Abstract
Bauhinia is a leguminous plant species found in almost every part of the world, including southern Africa. In this study, grain composition and protein body microstructure of two indigenous southern African Bauhinia species, B. galpinii and B. petersiana were determined. Protein (38 g/100 g) and fat (23 g/100 g) were the major constituents of Bauhinia. Bauhinia grains also contained substantial amounts of zinc (6 mg/100 g) and iron (3 mg/100 g) when compared to FAO/WHO standards. The parenchyma cells of Bauhinia showed spherical protein bodies with globoids inclusions and these were surrounded by lipids. However, the protein bodies of B. petersiana were smaller in size (7 ± 3 μm) than those of B. galpinii (13 ± 4 μm). The microstructure of protein bodies in Bauhinia is very similar to that of soya, suggesting that the processing technology developed for soya protein may be adopted for Bauhinia.
Keywords: Bauhinia, Soya, Composition, Microstructure, Protein
Introduction
Bauhinia is a leguminous plant species found in almost every part of the world, including southern Africa. The genus Bauhinia consists of about 300 species (Filho 2009). Bauhinia grains are protein-rich oilseeds (Arnold et al. 1985; Anhwange et al. 2005) similar to soya bean (Amonsou et al. 2011) and peanuts (Venkatachalam and Sathe 2006). Up to 33 % protein and 29 % lipid have been reported for B. monandra (Anhwange et al. 2005). Some Bauhinia species found in southern Africa are moderately to highly drought tolerant (Fanie and Venter 1996; Bosch 2006) and therefore, have some advantages over soya and peanuts as alternative food sources. B. galpinii also known as the “Pride of De Kaap” is widespread and can be found in many provinces in South Africa. This species is hardy to drought and moderate to frost (Palgrave and Palgrave 2002). B. pertersiana, known as the “wild coffee bean” or the “Kalahari white Bauhinia” is distributed across southern African Countries including Botswana, Zimbabwe, Namibia and part of South Africa. It is highly drought tolerant (Brummitt and Ross 1975). However, in comparison with peanuts and soya, the Bauhinia grains are much underutilised and under-researched. Traditionally, Bauhinia grains are consumed as roasted nut (Fanie and Venter 1996). Roasted grains are pounded and used to make a pleasant tasting meal (Fanie and Venter 1996). The knowledge of the grain composition may be important for the purposes of nutrition and grain utilisation.
Further, food microstructure is fundamental to the understanding of the functionality of a food material. Knowledge of the grain microstructure may be important in the extraction, processing and utilisation of protein (Aguilera 2005; Parada and Aguilera 2007). The physical localisation of protein bodies relative to either the lipids has been found to influence protein extractability (Shand et al. 2007) and functional properties such as protein digestibility (Aguilera 2005). Grain microstructure also affects grain hardness (Aguilera and Stanley 1999), an important parameter in milling operations and equipment design for processing. Depending on plant species, some variations in the protein body microstructures have been found among these oilseeds (Young et al. 2004; Amonsou et al. 2011). According to Amonsou et al. (2011), globoid and druse crystal inclusions found in marama protein bodies appeared to be absent in soya. The microstructure of protein bodies in Bauhinia grains is not known.
In the study, the chemical composition and microstructure of protein bodies in grains of Bauhinia species were determined.
Materials and methods
Materials
Two indigenous southern African Bauhinia species, B. petersiana and B. galpinii, were used. The grains of these species were gathered in 2012 by the National Botanical Garden in the Lowveld region, Nelspruit (Mpumalanga Province), South Africa. The plant specimens were deposited at the Herbarium at the University of KwaZulu-Natal, Pietermaritzburg, South Africa. Soya bean (Glycine max) obtained from PANNAR SEED (Greytown, South Africa) was used as a reference sample.
Physical properties of Bauhinia grains
The colour of Bauhinia grains were determined by visual observation in day light. The size of randomly selected grains (n = 50) was determined in three linear perpendicular dimensions using a micrometer screw gauge reading to an accuracy of 0.01 mm.
Flour preparation
Bauhinia grains were dehulled by crushing the grains in a laboratory grinder and the grain coats removed manually. The cotyledons were then milled and the resulting flours were stored at 4 °C until analysed.
Chemical analysis
Proximate composition
The moisture, fat, crude fibre and ash contents of grain flours were determined using the AOAC Methods no. 934.01, 920.39, 978.10 and 942.05, respectively (AOAC 2000). The protein content (N X 5.71) was determined by the Dumas method of combustion analysis (Method no. 990.03, AOAC 2000). Total carbohydrate was calculated by difference.
Mineral composition
Grain flours were digested as described in Amonsou et al. (2011) and the mineral content analysed by AOAC (1984)) using the Inductively Coupled Plasma (ICP) spectroscopy.
Microscopy
Sample preparation for Scanning Electron Microscopy, (SEM), Light Microscopy (LM) and Transmission Electron Microscopy (TEM)
Tissue blocks (1 mm3) cut from the surface of the interior part of the cotyledons were fixed in 0.05 M cacodylate buffer, pH 7.2, containing 3 % (w/v) glutaraldehyde for 48 h. These were then washed and postfixed in Osmium for 2 h. This was followed by washing with buffer and dehydration in a graded series aqueous ethanol.
For LM and TEM, fixed and dehydrated tissues in ethanol were further dehydrated twice in propylene oxide at 30 min intervals. These were then infiltrated with three different ratios of Epon to propylene oxide and embedded in Epon. The resin was polymerized at 70 °C for 24 h.
Sections (1 μm) were cut for LM using an ultramicrotome. These sections were stained separately with Ladd and Coomassie Brilliant Blue R 250 (Gahan 1984).
Ultrathin sections were cut for TEM. These sections were placed on copper grids and contrasted in 4 % aqueous uranyl acetate and Reynolds lead acetate, respectively.
Confocal Laser Scanning Microscopy (CLSM)
Fresh tissue sections (2–3 μm) were cut from the cotyledon inner surface. These sections were analysed with a ZEISS 710 confocal laser scanning microscope without any further treatment. The excitation wavelength was 405 nm. Fluorescing protein was detected after passing through a 420 μm long filter, with a pinhole set at 55 μm.
Statistical analysis
Analysis of variance (ANOVA) was done on chemical composition data. Means were compared by using the Fisher Least Significant Difference (LSD) test (p < 0.05). The mean size of protein bodies and their distribution per cell (n = 50 cells) were determined.
Results and discussion
Physical properties of Bauhinia grains
Bauhinia grains appeared similar in colour and shape, but different in size (Fig. 1). B. galpinii and B. petersiana grains appeared brown and elliptical. However, unlike B. petersiana grains, B. galpinii grains appeared slightly concave on one side and convex on the other. In terms of the size, the thickness of B. galpinii grains (2.4 ± 0.4 mm) was about twice that of B. petersiana grains. B. galpinii grains were also slightly longer (12.3 ± 0.7 mm) and wider (8.9 ± 0.9 mm) than B. petersiana grains (Length: 9.3 ± 0.6 mm; width: 6.5 ± 0.5 mm). The difference in size may influence the chemical composition of the Bauhinia species.
Fig 1.
Species of Bauhinia grains
Chemical composition of Bauhinia grains
The protein contents of B. petersiana and B. galpinii were only slightly low compared to soya (Table 1). But, B. galpinii contained slightly high protein compared to B. petersiana. This may be attributed to differences in grain size of B. galpinii and B. petersiana as described above. The two Bauhinia species and soya had much similar fat contents. But, the crude fibre contents of Bauhinia were low (approx. 1.5 g/100 g flour), about half that of soya.
Table 1.
Proximate composition of Bauhinia seed sand soya bean (g/100 g flour) a
| Samples | Moisture | Protein | fat | Crude fibre | Ash | Carbohydrateb |
|---|---|---|---|---|---|---|
| B. petersiana | 5.2a ± 0.1 | 37.7a ± 0.3 | 22.3a ± 0.4 | 1.6b ± 0.2 | 4.1a ± 0.1 | 29.1c ± 0.6 |
| B. galpinii | 8.0b ± 0.1 | 38.5b ± 0.4 | 24.2b ± 0.3 | 1.4a ± 0.1 | 4.5b ± 0.7 | 23.3b ± 0.4 |
| Soya | 7.5b ± 0.3 | 42.8c ± 0.1 | 22.8a ± 0.5 | 2.3c ± 0.4 | 5.2b ± 0.2 | 19.8a ± 0.5 |
aMean ± SD. Mean values with different superscript letters in columns are significantly different (p < 0.05)
bCarbohydrate by difference
In comparison with other Bauhinia species, the protein and fat contents of B. galpinii and B. petersiana appeared higher than those of B. malabrica (Vijayakumari et al. 1993). B. purpurea (Vijayakumari et al. 1997) and B. Tomentosa (Agbede 2007). But, the crude fibre and carbohydrate contents of the two Bauhinia species appeared low compared to B. purpurea (Vijayakumari et al. 1997). In this study, chemical analyses were done on the cotyledon. The authors who reported on B. purpurea did not specify whether the grain coat were removed before milling as this may have accounted for the difference in carbohydrate and/or fibre. Further, grain chemical composition may differ according to species, environmental factors and agricultural practices.
Potassium, phosphorus, magnesium and calcium were the major minerals in Bauhinia, similar to soya (Table 2). Bauhinia grains also contained substantial amounts of zinc (6 mg/100 g) and iron (3 mg/100 g) when compared to FAO/WHO standards. The mineral composition of Bauhinia appeared similar to oilseeds such as marama bean (Amonsou et al. 2011), peanuts (Wu et al. 1997) and other Bauhinia species such as the B. monandra (Anhwange et al. 2005).
Table 2.
Mineral composition of Bauhinia seeds compared to soya bean (mg/100 g flour) a
| Elements | B. Petersiana | B. Galpinii | soya |
|---|---|---|---|
| K | 629a ± 23 | 907b ± 33 | 1259c ± 23 |
| P | 775b ± 36 | 743b ± 18 | 600a ± 10 |
| Mg | 316b ± 17 | 361c ± 19 | 243a ± 14 |
| Ca | 325c ± 22 | 159b ± 12 | 144a ± 15 |
| Na | 39a ± 5 | 38a ± 7 | 62b ± 9 |
| Mn | 1.9a ± 0.1 | 4.2b ± 0.3 | 3.1c ± 0.4 |
| Cu | 1.8b ± 0.3 | 1.9b ± 0.1 | 1.1a ± 0.2 |
| Fe | 2.7b ± 0.4 | 2.3a ± 0.3 | 6.2c ± 0.1 |
| Zn | 7.5c ± 0.1 | 4.7a ± 0.2 | 5.1b ± 0.1 |
aMean ± SD is reported on dry basis. Mean with different superscript letters in rows are significantly different (p < 0.05)
Microstructure of protein in Bauhinia grains
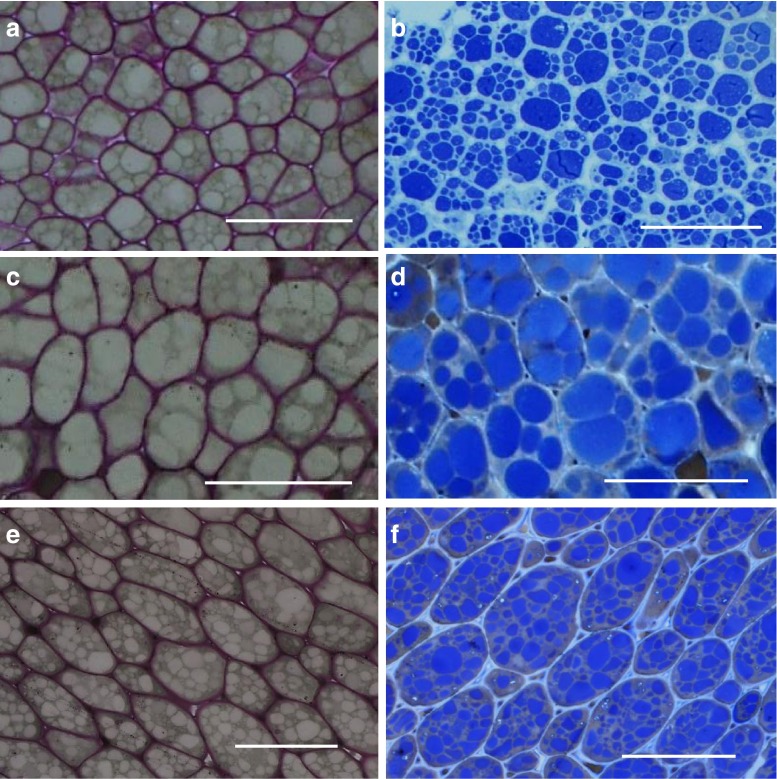
With LM, the parenchyma cells of Bauhinia and soya showed circular bodies in cross section (Fig. 2). The walls of these cells were stained purple with Ladd leaving the circular bodies unstained (Fig. 2a, c, e). Dwarte and Ashford (1982) reported a purple colour of the parenchymal cell walls in celery when these cells were stained with toluidine blue. The Ladd stain that was used in this study contained toluidine Blue and this may have reacted with cell wall components. Thus, staining with Ladd was useful to differentiate the cell walls in Bauhinia and soya parenchyma cells. To determine whether the unstained circular bodies were protein bodies, the tissue sections were stained with Coomassie Blue, a standard dye for protein (De Moreno et al. 1986; Hafiz 2005). Following this treatment, the circular bodies appeared distinct and stained blue within the cells in both Bauhinia and soya (Fig. 2b, d, f), suggesting that these were protein bodies. In a similar manner, the protein bodies in marama and soya stained blue when treated in the same way (Amonsou et al. 2011; Mosele et al. 2011).
Fig 2.
Light microscopy of Bauhinia seed and soya bean parenchyma cells stained with Ladd (a, c & e) and Coomassie Brilliant Blue (b, d & f). a & b: B.petersiana, c & d: B. galpinii, e & f: Soya bean. Bar: 50 μm. Cell walls are stained purple with Ladd and protein bodies are stained blue with Coomassie Brilliant Blue
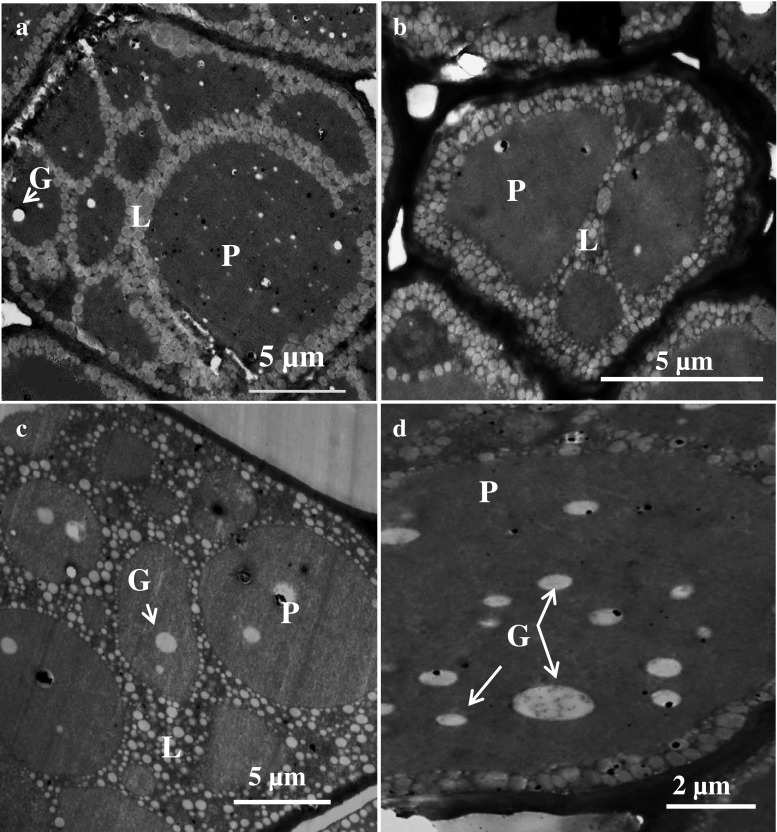
TEM of the Bauhinia grains also showed circular, electron dense protein bodies in cross-section surrounded by networks of lipid bodies similar to what was observed in the soya bean (Fig. 3). A similar organisational structure of protein bodies relative to the lipid bodies has been observed in marama bean and soya (Amonsou et al. 2011) and peanuts (Young et al. 2004). However, the distribution and size of these protein bodies in the parenchyma cells of the two Bauhinia species were different. B. petersiana seemed to contain more smaller protein bodies per cells (approx. 8 per cell, size: 7 ± 3 μm), than did B. galpinii (approx. 4 per cell, Size: 13 ± 4 μm). The protein bodies in parenchymal cells of B. petersiana also appeared to be of a regular pattern consisting of one big and single protein body occurring together with smaller ones per cell (Fig. 2a, b; Fig. 3a), the pattern which is unique and different from those in the parenchyma cells of B. galpinii and soya. The size of protein bodies in Bauhinia is within the range reported in the literature for most oilseeds including soya (Lott 1981; Young et al. 2004; Amonsou et al. 2011).
Fig. 3.
Transmission electron microscopy of protein bodies in Bauhinia seed and soya bean a: B. Petersiana, b: B. Galpinii, c: Soya bean, d: Globoids (G) in protein body of B. galpinii, L lipid bodies, P protein bodies
Further, the protein body inclusions were found in Bauhinia grains and soya bean (Fig. 3). According to Lott and Buttrose (1978)), the protein bodies in seeds may contain inclusions, namely the globoid crystals, which constitute a storage site for phosphorus deposited as insoluble phytate (Martinez 1979). Protein body inclusions have also been reported in oilseed such as peanuts (Young et al. 2004) and marama bean (Amonsou et al. 2011). Previous research on the elemental analysis of protein body inclusions showed that the inclusions contained mainly phosphorus, potassium, magnesium and calcium (Lott and Spitzer 1980; Lott and Buttrose 1978). These minerals in Bauhinia grains (Table 2) may have originated from the globoid sites.
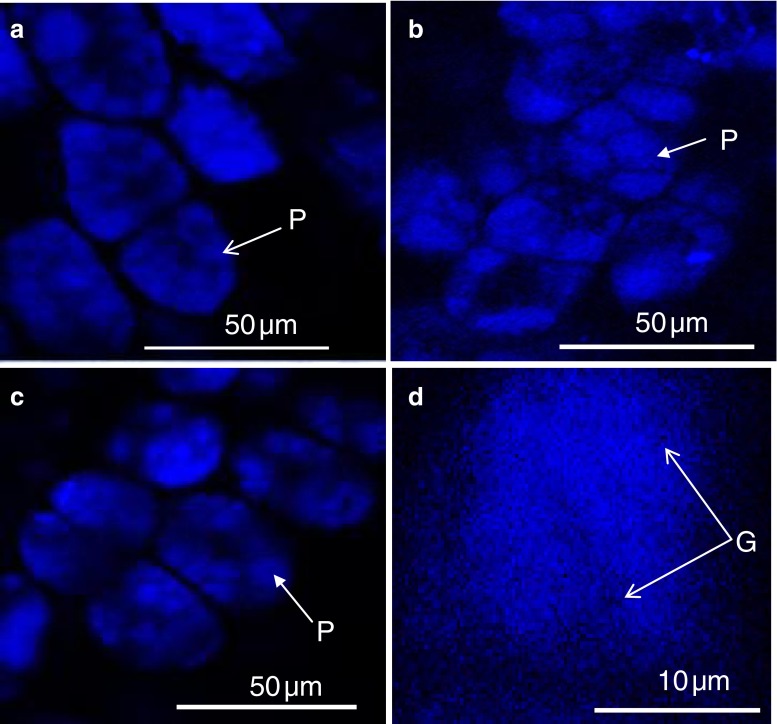
The protein bodies in the Bauhinia were further studied with CLSM. Confocal microscopy revealed the presence of autoflorescent protein bodies within the parenchyma cells of Bauhinia and soya (Fig. 4a, b, c). These protein bodies appeared in a cluster and are similar to those observed with TEM and LM in terms of their spherical shape. Inclusions that did not fluoresce were also found in protein bodies (Fig. 4 d). These are possibly the globoids inclusions, thus confirming the TEM result (Fig. 3).
Fig. 4.
Confocal laser scanning microscopy of protein bodies in Bauhinia seeds and soya bean parenchyma cells a: B. petersiana, b: B. galpinii, c: Soya bean, d: Globoids (G) in a single protein bodies of B. galpinii
Conclusions
B. galpinii and B. petersiana contain substantial amounts of protein, fat and micronutrients such as the zinc and iron. The protein bodies in Bauhinia grains are very similar to those in soya in terms of their spherical shape and localization within the parenchyma cells. The great similarity in the protein body microstructure between Bauhinia grains and soya suggests that the processing technology developed for soya protein may be adopted for Bauhinia.
Acknowledgments
We thank Mrs Shirley Mackellar and Miss Nelisha Murugan of the Microscopy and Microanalysis unit, University of KwaZulu-Natal, South Africa for their assistance with the microscopy part of this work.
References
- Agbede OJ. Chemical analysis of leaf meal and processed grain flours of an aesthetic plant: Bauhinia tomentosa. J Food Agric Environ. 2007;5:233–235. [Google Scholar]
- Aguilera JM. Why food microstructure. J Food Eng. 2005;67:3–11. doi: 10.1016/j.jfoodeng.2004.05.050. [DOI] [Google Scholar]
- Aguilera JM, Stanley DW. Microstructure principles of food processing and engineerin. 2. Gaithersburg: Aspen Publishers, Inc; 1999. pp. 259–310. [Google Scholar]
- Amonsou E, Taylor J, Minnaar A. Microstructure of protein bodies in marama bean species. LWT-Food Sci Technol. 2011;44:42–47. doi: 10.1016/j.lwt.2010.06.021. [DOI] [Google Scholar]
- Anhwange BA, Ajibola VO, Oniye SJ. Nutritional potential of the seeds of Bauhinia monandra (Linn) J Food Technol. 2005;3:204–208. [Google Scholar]
- Official methods of analysis. 14. Arlington: Association of Official Analytical Chemists; 1984. [Google Scholar]
- Official methods of analysis. 17. Rockville: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Arnold TH, Wells MJ, Wehmeyer AS. Khoisan food plants: taxa with potential for future economic exploitation. In: Wickens GE, Goodin JR, Field DV, editors. Plants for arid lands. London: Chapman and Hall; 1985. pp. 69–86. [Google Scholar]
- Bosch CH (2006) Bauhinia petersiana Bolle, in Cereals and pulses/Céréales et legumes secs [CD-Rom], ed. Brink M, Belay G, PROTA 1. PROTA, Wageningen, Netherlands
- Brummitt RK, Ross JH. The relationship of Bauhinia petersiana and B. Macrantha (Leguminosae-Caesalpinioideae) Kew Bull. 1975;30:593–595. doi: 10.2307/4102899. [DOI] [Google Scholar]
- De Moreno MR, Smith JF, Smith RV. Mechanism studies of coomassie blue and silver staining of proteins. J Pharm Sci. 1986;75:907–911. doi: 10.1002/jps.2600750919. [DOI] [PubMed] [Google Scholar]
- Dwarte D, Ashford AE. The chemistry and microstructure of protein bodies in celery endosperm. Bot Gaz. 1982;143:164–175. doi: 10.1086/337285. [DOI] [Google Scholar]
- Fanie T, Venter JA (1996) Making the Most of the Indigenous trees, du Plessis E (ed), Brisa Publication, Pretoria, South Africa
- Filho VC. Chemical composition and biological potential of plants from the genus Bauhinia. Phytother Res. 2009;23:1347–1354. doi: 10.1002/ptr.2756. [DOI] [PubMed] [Google Scholar]
- Gahan BP. Plant histochemistry and cytochemistry: An Introduction. London: Academic; 1984. pp. 201–243. [Google Scholar]
- Hafiz A. Principle and reaction of protein extraction, purification and characterisation. Boca Raton: CRC Press; 2005. pp. 84–89. [Google Scholar]
- Lott JNA (1981) Protein bodies in seeds. Nordic J Bot 1:421-432
- Lott JNA, Buttrose MS. Location of reserves of mineral element in seed protein bodies: macadamia, walnut, and hazel nut. Can J Bot. 1978;56:2072–2082. doi: 10.1139/b78-247. [DOI] [Google Scholar]
- Lott JNA, Spitzer E. X-ray analysis studies of elements stored in protein body globoid crystals of Triticium grain. Plant Physiol. 1980;66:494–499. doi: 10.1104/pp.66.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez HW (1979) The importance of functionality of vegetable protein in food, in Soy protein and human nutrition, ed. Wilck H, Hokpin TD and Waggle HD, Academic Press, London, pp. 53–77
- Mosele MM, Hansen ÅS, Hansen M, Schulz A, Martens HJ (2011) Proximate composition, histochemical analysis and microstructural localisation of nutrients in immature and mature seeds of marama bean (Tylosema esculentum): an underutilised food legume. Food Chem 127:1555–1561
- Palgrave CK, Palgrave CM (2002) Keith coates palgrave’s trees of southern africa, 3rd edn. Struik, Cape Town
- Parada J, Aguilera JM. Food microstructure affects the bioavailability of several nutrients. J Food Sci. 2007;72:21–32. doi: 10.1111/j.1750-3841.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- Shand PJ, Ya H, Pietrasik Z, & Wanasundara PKJPD (2007) Physicochemical and textural properties of heat-induced pea protein isolate gel. Food Chem 102:119–130
- Venkatachalam M, Sathe S. Chemical composition of selected edible nut seeds. J Agric Food Chem. 2006;54:4705–4714. doi: 10.1021/jf0606959. [DOI] [PubMed] [Google Scholar]
- Vijayakumari K, Siddhuraju P, Janardhanan K. Chemical composition and nutritional potential of the tribal pulse (Bauhinia malabarica Roxb) Plant Foods Hum Nutr. 1993;44(1):291–298. doi: 10.1007/BF01088325. [DOI] [PubMed] [Google Scholar]
- Vijayakumari K, Siddhuraju P, Janardhanan K. Chemical composition, amino acid content and protein quality of the little-known legume Bauhinia purpurea. J Sci Food Agric. 1997;73:279–286. doi: 10.1002/(SICI)1097-0010(199703)73:3<279::AID-JSFA713>3.0.CO;2-H. [DOI] [Google Scholar]
- Wu WH, Lu JY, Jone AR, Mortley DG, Loretan PA, Bonsi CK, Hill WA. Proximate composition, amino acid profile, fatty acid composition and mineral content of peanut seeds hydroponically grown at elevated CO2 levels. J Agric Food Chem. 1997;45:3863–3866. doi: 10.1021/jf970077f. [DOI] [Google Scholar]
- Young CT, Pattee EH, Schadel EW, Sander HT. Microstructure of peanut (Arachishypogaea L. cv ‘NC 7’) cotyledons during development. LWT-Food Sci Technol. 2004;37:439–445. doi: 10.1016/j.lwt.2003.10.016. [DOI] [Google Scholar]