type of samples, increased slowly followed by comparatively a sharp rise at the end of the storage (Fig. 2). However, on storage up to 12 days, irrespective of the type of samples there was no appreciable difference in the degree of proteolysis; with progressing storage period to 16 days, control sample showed a rapid increase in proteolysis, which was markedly higher (P < 0.05) than that of treated samples. With advancing storage up to 16 days, there was no significant variation in soluble nitrogen content among the treated samples. Further increasing storage period to 18 days, sample T1 showed a significantly (P < 0.05) sharp increase in proteolysis and thereby in soluble nitrogen content compared to the sample T2 and T3. Upon subsequent storage up to 20 and 22 days, similar observation was made for sample T2 and T3 respectively. It was evident that control sample exhibited rapid proteolysis compared to the treated samples. Elevation of the level of thymol was found significantly beneficial in controlling the rate of proteolysis in cottage cheese during storage. This could be attributed to the antimicrobial activity of thymol against a diverse sort of spoilage microorganisms responsible for the proteolysis with a concomitant increase in soluble nitrogen.
Changes in microbiological quality
Psychrotrophs
Psychrotrophic count of the fresh control sample was 3.841985 log cfu/g, while the treated samples added with 30, 40 and 50 ppm thymol showed a psychrotrophic count of 3.766413, 3.68842 and 3.574031 log cfu/g, respectively with a corresponding reduction in initial count by around 16, 30 and 46 %, respectively compared to the control sample. Numerous studies show that thymol and other essentials oils have strong bactericidal effect. The reduction was perhaps on account of the bactericidal effect of thymol (Wan et al. 1998; Hammer et al. 1999; Koga et al. 1999; Thuille et al. 2003). With the progress in storage, gradual increase in psychrotrophic count was observed irrespective of the treatment (Fig. 2). It was also found that upon storage for 12 and 16 days, comparatively a sharp rise in psychrotrophic count was observed in control as well as in T1 sample, whereas upon storage for 18 and 20 days, sample T2 and T3 showed a similar observation for psychrotrophic growth. Towards the end of storage period, each sample showed significantly faster growth of psychrotrophs compared to the earlier storage period. Rapid increase in psychrotrophic count was observed in control sample compared to the samples added with thymol and with the rise in its usage level, rate of increase in psychrotrophs was found to decline. Comparatively the lower growth of psychrotrophs in samples added with thymol seems due to the strong antimicrobial action of this compound.
Yeasts and molds
Table 1 clearly depicts the effect of addition of different levels of thymol on the onset of yeasts and molds in cottage cheese during storage. The fresh control sample showed an average yeasts and molds count of 0 to 0.30103 log cfu/g, while the treated samples exhibited no occurrence of yeasts and molds on storage up to 12 days. The occurrence of yeasts and molds in the treated samples was noticed during 12 to 16 days of storage. Thereafter with progressing storage period, yeasts and molds count gradually increased, which was followed by a sharp rise towards the end of storage period regardless of treatment.
Table 1.
Effect of different levels of thymol on (A) Yeasts and molds count and (B) Coliforms (log cfu/g) of direct acidified cottage cheese during storage at 4–5 °C
| Days | Control | Levels of thymol (ppm) | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| (A) Yeasts and molds count | ||||
| 0 | 0–0.30103 | −ve | −ve | −ve |
| 4 | 0.778151 | −ve | −ve | −ve |
| 8 | 1.113943 | −ve | −ve | −ve |
| 12 | 1.977724 | 0–0.69897 | 0–0.30103 | −ve |
| 16 | 2.64836 | 1.832509 | 1.681241 | 0–0.778151 |
| 18 | ND | 2.544068 | 2.049218 | 1.875061 |
| 20 | ND | ND | 2.824776 | 2.133539 |
| 22 | ND | ND | ND | 2.89487 |
| (B) Coliforms | ||||
| 0 | <1 | <1 | <1 | <1 |
| 4 | 1.447158 | 1.30103 | <1 | <1 |
| 8 | 1.875061 | 1.748188 | 1.380211 | 1.20412 |
| 12 | 2.491362 | 2.09691 | 1.778151 | 1.447158 |
| 16 | 3.875061 | 2.565848 | 2.041393 | 1.778151 |
| 18 | ND | 3.515874 | 2.352183 | 2.120574 |
| 20 | ND | ND | 3.164353 | 2.447158 |
| 22 | ND | ND | ND | 3.50515 |
T1, T2 and T3 imply samples treated with 30, 40 and 50 ppm of thymol (30 % soln in butteroil), respectively. ND Not detected
Owing to the antifungal action, addition of thymol in cottage cheese was observed to exhibit a significant inhibitory effect against the growth of yeasts and molds during storage. With the elevation in concentration of thymol, occurrence of yeasts and molds was considerably delayed. The study also demonstrated that sample T3 showed relatively lower yeasts and molds growth followed by T2 and T1 samples, respectively. Yeasts and molds count of the fresh cultured cottage cheese should not exceed 1 log cfu/g (Robinson 1981). However, yeasts and molds count of the fresh samples was below this limit; during storage period, it gradually increased causing spoilage of the product. Further break down of lactose into lactic acid that happened harshly towards the end of storage period intensified the proliferation of yeasts and molds.
Coliforms
Use of thymol was quite effective in controlling the growth of coliforms in cottage cheese during storage. Initially, coliforms counts of the samples were below 1 log cfu/g. The count remained within 1 log cfu/g in T2 and T3 stored up to 4 days. (Table 1). While the corresponding count in the control and T1 samples reached to 1.447158 and 1.30103 log cfu/g, respectively. Afterward each sample showed a gradual increase in coliforms count followed by a rapid growth being observed at the end of storage. On the 16th day of storage, the control sample showed noticeably higher coliforms count compared to the treated samples; while on the 18th and 20th day, the sample T1 and T2, respectively also exhibited a similar observation when compared with the T3 sample.
Although the antimicrobial activity of phenolic compounds is well established, the inhibitory mechanisms of these compounds on microorganisms have not yet been elucidated well. Indeed, it has been proposed that phenolics of essential oils generally attack the cytoplasmic membrane releasing intracellular constituents due to a weakening or destruction of permeability barrier of the cell membranes (Vas 1953). It is also advocated that the antimicrobial action of essential oils might be due to impairment of a variety of enzyme systems, including those involved in energy production and structural component synthesis of microorganisms (Conner and Beuchat 1984a, b). Leakage is found to be a general phenomenon induced by many antibacterial substances (Hugo 1991). Phenolics generally cause static rather than outright toxic effects. Cell membranes that leak or function poorly would not necessarily be lethal, but would most probably cause a slowing of metabolic processes, such as cell division (Darvill and Albersheim 1984). Juven et al. (1994) postulated that inhibition of S. typhimurium by thymol was due to a reaction of the compound with proteins in the cytoplasmic membrane of the microorganism. This causes alterations in permeability of the membrane resulting in possible leakage and affects the proton motive force. Unlike many antibiotics, the hydrophobic constituents of essential oils are capable of gaining access to the periplasm of Gram-negative bacteria through the porin proteins of the outer membrane (Helander et al. 1998). It is clearly demonstrated that oregano essential oils as well as thymol and carvacrol disrupt the cell membrane, causing an increased permeabilization to the nuclear stain EB. Thymol and carvacrol have also been reported to disintegrate the outer membrane of E. coli and S. typhimurium at levels close to the minimum inhibitory concentration (MIC) (Helander et al. 1998).
The antimicrobial activity of essential oils is mainly attributed to the phenols they contain. Thymol has been tested for their antimicrobial activity against B. subtilis, S. enteritidis, S. Aureus, P. aeruginosa, Proteus and E. coli and it was found that they inhibited all microorganisms at dilutions as low as 1:2000 (Katayama and Nagai 1960).
Conclusion
In the present investigation the effect of different levels of thymol on the direct acidified cottage cheese was carried out to increase its shelf life. Among three different levels i.e. 30, 40, 50 ppm thymol, it was observed that with increasing the level of thymol, cottage cheese underwent moderately minimum changes. However, though addition of 50 ppm thymol in cottage cheese instead of 40 ppm, enhanced the shelf life only by 2 days, it imparted more pronounced medicinal flavour to the product. While use of 40 ppm thymol improved the keeping quality of cottage cheese by 8 days against the control sample without any noticeable adverse effect on the typical flavour of cottage cheese. Hence, addition of thymol @ 40 ppm in cottage cheese was proved effective to extend the shelf life of the product from 12 to 20 days with an additional increase of shelf life by 67 % as compared to the control sample.
References
- ADSA (2004) Cottage cheese score card, 2003 rules. In: 83rd Annual Collegiate Dairy Products Evaluation Contest. Committee on Dairy Product Evaluation, American Dairy Science Association. 1111 N. Dunlap Avenue Savoy. IL 61874. USA. pp 16
- Angevine NC. Keeping quality of cottage cheese. J Dairy Sci. 1959;42:2015–2019. doi: 10.3168/jds.S0022-0302(59)90850-1. [DOI] [Google Scholar]
- Horwitz W, editor. Vol. II. 17. Washington: Association of Official Analytical Chemists; 2000. Association of official analytical chemists. Official methods of analysis. [PubMed] [Google Scholar]
- Conner DE, Beuchat LR. Sensitivity of heat stressed yeasts to essential oils of plants. Appl Environ Microbiol. 1984;47:229–233. doi: 10.1128/aem.47.2.229-233.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner DE, Beuchat LR. Effects of essential oils of plants on growth of food spoilage yeasts. J Food Sci. 1984;49:429–434. doi: 10.1111/j.1365-2621.1984.tb12437.x. [DOI] [Google Scholar]
- Darvill AG, Albersheim P. Phytoalexins and their elicitors: a defence against microbial infection in plants. Annu Rev Plant Physiol. 1984;34:243–275. doi: 10.1146/annurev.pp.35.060184.001331. [DOI] [Google Scholar]
- Davidson PM. Chemical preservatives and natural antimicrobial compounds. In: Doyle MP, Beuchat LR, Montville TJ, editors. Food microbiology fundamentals and frontiers. New York: ASM; 1997. pp. 520–556. [Google Scholar]
- Deeth HC, Fitzgerald CH. Lipolysis in dairy products: a review. Aust J Dairy Technol. 1976;31:53–64. [Google Scholar]
- Emmons DB, Tuckey SL. Cottage cheese and other cultured milk products. New York: Has Pfizer & Co, Inc; 1967. [Google Scholar]
- Evanston IL (1990) System of Statistics (SYSTAT). Evanston IL (ed), Wilkinson
- Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- Harmon LG, Smith CL. Spoilage organisms in cottage cheese. J Dairy Sci. 1956;39:9–15. [Google Scholar]
- Harmon LG, Smith CL. The influence of environment and processing in spoilage organisms in cottage cheese. J Dairy Sci. 1956;19:252–255. [Google Scholar]
- Helander IK, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, von Wright A. Characterization of the action of selected essential oil components on Gram negative bacteria. J Argil Chem. 1998;46:3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- Houghtby GA, Maturin LJ, Koenig EK. In: Microbiological count methods. 16. Marshall RT, editor. Washington: American Public Health Association (APHA); 1993. pp. 213–246. [Google Scholar]
- Hugo WB. A review: a brief history of heat and chemical preservation and disinfection. J Appl Bacteriol. 1991;71:9–18. doi: 10.1111/j.1365-2672.1991.tb04581.x. [DOI] [PubMed] [Google Scholar]
- ISO 8586:1993E. Sensory analysis-methodology-general guidance. International Organization for Standardization, Geneva, Switzerland
- ISO 8586–1:1993E. Sensory analysis-general guidance for the selection, training and monitoring of assessors. International Organization for Standardization, Geneva, Switzerland
- ISO 8589:1985. Sensory analysis-general guidance for the design of test rooms. International Organization for Standardization, Geneva, Switzerland
- Johnson P. Cottage cheese: a primer for high quality and long shelf life. Am Dairy Rev. 1979;41:400. [Google Scholar]
- Juven BJ, Kanner J, Schved F, Weisslowicz H. Factors that interest with the antibacterial action of thyme essential oil and its active constituent. J Appl Bacteriol. 1994;76:626–631. doi: 10.1111/j.1365-2672.1994.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Katayama T, Nagai I. Chemical significance of the volatile components of spices in the food preservative viewpoint. VI. Structure and antibacterial activity of terpenes. Bull Jpn Soc Sci Fisheries. 1960;26:29–32. doi: 10.2331/suisan.26.29. [DOI] [Google Scholar]
- Koga T, Hirota N, Takumi K. Bactericidal activities of essential oils of basil and sage against a range of bacteria and the effect of these essential oils on Vibrio parahaemolyticus. Microbiol Res. 1999;154:267–273. doi: 10.1016/S0944-5013(99)80024-X. [DOI] [PubMed] [Google Scholar]
- Kokkini S, Karousou R, Dardioti A, Krigas N, Lanaras T. Autumn essential oils of Greek oregano. Phytochemistry. 1997;44:883886. doi: 10.1016/S0031-9422(96)00576-6. [DOI] [Google Scholar]
- Kosikowski FV. Cottage cheese. In: Kosikowski FV, editor. Cheese and fermented milk foods. 2. New York: F. V. Kosikowski and Associates; 1982. pp. 109–143. [Google Scholar]
- Leung AY. Encyclopedia of common natural ingredients used in foods, drugs and cosmetics. New York: Wiley; 1978. pp. 309–311. [Google Scholar]
- Luck H, Mosteret JF, Husmann RA. Shelf life of perishable dairy products. S Afr J Dairy Technol. 1977;9:25–27. [Google Scholar]
- Makhal S, Kanawjia SK. Preservation of cottage cheese: a review. Indian J Dairy Sci. 2003;56:1–12. [Google Scholar]
- Makhal S, Kanawjia SK. Microbial metabolites in preserving dairy products—an overview. J Food Dairying Home Sci. 2003;23:157–171. [Google Scholar]
- Makhal S, Kanawjia SK. Phytophenols in preservation of dairy products: fads and fantasy to facts and figures. Indian J Dairy Biosci. 2003;14:82–87. [Google Scholar]
- Makhal S, Mandal S, Kanawjia SK. MicroGARD™ in food preservation: a shifted paradigm towards natural preservatives. Indian J Dairy Biosci. 2003;14:70–78. [Google Scholar]
- Makhal S, Giri A, Kanawjia SK (2011) Effect of κ-carrageenan and tetrasodium pyrophosphate on the yield of direct acidified cottage cheese. J Food Sci Technol. doi:10.1007/s13197-011-0438-5 [DOI] [PMC free article] [PubMed]
- Robinson RK. Dairy microbiology. Vol. 2. London: Elsevier Science Publishers Ltd; 1981. [Google Scholar]
- Robinson RK. A colour guide to cheese and fermented milk. London: Chapman and Hall; 1995. [Google Scholar]
- Scafer JG. Cottage cheese sanitation practice. Am Milk Rev. 1958;20:42–44. [Google Scholar]
- Shakeel-Ur-Rehman, Farkye NY, Drake MA. Reduced fat cheddar cheese from a mixture of cream and liquid milk protein concentrate. Int Dairy J. 2003;56:94–98. doi: 10.1046/j.1471-0307.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- Sikkema J, de Bont JAM, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriyarachchi RV, Fleet HG. Occurrence and growth of yeast in yogurts. Appl Environ Microbiol. 1981;42:574–577. doi: 10.1128/aem.42.4.574-579.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuille N, Fille M, Nagl M. Bactericidal activity of herbal extracts. Int J Hyg Environ Health. 2003;206:217–221. doi: 10.1078/1438-4639-00217. [DOI] [PubMed] [Google Scholar]
- Ultee A, Kets EPW, Smid EJ. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 1999;65:4606–4610. doi: 10.1128/aem.65.10.4606-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheese varieties and descriptions. USDA Agriculture Handbook 54. Washington: US Department of Agriculture; 1953. [Google Scholar]
- Vas K. Mechanisms of antimicrobial action-interference with the cytoplasmic membrane. Agrokém Talajt. 1953;2:1–16. [Google Scholar]
- Vazquez BI, Fente C, Franco CM, Vazquez MJ, Cepeda A. Inhibitory effects of eugenol and thymol on Penicillium citrinum strains in culture media and cheese. Int J Food Microbiol. 2001;67:157–163. doi: 10.1016/S0168-1605(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Walstra P, Jenness R. Dairy chemistry and physics. New York: John Wiley; 1984. [Google Scholar]
- Wan J, Wilcock A, Coventry MJ. The effect of essential oils of basil on the growth of Aeromonas hydrophila and Pseudomonas fluorescens. J Appl Microbiol. 1998;84:152–158. doi: 10.1046/j.1365-2672.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- Wendorff WL, Wee C. Effect of smoke and spice oils on growth of molds on oil-coated cheese. J Food Prot. 1997;60:153–156. doi: 10.4315/0362-028X-60.2.153. [DOI] [PubMed] [Google Scholar]