Abstract
The effectiveness of different postharvest wash treatments at two levels (10 and 20 g/l) for different dipping times was determined to remove chlorpyrifos from date fruits treated at concentration of 2 mg/l. The recovered amount of chlorpyrifos was extracted based on the solid phase extraction (SPE) method and then analyzed by gas chromatography with mass spectrometry (GC-MS). The results demonstrate that the removal of chlorpyrifos increased in the order of acetic acid (AA)> citric acid (CA)> hydrogen peroxide (H2O2)> potassium permanganate (KMnO4)> running water (H2O), and the percent of pesticide residue on date fruits depended on the concentration of tested washing treatments and dipping time without the formation of the toxic by-product, chlorpyrifos-oxon. Kinetic studies revealed that chlorpyrifos was found to be more easily removable from date fruits treated with the tested chemical solutions with t1/2 values of 12–29 min compared with roughly 53 min in case of running water. The impact of these washing treatments on quality of date fruits illustrated that all treatments exerted a little negative effect on total sugars content but H2O2 and KMnO4 at level of 2 % had more drastic effect. Whereas, running water, 10 and 20 g/l CA caused significant increases in total phenolic contents, during all the tested contact times compared with control. Except the insignificant effect of KMnO4 treatments, antioxidant capacity of date fruits tended to increase in all wash treatments, when the contact times were 5 or 15 min.
Keywords: Chlorpyrifos, Washing, Date fruits, Antioxidant capacity
Introduction
Pesticides are widely used in agriculture to control a variety of pernicious organisms that spoil the crops. They provide unquestionable benefit for agricultural production, even though, as a consequence, low amounts of some residues may persist in the food supply and could constitute a potential chronic toxicity and in some cases, acute toxicity for humans (Ekström et al. 1996; Osman and Al-Rehiayani 2003).
Chlorpyrifos (O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate) is a broad spectrum organophosphorus (OP) insecticide, widely used in agriculture and public health, and possesses low water solubility (1.40 mg/l) and high octanol-water partition coefficient (Kow = 4.70) (Tomlin 2002). It acts as non-systemic insecticide with contact, stomach, and respiratory action and used to control coleoptera, diptera, homoptera and lepidoptera in soil or on foliage in over 100 crops, including pome fruit, stone fruit, citrus fruit, bananas, vines, vegetables, etc. (Tomlin 2002). Despite recent restrictions on further production for use, chlorpyrifos remains the most widely used organophosphate pesticide, and there is increasing concern over the potential consequences of fetal and childhood exposures (Song et al. 1998). The acute toxicity of chlorpyrifos is mediated through inhibition of cholinesterase by the active metabolite chlorpyrifos oxon, and the consequent accumulation of the neurotransmitter acetylcholine (ACh) in synaptic junctions leads to excessive stimulation of postsynaptic cells causing cholinergic toxicity (Ecobichon 1996), but new evidence suggests that chlorpyrifos itself may influence DNA synthesis (Dam et al. 2000), brain cell replication (Crumpton et al. 2000), induce intracellular oxidative stress (Osman 1999) and thereby disrupting normal cellular development and differentiation (Bebe and Panemangalore 2003).
A number of physical–chemical and conventional methods, such as Fenton oxidation (Wang and Lemley 2002), biotreatment (Liu et al. 2004), titanium dioxide catalytic treatment (Kouloumbos et al. 2003), powdered activated carbon filtration and reverse osmosis (Heijman and Hopman 1999) have been demonstrated to be highly effective for the removal of organic chemicals including pesticides. Those techniques mainly focused on pesticides dissolved in aqueous solutions. However, they are less effective or unsuitable for removal of residual pesticides adhering on vegetable surface. Products currently used as food additives or for sanitary purposes could be selected for washings. Citric acid is authorized as food additive in the EU (E330 antioxidant) and used as an acidulant and synergistic antioxidant in pharmaceutical preparations, while hydrogen peroxide (H2O2) is often added as an oxidant for the treatment of both drinking-water and wastewater, control of surplus chlorine contents or for the removal of residual ozone contents in processed waters as well as in the US Pharmacopeia (at 30 g/l) as a common disinfectant (Pugliese et al. 2004). Potassium permanganate (KMnO4) is an antiseptic and disinfectant that can be employed for drinking water treatment at 0.10 g/l; additionally, it reacts with ethylene delaying the maturation of fruits.
Date palms (Phoenix dactylifera L.) are a staple food in the diet of many countries and are considered as the major fruit of the Near East and North Africa where they are consumed in large quantities fresh, dried, or in various processed forms since they are rich in carbohydrate (mainly glucose, fructose and a small amount of sucrose), protein and minerals (Considine 1982; Al-Showiman and Fayadh 1990). Sugars contribute the most prevalent single component and in the ancient date production countries the date has been used more as a sugar source than as a fruit due to their high carbohydrate content (70–80 %). Also, date fruits serve as a good source of natural antioxidants, where fruits contain various phenolic compounds, such as protocaechuic, p. hydroxy benzoic, vanillic, syringic, caffeic, coumaric and ferulic (Al-Farsi et al. 2005), which contribute significantly to total antioxidant activity, that may have many beneficial effects for human health (Besbes et al. 2009). Moreover, date juice concentrates are good source of tannins and ascorbic acid (Kulkarni et al. 2010). The strong relationships between total phenolic and flavononid contents suggest that these compound play important role in antioxidant activities (Kosanić et al. 2011).
In the Kingdom of Saudi Arabia (KSA), dates are one of the most important crops because of their religious and nutritional significance. Saudi Arabia is considered one of the largest date producer in the world, with production amounting to 970,488 tones per year and the number of date palm trees is over 18 million (Anon 2004). As a result of its high economic value as well as the large number of pests that infest dates during growth, significant quantities of pesticides are often necessary for the protection of this crop. A number of pesticides are registered by the Ministry of Agriculture in Saudi Arabia to control these pests, which infest dates and causes severe damage (Al-Rehiayani and Osman 2003 and 2005). Chlorpyrifos is widely applied to date palm in KSA at rate of 250 ml/100 L to control fruit warm, caterpillars, mites and aphids. This leads to pesticide residues on (or in) the fruits at harvest. Unfortunately, no data are available on chlorpyrifos removing from date fruits, although it has been reported in fruits and vegetables (Krol et al. 2000), nectarine (Pugliese et al. 2004), rice grains (Kushi et al. 1999), and water (Sherrard et al. 2004).
The search for means to improve the production of dates in KSA is always the target of scientists, politicians and businessmen, who seek new techniques to enhance the quality and safety of this product. Therefore, the present study was carried out to evaluate the effectiveness of running water (H2O), hydrogen peroxide (H2O2), potassium permanganate (KMnO4), citric acid (CA) and acetic acid (AA) at concentrations of 10 and 20 g/l for different contact times as simple wash treatments for reducing chlorpyrifos residues from date fruits and the possibility of toxic by-products formation by these treatments was investigated by gas chromatography- mass spectroscopy (GC–MS). In relation to the importance of antioxidant compounds in date fruits, the objectives of this research was also to study the effect of these wash treatments on total sugar, total phenolic contents and antioxidant capacity in dates.
Materials and methods
Chemicals
Analytical grade standard for chlorpyrifos, (O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate), was obtained from Chemservice, USA, with purity of 99 % purity, while formulated chlorpyrifos (48 g a.i./l, EC) was purchased from the local market of Al-Qassim region, KSA. Certified HPLC-grade of actone, methanol, cyclohexane and ethyl acetate were purchased from BDH Company, while the Water spe-20G Column Processor designed vacuum manifold capable of processing up to 20 solid phase extraction (SPE) columns and SPE columns (Waters spe™, C18, 500 mg per column) were purchased from Waters, USA. Acetic acid (100 %), citric acid (trisodium salt dehydrate), hydrogen peroxide (30 %) and potassium permanganate (>99 %) were obtained from BDH, Bio-Rad Lab., WinLab and Sigma Companies, respectively. Ultra-pure deionized water of 15 MΩ cm resistivity was obtained from a water purification system (PURELAB Option-R, ELGA, UK) and used throughout this study. All other chemicals used in this study were of the highest grade available.
Date fruits treatment
The date fruits (Phoenix dactylifera L.) used in this experiment were the Succary variety, the most preferred date variety grown in Al-Qassim region, were obtained from organic farming without use of pesticides provided by a rural cooperative located in Al-Qassim region, KSA. They were harvested in September 2009 and were untreated post-harvesting. Tamr samples (fully ripe date fruits, about 25 weeks after pollination) were collected randomly with no preference to size, color, appearance or firmness and then divided into 10 equal sizes (3 × 1 kg each). The 0. 42 ml of formulated chlorpyrifos (48 % w/v, EC, Sulpher Mills Limited Company) was mixed with 10 liters ultra-pure deionized water to give a concentration of 2 mg/l. Fresh and unblemished pesticide-free fruits were immersed into chlorpyrifos solution for 2 min with gentle rotation by hand. Date samples with pesticide on the surface were then air-dried for about 24 h under room conditions.
Removal of residual pesticide from date fruits
Removal of chlorpyrifos on date fruits was studied at concentration of 1 and 2 % of either acetic acid (AA), citric acid (CA), hydrogen peroxide (H2O2) or potassium permanganate (KMnO4), and four different contact times (5, 15, 30, and 60 min) at room temperature (25 ± 1 °C). Triplicate random date samples spiked with chlorpyrifos were divided into the following treatment groups: control (no wash); rinsing in running tap water; AA, CA, H2O2 and KMnO4.
Sample preparation and solid-phase extraction
Samples were chopped and a subsample (10 g) was weighed into 50 ml Teflon centrifuge tube and extracted with 20 ml acetone using a homogenizer (Euroturax, IKA Labortechnik Staufen, Germany) at full speed for 2 min. The extract was centrifuged at 3000 rpm for 5 min and 15 ml of the supernatant was transferred to a clean graduated cylinder. Solid-phase extraction was carried out using SPE columns preconditioned by passing 6 ml of ethyl acetate followed by 6 ml of methanol and then 8 ml of ultra-pure deionized water according to Štanbaher and Zupančič-Kralj (2003). The sorbent was never allowed to dry during the conditioning and sample loading steps. Then the extraction columns were fitted with detachable 70-ml polypropylene reservoirs to contain the diluted sample extract. The extract was transferred to the reservoir, which was partially filled with ultra-pure deionized water and then water was added to the top. Sample loading was performed under vacuum at flow rates of 5 ml min−1. After the passage of the extract, the column was dried by vacuum aspiration under increased vacuum for 30 min. The pesticides were eluted with three 2-ml aliquots of ethyl acetate–acetone at the ratio of 90:10 (v/v). The eluates were collected in 10 ml tubes under gravity flow only. After all the elution solvent had passed through the extraction column, the residual solvent was forcibly removed from the column. The eluate was evaporated to less than 1 ml using gentle stream of nitrogen and then the solvent was exchanged to acetone by adding two 2-ml portions of acetone and evaporated to low volume after each addition. The extract was quantitatively transferred to 2 ml clean vials and completed with ethyl acetate/cyclohexane (1:1) to 1 ml.
Recovery studies
We confirmed that date fruits used in the recovery test were chlorpyrifos free. For recovery studies subsamples of known blanks (10 g) were spiked prior to extraction by addition of 2 ml of chlorpyrifos pesticide standard solutions in acetone to give 0.01, 0.02, 0.20 or 0.50 mg/kg. They were then prepared according to the proposed procedure as described previously and then absolute recovery and precision (expressed as relative standard deviation) were measured by analyzing three samples. The recovery values ranged from 87 % to 92 %, and precision was less than 10 %. Limits of detection (LOD) and quantitation (LOQ) were calculated from the signal-to-noise ratios obtained by analysing unspiked samples (n = 10); LOD and LOQ were taken to be the concentrations of pesticide resulting in a signal-to-noise ratio of 3 and 10, respectively. The LOD (0.15 μg/kg) and LOQ (0.50 μg/kg) for chorpyrifos in date fruits are much lower than the maximum residue level (MRL) allowed for date fruits in KSA (0.02 mg/kg) according to KSA regulations (El-Saeid and Al-Dosari, 2010).
Gas chromatography–mass spectrometry (GC-MS)
Gas chromatography (Model GC 450, Varian Inc., The Netherlands) with a mass spectrometry (MS 220.41) detector equipped with split/splitless injector with electronic pressure control was employed. A Fused silica CP-Sil 8 CB-LB/MS capillary column (30 m × 0.25 mm i.d) was used in combination with the following oven temperature programme: initial temperature 50 °C, 5 °C/min ramp to 290 °C held for 10 min. The injector temperature was 280 °C. The carrier gas (helium, 99.999 %) flow rate was set to a constant head pressure of 200 kPa at flow rate of 1.0 ml min−1 with split ratio of 1: 20 min. The mass spectrometer was operated in electron ionization mode with a transfer line temperature of 280 °C, manifold temperature 40 °C, ion trap temperature 200 °C, ion source 240 °C and selected ion monitoring (SIM) mode. The ion energy for electron impact (EI) was kept at 70 eV. MS Workstation version 6.9.1. was used for data acquisition. For positive identification, both retention time (Rt) and the presence of five fragment ions (z/m ions: 352, 314, 258, 197 and 97) were considered.
Calibration was achieved by preparing matrix calibration standards from the extracts of blank samples in order to compensate for matrix effect. Analytes were quantified by using a 3-point calibration with those matrix matched calibration standards corresponding to the spiked concentration.
Extraction of sugars and phenolics
Fruit sample was weighed (1 g) into 50 ml Teflon centrifuge tube and extracted with 25 ml 80 % ethanol using a homogenizer (Euroturax, IKA Labortechnik Staufen, Germany) at full speed for 2 min. The extract was centrifuged at 4000 rpm for 10 min. The supernatant was used to measure total sugars (TS), total phenolics (TP) and antioxidant capacity (AC).
Total sugars
Ethanol extracts were used to determine TS according to the phenol-sulfuric colorimetric method of Dubois et al. (1956) using glucose (Sigma) as standard, the results were expressed as g/100 g of fresh weight.
Total phenolics
Total phenolics content (TP) was determined according the method of Singleton and Rossi (1965) using the Folin-Ciocalteu reagent. In brief, 0.1 ml of extract was added to 7.9 ml of distilled water, 0.5 ml of Folin-Ciocalteu reagent, 1.5 ml of sodium carbonate solution (200 g/l) and then mixed vigorously. The mixture was allowed to stand for 1 h at the room temperature and then the absorbency was measured at a wavelength of 765 nm. Gallic acid was used as a standard and the results were expressed as mg gallic acid equivalents (GAE)/100 g sample.
Antioxidant capacity
Antioxidant capacity (AC) or free radical scavenging activity was determined according to Brand-Williams et al. (1995) using 1,1-diphenyl-2-picryl-hydrazil (DPPH) reagent. DPPH is a widely used stable free radical to evaluate antioxidant activities of bioactive compounds and food extracts (Gao et al. 2011). In brief, 1.5 ml of freshly prepared methanolic DPPH solution (0.02 mg/ml) was added to 0.75 ml of 80 % ethanol extract and then stirred. The decolourizing process was recorded after 5 min of reaction at a wavelength of 517 nm and compared with a blank control using the Spectrophotometer. The DPPH radical scavenging activity of the extracts was measured using the Trolox standard curve. Results were expressed as μmol Trolox equivalent (TE) antioxidant capacity/100 g sample.
Statistical analysis
Treatments were done in triplicate for each time. Data were calculated as mean ± SD analyzed using ANOVA. A probability of 0.05 or less was considered significant. The statistical package of the Costat Program (1986) was used for all chemometric calculations.
Results and discussion
Removing of chlorpyrifos by different wash treatments
In the present study, the effects of some wash treatments on chlorpyrifos residues in the date fruits were investigated by using gas chromatography–mass spectrometry. These methods include washing by either running tap water and two levels (10 and 20 g/l) of either AA, CA, H2O2 or KMnO4. The amount of chlorpyrifos was significantly decreased exponentially as the contact time increased in the fruits treated with different wash treatments (Table 1). Also, there were significant variations between all the tested treatments and control (chlorpyrifos only) with respect to their abilities to remove chlorpyrifos within the contact times as well as there were significant variations between all treatments when the contact time was 5 min and the remaining contact times. Also, the data show that the washing by running tap water proved the least effective, showing 37.08 %, 42.46 %, 51.77 %, and 51.93 % loss when the contact times were 5, 15, 30 and 60 min, respectively, compared with no wash treatment (control). It can be observed that the reduction by washing process with water in this study were higher those of Abou-Arab (1999) and Cengiz et al. (2006) who reported that 9–23 % of the initial pesticide levels were reduced by washing fruits by tap water. The results are also differed to those of Krol et al. (2000) who found that rinsing fruits and vegetables with tap water for 15–30 s produced significant reductions in residue levels of malathion, iprodione and other pesticides but not of chlorpyrifos. On the other hand, washing rice grains with water removed approximately 60 % of the chlorpyrifos residues (Lee et al. 1991).
Table 1.
Effect of different wash treatments on removing of chlorpyrifos from date fruits
| Wash treatment | Contact time (min) | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 15 | 30 | 60 | |||||
| Chlorpyrifos levels (μg/kg) | Remove (%) | Chlorpyrifos levels (μg/kg) | Remove (%) | Chlorpyrifos levels (μg/kg) | Remove (%) | Chlorpyrifos levels (μg/kg) | Remove (%) | |
| Chlorpyrifos (T1) | 32.9 ± 2.20aH | – | 32.8 ± 4.55aF | – | 32.7 ± 3.11aF | – | 32.2 ± 4.87aG | – |
| Chlorpyrifos+ Running water (T2) | 20.7 ± 1.50bG | 37.08 | 18.9 ± 2.18aE | 42.54 | 15.8 ± 1.53aE | 51.77 | 15.5 ± 2.23aF | 51.93 |
| Chlorpyrifos+ 1 % Acetic acid (T3) | 6.2 ± 0.63cB | 81.12 | 3.6 ± 0.21bB | 88.91 | 2.6 ± 0.25bB | 91.93 | 0.51 ± 0.10aAB | 98.42 |
| Chlorpyrifos+ 2 % Acetic acid (T4) | 4.2 ± 0.21cA | 87.23 | 2.2 ± 0.27bA | 93.36 | 0.27 ± 0.11aA | 99.17 | 0.16 ± 0.05aA | 99.5 |
| Chlorpyrifos+ 1 % Citric acid (T5) | 9.1 ± 0.76cC | 72.46 | 6.9 ± 0.22bC | 78.85 | 3.1 ± 0.27aB | 90.43 | 2.5 ± 0.34aC | 92.33 |
| Chlorpyrifos+ 2 % Citric acid (T6) | 7.3 ± 0.99cB | 77.81 | 5.2 ± 0.85bB | 84.12 | 2.5 ± 0.44aB | 92.39 | 1.34 ± 0.35aBC | 95.84 |
| Chlorpyrifos+ 1 % H2O2 (T7) | 17.3 ± 2.53bF | 47.33 | 8.6 ± 1.05aD | 73.79 | 7.8 ± 0.68aD | 76.30 | 7.2 ± 1.23aE | 77.80 |
| Chlorpyrifos+ 2 % H2O2 (T8) | 13.2 ± 3.11bE | 59.82 | 6.7 ± 1.50aC | 79.55 | 6.0 ± 0.87aC | 81.56 | 5.1 ± 1.01aD | 84.13 |
| Chlorpyrifos+ 1 % KMnO4 (9) | 12 ± 1.21cE | 63.56 | 9.7 ± 1.06bD | 70.34 | 8.4 ± 1.22aD | 74.40 | 8.0 ± 1.02aE | 75.16 |
| Chlorpyrifos+ 2 % KMnO4 (T10) | 10.2 ± 0.67cD | 68.97 | 8.8 ± 0.67bD | 73.12 | 5.3 ± 0.90aC | 83.67 | 5.2 ± 3.54aD | 83.79 |
Each value is the mean ± S.D of three replicates with 6 determinations
Means having different letter in a row (or column) are significantly different (P < 0.05)
In the present study, rinsing at 1 % AA removed 81.12, 88.91, 91.93 and 98.42 % of residual pesticide, while rinsing at higher concentration of AA (20 g/l) increased the efficiency in pesticide removal where the percentages of chlorpyrifis removing were 87.23, 93.36, 99.17 and 99.5 % when the contact times were 5, 15, 30 and 60 min, respectively. In case of CA, washing with 1 g/l CA solution caused 72.46, 78.85, 90.43 and 92.33 % loss in chlorpyrifos, while at the higher concentration, the percentages of chlorpyrifos removal were 77.81, 84.12, 92.39 and 95.84 % when the contact times were 5, 15, 30 and 60 min, respectively. Also, the results showed that when date fruits contaminated with 2 mg/l of chlorpyrifos and then washed with 10 g/l H2O2 solution the percentages of chlorpyrifos removal were 47.33, 73.79, 76.30 and 77.80 %, while at the concentration of 20 g/l the percentages of chlorpyrifis removal were 59.82, 79.55, 81.56 and 84.13 % when the contact times were 5, 15, 30 and 60 min, respectively. In the case of date fruits treated with chlorpyrifos and then washed with 10 g/l KMnO4, roughly 64 % of the available chlorpyrifos was removed within the first 5 min, a percentage that increased to about 70–75 % within the 15–60 min. In the case of date fruits treated with 20 g/l KMnO4, 69–73 % of the available chlorpyrifos was removed within the first 15 min; this increased to about 84 % within 30–60 min. The washing with water or soaking in solutions of salt and some chemicals e.g. chlorine, chlorine oxide, H2O2, ozone, AA, hydroxyl peracetic acid, iprodione and detergents are reported to be highly effective in reducing the level of pesticides (Bajwa and Sandhu 2011). The present findings are in accordance with that reported by Zhang et al. (2007) who found that washing with AA solutions (at 100 g/l concentration for 20 min) caused 79.8 %, 65.8 %, 74.0 % and 75.0 % loss of chlorpyrifos, p,p'-DDT, cypermethrin, chlorothalonil from cabbage, while washing by tap water (for 20 min) caused 17.6 %, 17.1 %, 19.1 % and 15.2 % loss. On the other hand, washing nectarines treated with chlorpyrifos, fenarimol, iprodione, malathion, methidathion, myclobutanil, parathion and pirimicarb with aqueous CA, H2O2, KMnO4, sodium hypochlorite, sodium metabisulfite, and urea solutions produced results without significant differences with those obtained with the tap water (Pugliese et al. 2004). Oxidizing (hydrogen peroxide, potassium permanganate and sodium hypochlorite) and reducing reagents (sodium metabisulfite) were not more effective than tap water washing; thus, the main mechanism for removing the residues is dissolution and chemical degradation is unappreciable. From the results obtained in this research work and assuming the criterion that a treatment is efficient in degrading pesticides if a removal percentage of above 70 % is obtained (Ormad et al. 2008). So it is recommended to use such simple and non-toxic washing treatments to reduce such residues in fruit samples (Krol et al. 2000). The present study revealed that removing of chlorpyrifos from date fruits depends on the levels of tested chemical solutions and the contact times which are in accordance with that by Abou-Arab (1999) who reported that the loss of HCB, lindane, p,p'-DDT, dimethoate, profenofos and pirimiphos-methyl depends on the levels of acetic acid and NaCl solutions. Also, the amount of pesticide removed by washings is related to its water solubility and octanol–water partition coefficient (Pugliese et al. 2004).
One of the health concerns of using oxidants to degrade pesticide is the formation of toxic intermediates. The present study investigated the efficacy of two oxidizing agentss (KMnO4 and H2O2) to remove chlorpyrifos from date fruits. KMnO4 and H2O2 were assayed for washings have redox properties, and thus, can modify the chemical structure of the selected pesticides creating a derived by-product that is more toxic than the parent pesticide. If it is the case, such washing treatment should not be utilized to reduce the pesticide residue levels in fruits. It is well known that some organophosphorus pesticides containing P = S bonds (actually organothiophosphorus pesticides) such as chlorpyrifos reacts with oxidative reagents producing its respective oxygen analogs (e.g. chlorpyrifos-oxon), which are more potent as mammalian acetylcholinesterase inhibitors than the parent forms (Amdur et al. 1991). The possible formation of toxic by-products by either KMnO4 or H2O2 that are oxidative reagents was investigated by gas chromatography-massspectrometry (GC-MS) in SCAN mode by monitoring m/z ions: 109, 197, 242, 270 and 298 for chlorpyrifos oxon. Only single peak at a retention time of 12.14 min corresponding to chlorpyrifos was observed in the GC-MS chromatogram and there is no intermediate or dead-end product detected using the analytical method described in the present study (Fig. 1). No toxic by-products (chlorpyrifos-oxon, malaoxon, methidaoxon and methyl paraoxon) were identified in the extracts of the washed samples for the washing-time and low concentrations of sodium hypochlorite, KMnO4 and H2O2, but at high levels oxons from the organophosphorus pesticides were identified (Pugliese et al. 2004). There are many factors that affect the activity of the oxidative reagents to form oxons such as solvent, pH, identity of the pesticide, levels of reagents, the reagent it self, reaction time, temperature, endogenous matrix compounds (Pugliese et al. 2004). Advanced oxidation processes (AOPs) are the hydroxyl radical-mediated oxidations which utilize hydroxyl radicals (•OH) as their primary oxidizing species and have proven to be very effective in treating a wide variety of organic contaminants leading not only to their destruction, but also, given sufficient conditions, to their complete mineralization with no more toxic compound can be produced during the degradation process (Glaze et al. 1987; Buxton et al. 1988; Benitez et al. 2002). Furthermore, the radicals produced from these oxidants can substitute halogens attached to aromatic rings, thus generating biodegradable compounds, although the reaction takes place in a slow rate (Haag and Yao 1992). Products of chlorpyrifos degradation include 3,5,6-trichloro-2-pyridinol which subsequently breaks down to organochlorine compounds and carbon dioxide (The Royal Society of Chemistry 1988). H2O2 enhanced the photodegradation of parathion through the reaction between UV generated hydroxyl radical and parathion yielding several organic byproducts, of which the paraoxon, 4-nitrophenol, O,O,O-triethyl thiophosphate and O,O-diethyl-methyl thiophosphate (Wu and Linden 2008). A higher concentration of H2O2 results in a higher steady-state hydroxyl radical concentration and thus increases the availability of hydroxyl radical to degrade parathion (Wu and Linden 2008). Also, H2O2 acts as a hydroxyl radical scavenger producing a much less reactive •HO2 radical. This scavenging effect becomes significant at higher hydrogen peroxide concentrations and thus less hydroxyl radical is available for degrading parathion.
Fig. 1.
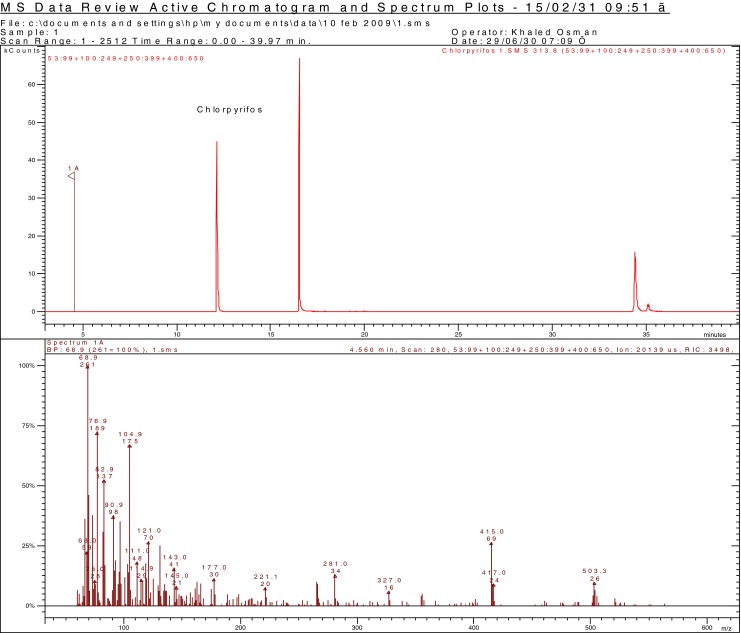
GC-MS chromatogram corresponding to date fruits immersed in 2 mg/kg chlorpyrifos and washed by different treatment
Kinetic studies
A biphasic model was assumed in order to carry out the statistical study of the loss of chlorpyrifos according to the Eq. 1.
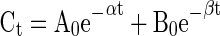 |
1 |
where Ct is the recovered amount of chlorpyrifos at t min, A0 and B0 are the concentrations of chlorpyrifos at zero time, while α and β are the disappearance rate constants for the first and second phases, respectively. The half-life (t1/2) of the exponential decay was calculated according to the Eq. 2.
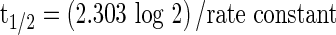 |
2 |
The data fitting results in case of different wash treatment using second order kinetic showed that coefficients of determination (R2) ranged from 0.988 to 0.999 (Table 2). The biphasic model is characterized by a rapid phase (first phase), and a much slower phase (second phase). This is clearly reflected in the half-live values (t1/2), where t1/2 values of chlorpyrifos were 53.33, 13.86, 12.60, 21.01, 19.25, 12.60, 12.16, 28.88 and 27.73 min, while, t1/2 values in the second phase model were 138.65, 30.14, 17.33, 33.01, 46.22, 15.07, 21.01, 57.77 and 53.33 min when date fruits were contaminated with chlorpryrifos at concentration of 2 mg/l and treated with running water, 10 AA, 20 AA, 10 CA, 20 CA, 10 H2O2, 20 H2O2, 10 KMnO4 and 20 mg/l KMnO4, respectively (Table 2). The fate of chlorpyrifos in aquatic systems has been especially well-studied, with t1/2 ranged from 4.7 h to 7 h (Schaefer and Dupras 1970; Knuth and Heinis 1992). Also, The rate of reaction of chlorpyrifos (vide supra) with HO• in the gas phase at high temperatures was studies with t1/2 of 1.4 h (Hebert et al. 2000).
Table 2.
Kinetic parameters for the chlorpyrifos dissipation in date fruits in presence of different wash treatments
| Wash treatmenta | Kinetic parameters | ||||||
|---|---|---|---|---|---|---|---|
| A0 (μg/kg) | B0 (μg/kg) | α (min-1) | β (min-1) | t1/2α (min) | t1/2β (min) | Regression coefficient (R2) | |
| T2 | 21.90 | 16.05 | 0.013 | 0.005 | 53.33 | 138.65 | 0.988 |
| T3 | 7.60 | 4.60 | 0.050 | 0.023 | 13.86 | 30.14 | 0.999 |
| T4 | 5.25 | 0.35 | 0.055 | 0.040 | 12.60 | 17.33 | 0.999 |
| T5 | 10.60 | 3.80 | 0.033 | 0.021 | 21.01 | 33.01 | 0.997 |
| T6 | 8.25 | 3.65 | 0.036 | 0.015 | 19.25 | 46.22 | 0.999 |
| T7 | 21.05 | 9.05 | 0.055 | 0.046 | 12.60 | 15.07 | 0.999 |
| T8 | 16.40 | 7.10 | 0.057 | 0.033 | 12.16 | 21.01 | 0.999 |
| T9 | 13.10 | 8.85 | 0.024 | 0.012 | 28.88 | 57.77 | 0.999 |
| T10 | 11.70 | 5.50 | 0.025 | 0.013 | 27.73 | 53.33 | 0.994 |
asee Table 1
A0 and B0 are the concentrations of chlorpyrifos at zero time, while α and β are the disappearance rate constants for the first and second and phases
t1/2α and t1/2β are the half-life times for the first and second and phases
Each value is the mean of three replicates with 6 determinations
The present findings are in accordance with those of many investigators who reported that the kinetics of pesticide degradation is commonly biphasic with a very rapid degradation rate at the beginning followed by a very slow prolonged dissipation (Alexander 1994; Jones et al. 1996; Rigas et al. 2007; Osman et al. 2009). The relative importance of the phases depends on the availability of the pollutants, hydrophobicity, and affinity for organic matter. On the contrary, first order removal rate of 0.039 h−1 was recorded for chlorpyrifos simulated stormwater runoff treated in constructed wetland mesocosms (Sherrard et al. 2004).
Effect of different wash treatments on some date fruits quality parameters
The effect of wash treatments on quality parameters of date fruits is of interest since the wash treatment may be performed by dates’ processors and consumers. Quality of fruits and vegetables is greatly affected by post-harvest treatments and storage (Ismail et al. 2008) by affecting in the nutritional and sensory quality of the product (Laurila and Ahvenainen 2002). Unfortunately, few studies dealt with the effect of wash treatments on date fruits quality parameters. Thus, research is needed to investigate the effect of current wash treatments on TS, TP and CA, and ultimately devise ideal conditions of wash treatments suitable for the fruit. The mean content of TS from the date fruit extracts is reported as % of fresh weight in Table 3. All of the treatments exerted a little negative effect on total sugars, but H2O2 and KMnO4 at level of 20 g/l had more drastic effect. The loss of sugars from date fruits was increasing by extending the wash time. Sugars in Succary date fruits are fructose (9.17 %), glucose (12.45 %) and sucrose (35.94 %) (Al-Humaid et al. 2010). These soluble sugars are easily to dissolve in washing solutions and continuously lost from fruits by extending wash time.
Table 3.
Effect of wash treatments on total soluble sugars of date fruits
| Wash treatmenta | Contact time (min) | |||
|---|---|---|---|---|
| 5 | 15 | 30 | 60 | |
| T1 | 68.4 ± 0.35aB | 69.2 ± 1.25aD | 68.4 ± 0.89aBC | 69.2 ± 3.25aD |
| T2 | 67.3 ± 1.84aB | 66.1 ± 0.78aD | 67.4 ± 2.19aBC | 67.5 ± 1.34aD |
| T3 | 67.0 ± 1.63aB | 68.4 ± 4.31aD | 67.7 ± 3.75aBC | 68.4 ± 2.33aC |
| T4 | 65.9 ± 2.33aB | 66.8 ± 2.03aCD | 65.2 ± 4.45aB | 64.6 ± 1.06aC |
| T5 | 66.5 ± 2.40bB | 65.6 ± 0.0bC | 60.4 ± 0.0aA | 61.7 ± 0.14aB |
| T6 | 65.0 ± 0.0bAB | 64.2 ± 0.0bBC | 62.6 ± 7.35aAB | 62.2 ± 1.00aBC |
| T7 | 65.6 ± 1.70bB | 63.2 ± 0.57bAB | 62.8 ± 0.0bA | 62.3 ± 0.0aBC |
| T8 | 62.5 ± 0.0aA | 62.3 ± 4.74aAB | 62.6 ± 3.61aA | 58.4 ± 1.41aA |
| T9 | 65.3 ± 0.41bB | 65.4 ± 0.26bC | 61.9 ± 0.92bA | 56.4 ± 0.0aA |
| T10 | 61.5 ± 0.78aA | 60.9 ± 0.0bA | 60.2 ± 4.38bA | 55.2 ± 3.54aA |
asee Table 1
All the given values are means of three determinations ± standard deviation
Data are expressed as g/100 g of fresh weight
Means having different letter in a row (or column) are significantly different (P < 0.05)
The effect of wash treatments on TP as antioxidant compounds is illustrated in Table 4. All wash treatments tended to increase TP contents after 5 min of contact, except 10 g/l CA and 20 g/l H2O2 treatments as compared with control (chlorpyrifos only). However, TP levels of all wash treatments tended to decrease by increasing the contact time. Significant increase in TP contents by running water, 10 g/l AA and 20 g/l CA during all the tested the contact times compared with control value. Postharvest application of several compounds, e.g. NaCl, NaOH and acetic acid, have also been reported to accelerate ripening of dates; however, the taste and color of fruit were generally inferior to those naturally ripened on the tree (Kalra and Jawanda 1974; Shamshiri and Rahemi 1999). The ripening agents possibly disrupt the epidermal cell and the protoplasm thereby releasing and activating the enzymes (Saleem et al. 2005). The increase in the TP in dates by some wash treatments could be due to ethylene action. This hormone stimulates activity of phenylalanine ammonia lyase, a key enzyme in biosynthesis of phenolic compounds and accumulation of phenolic constituents (Hwang et al. 1994; Ritenour et al. 1995). Also, the comparatively higher value of DPPH activity may be due to the high content of phenolic compounds (Sasidharan and Menon 2011).
Table 4.
Effect of wash treatments on total phenolics of date fruits
| Wash treatmenta | Contact time (min) | |||
|---|---|---|---|---|
| 5 | 15 | 30 | 60 | |
| T1 | 149 ± 4.0aB | 149 ± 2.2aB | 142.5 ± 0.9aBC | 136 ± 0.7bAB |
| T2 | 200 ± 0.0bE | 171 ± 12.0aD | 168 ± 7.7aE | 165 ± 21.2aD |
| T3 | 163 ± 12.0bD | 162 ± 5.0bCD | 160.5 ± 30.4bDE | 151 ± 5.7aC |
| T4 | 166 ± 3.1cC | 168 ± 0.0dE | 119 ± 1.4aA | 129 ± 8.4bA |
| T5 | 147 ± 0.0cB | 145 ± 13.4cB | 133 ± 5.0bB | 125 ± 5.0aA |
| T6 | 158 ± 1.4bC | 158 ± 0.7bC | 158 ± 0.7bDE | 143 ± 0.0aBC |
| T7 | 160 ± 3.5cC | 144 ± 5.7bB | 142 ± 5.7bBC | 128 ± 1.4aA |
| T8 | 136 ± 5.7bA | 127 ± 8.5aA | 128 ± 2.1aA | 129.5 ± 6.4aA |
| T9 | 169 ± 0.0dD | 148 ± 4.2cB | 141 ± 9.9aBC | 143.5 ± 7.1aBC |
| T10 | 150 ± 2.3bB | 149 ± 2.3bB | 149 ± 0.71bC | 139.5 ± 9.9aB |
asee Table 1
All the given values are means of three determinations ± standard deviation
Data are expressed as mg gallic acid equivalents (GAE)/100 g sample
Means having different letter in a row (or column) are significantly different (P < 0.05)
The AC of date fruits was highly increased when the fruits were immersed in 20 g/l AA for 5 min followed by 10 g/l AA for 5–15 min and then 10 g/l CA compared to control value (Table 5). Also, when fruits treated with H2O2 at either 1 or 20 g/l, the AC increased for all the tested contact times except at 10 g/l for 60 min where the AC value is similar to control value. On the other hand, KMnO4 treatment at either 10 or 20 g/l for 60 min as contact time exerted a negative effect on this attribute. Soaking strawberry in salicylic acid at 2 mM was found to be effectively to increase total antioxidant potential, ascorbic acid content, total soluble solids and prevented fungal contaminations (Asghari and Aghdam 2010). It is established that the AC of dates was due mainly to the presence of water-soluble compounds with potent free radical-scavenging effects, including the phenolic compounds (mainly cinnamic acids) and flavonoids (flavones, flavonols and flavanones) (Wang et al. 1996; Connor et al. 2002; Vayalil 2002; Guo et al. 2003; Mansouri et al. 2005; Biglari et al. 2008). Significant correlation between AC and TP content in date palm fruits has been established by many investigators (Allaith 2008; Biglari et al. 2008).
Table 5.
Effect of wash treatments on antioxidant capacity of date fruits
| Wash treatmenta | Contact time (min) | |||
|---|---|---|---|---|
| 5 | 15 | 30 | 60 | |
| T1 | 239 ± 3.7aA | 239 ± 6.7aA | 239 ± 6.7aAB | 239 ± 9.6aD |
| T2 | 270 ± 3.5cD | 258 ± 9.8bC | 251 ± 4.9bCD | 225 ± 14.9aBC |
| T3 | 300 ± 0.0dE | 285 ± 10.9cC | 235 ± 3.4bA | 211 ± 6.2aA |
| T4 | 319 ± 0.0cF | 256 ± 10.3bB | 248 ± 10.3bBC | 207 ± 6.4aA |
| T5 | 277 ± 6.2cD | 258 ± 10.6bB | 234 ± 0.0aA | 232 ± 7.0aCD |
| T6 | 247 ± 4.1bBC | 240 ± 13.2abA | 235 ± 6.4aA | 234 ± 1.4aCD |
| T7 | 270 ± 6.1bD | 258 ± 6.9bB | 263 ± 6.4b | 238 ± 9.7aD |
| T8 | 256 ± 0.7aC | 256 ± 6.4aB | 260 ± 9.9aD | 256 ± 14.9aE |
| T9 | 235 ± 3.4bA | 237 ± 10.7bA | 234 ± 0.0bA | 223 ± 14.1aB |
| T10 | 244 ± 9.2cAB | 235 ± 13.2bA | 234 ± 13.4bA | 223 ± 7.8aB |
asee Table 1
All the given values are means of three determinations ± standard deviation
Data are expressed as μmol Trolox equivalent (TE) antioxidant capacity per 100 g fresh date.
Means having different letter in a row (or column) are significantly different (P < 0.05)
Conclusion
Washing with water and various chemical solutions for domestic use are necessary to decrease the intake of pesticide residues. Since the wash treatment dosage and treatment time could impact the efficiency in pesticide removal, the better removal efficiency could be obtained through optimizing these parameters. Chlorpyrifos was significantly removed more from date fruits in all the tested wash treatments at all the tested time intervals compared with unwashed-fruits (treated with chlopyrifos only). By the end of the experiment almost complete chlorpyrifos removing occurred in AA-washed fruits. Citric acid washing came next in importance to washing by acetic acid solutions, giving removal 92–96 % of the compound removing at the end of the experiment. By contrast, contaminated fruits treated with H2O2 and KMnO4 gave 78–84 and 75–84 % of the compound removing at the end of the experiment, respectively. Although, tap water wash treatment significantly reduced the tested pesticide residual levels on date fruits compared with no wash treatment, but still less effective than the other treatments. The removing pattern could be attributed to the low water solubility and high octanol-water partition coefficient of chlorpyrifos. Also, the present study revealed that there was a gradual increase in the percent of reduction due to the increase of concentrations of AA, CA, H2O2 or KMnO4 for same-contact time treatment as well as extension of contact time could increase the efficiency in pesticide removing.
Kinetic studies revealed that chlorpyrifos was found to be more easily removable from date fruits treated with the tested chemical solutions with t1/2 values of 12–29 min compared with roughly 53 min in case of running water. By the end of the experiment almost complete chlorpyrifos removal occurred when date fruits were washed with acetic acid followed by citric acid and then hydrogen peroxide and potassium permanganat. The rate of chlorpyrifos removing by chemical solutions was almost 1.85–4.39 times faster than running water.
All of wash treatments exerted a little negative effect on total sugars, but H2O2 and KMnO4 at level of 20 g/l had more drastic effect. Although results revealed some significant changes in the phenolic contents and antioxidant capacity associated with wash treatments, overall, contact times and did not account for all of the observed variation in the these attributes of date fruits.
Results of this work, however, provided some basic concepts that can be helpful in post-harvest methods for consumer and industrialization of the date fruit. Therefore, the present study validated that water and/or acetic acid, citric acid, hydrogen peroxide or potassium permanganate solutions as wash treatments are safe and promising processes for the removal of the chlorpyrifos from date fruits surface under domestic conditions. Results found in the present study must not be extrapolated to other pesticides, crops or conditions.
References
- Abou-Arab AAK. Behavior of pesticides in tomatoes during commercial and home preparation. Food Chem. 1999;65:509–514. doi: 10.1016/S0308-8146(98)00231-3. [DOI] [Google Scholar]
- Alexander M. Biodegradation and bioremediation. San Diego, CA, USA: Academic; 1994. [Google Scholar]
- Al-Farsi M, Alasalvar C, Morris A, Baron M, Shahidi F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem. 2005;53:7592–7599. doi: 10.1021/jf050579q. [DOI] [PubMed] [Google Scholar]
- Al-Humaid AI, Mousa HM, El-Mergawi RA, Abdel-Salam AM. Chemical composition and antioxidant activity of dates and dates-camel-milk mixtures as a protective meal against lipid peroxidation in rats. Am J Food Technol. 2010;5:22–30. doi: 10.3923/ajft.2010.22.30. [DOI] [Google Scholar]
- Allaith AA. Antioxidant activity of Bahraini date palm (Phoenix dactylifera L.) fruit of various cultivars. International J Food Sci Technol. 2008;43:1033–1040. doi: 10.1111/j.1365-2621.2007.01558.x. [DOI] [Google Scholar]
- Al-Rehiayani S, Osman KA. Residual levels of preharvest-sprayed amitraz in date fruits. J Pest Cont Environ Sci. 2003;11:1–12. [Google Scholar]
- Al-Rehiayani S, Osman KA. Fate of preharvest-prayed dicofol in date fruits: residue analysis by HPLC. Agric Marine Sci Sultan Qaboos Univ. 2005;10:21–26. [Google Scholar]
- Al-Showiman SS, Fayadh JM. Chemical composition of date palm (Phoenix dactylifera L.) J Chem Soc Pak. 1990;12:84–103. [Google Scholar]
- Amdur MO, Doull J, Klaassen CD (1991) Casarett’s and Doull’s toxicology: the basic science of poisons. 4th edn, Pub. McGraw Hill, Inc.
- Anon (2004) The fruit of the desert. Saudi sate market, Al-butain Agricultural Cooperative Association (BACA), Issue June 7, 2004. Available at: http://www.dates.com.sa/SaudiDates/SaudiDates.htm. 16/01/2010.
- Asghari M, Aghdam MS. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trend Food Sci Tech. 2010;10:502–509. doi: 10.1016/j.tifs.2010.07.009. [DOI] [Google Scholar]
- Bajwa U, Sandhu KS (2011) Effect of handling and processing on pesticide residues in food- a review. J Food Sci Technol. doi:10.1007/s13197-011-0499-5 [DOI] [PMC free article] [PubMed]
- Bebe FN, Panemangalore N. Exposure to low doses of endosulfan and chlorpyrifos modifies endogenous antioxidants in tissue of rats. J Environ Sci Health B. 2003;38:349–363. doi: 10.1081/PFC-120019901. [DOI] [PubMed] [Google Scholar]
- Benitez FJ, Acero JL, Real FJ. Degrading of carbofuran by using ozone, UV, radiation and advanced oxidation processes. J Hazard Mater. 2002;B89:51–65. doi: 10.1016/S0304-3894(01)00300-4. [DOI] [PubMed] [Google Scholar]
- Besbes S, Drira L, Blecker C, Deroanne C, Attia H. Adding value to hard date (Phoenix dactylifera L.): compositional, functional and sensory characteristics of date jam. Food Chem. 2009;112:406–411. doi: 10.1016/j.foodchem.2008.05.093. [DOI] [Google Scholar]
- Biglari F, Alkarkhi AFM, Easa AM. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chem. 2008;107:1636–1641. doi: 10.1016/j.foodchem.2007.10.033. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lelbensmittel Wissenschaften und Technologie. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical review of rate constants for reactions hydrated electrons, hydrogen atoms and hydroxyl radicals in aqueous solution. Phys Chem Ref Data. 1988;17:513–886. doi: 10.1063/1.555805. [DOI] [Google Scholar]
- Cengiz MF, Certel M, Karakas B, Gocmen H. Residue contents of DDVP (Dichlorvos) and diazinon applied on cucumbers grown in greenhouses and their reduction by duration of a pre-harvest interval and post-harvest culinary applications. Food Chem. 2006;98:127–135. doi: 10.1016/j.foodchem.2005.05.064. [DOI] [Google Scholar]
- Connor AM, Luby JJ, Hancock JF, Berkheimer S, Hanson EJ. Changes in fruit antioxidant activity among blueberry cultivars during cold temperature storage. J Agric Food Chem. 2002;50:893–898. doi: 10.1021/jf011212y. [DOI] [PubMed] [Google Scholar]
- Considine D. Foods and food production encyclopedia. New York: Van Nostrand Reinhold; 1982. pp. 542–550. [Google Scholar]
- Costat Program (1986) Costat statistical package for analysis of variance, version 2, Cohort Software, Minneapolis, MN, USA
- Crumpton TL, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: effects on nuclear transcription factor involved in cell replication and differentiation. Brain Res. 2000;857:87–98. doi: 10.1016/S0006-8993(99)02357-4. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Dev Brain Res. 2000;121:179–187. doi: 10.1016/S0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analyt Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Ecobichon DJ. Toxic effects of pesticides. In: Klaassen CD, editor. Casarett and Doull’s toxicology. New York: McGraw-Hill; 1996. pp. 643–698. [Google Scholar]
- Ekström G, Hemming H, Palborg M. Swedish pesticide risk reduction 1985–1995: food residues, health hazard and reported poisoning. Rev Environ Contam Toxicol. 1996;147:119–139. doi: 10.1007/978-1-4612-4058-7_4. [DOI] [PubMed] [Google Scholar]
- El-Saeid MH, Al-Dosari SA. Monitoring of pesticide residues in Riyadh dates by SFE, MSE, SFE, GC techniques. Arab J Chem. 2010;3:179–186. doi: 10.1016/j.arabjc.2010.04.007. [DOI] [Google Scholar]
- Gao H, Cheng N, Zhou J, Wang BN, Deng JJ, Cao W (2011) Antioxidant activities and phenolic compounds of date plum persimmon (Diospyros lotus L.) fruits. J Food Sci Technol. doi:10.1007/s13197-011-0591-x [DOI] [PMC free article] [PubMed]
- Glaze WH, Kang JW, Chapin DH. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci Eng. 1987;9:335–342. doi: 10.1080/01919518708552148. [DOI] [Google Scholar]
- Guo A, Yang J, Wei J, Li Y, Xu J, Jaing Y. Antioxidant activities of peel, pulp and seed fractions of common fruit as determined by FRAP assay. Nutr Res. 2003;23:1719–1726. doi: 10.1016/j.nutres.2003.08.005. [DOI] [Google Scholar]
- Haag WR, Yao CC. Rate constants for reaction of hydroxyl radicals with several drinking water contaminants. Environ Sci Technol. 1992;26:1005–1013. doi: 10.1021/es00029a021. [DOI] [Google Scholar]
- Hebert VR, Hoonhout C, Miller GC. Use of stable tracer studies to evaluate pesticide photolysis at elevated temperatures. J Agric Food Chem. 2000;48:1916–1921. doi: 10.1021/jf990699w. [DOI] [PubMed] [Google Scholar]
- Heijman SGJ, Hopman R. Activated carbon filtration in drinking water production: model prediction and new concepts. Colloid Surface A. Physicochem Eng Aspect. 1999;151:303–310. doi: 10.1016/S0927-7757(98)00643-8. [DOI] [Google Scholar]
- Hwang JH, Myoung-Won K, Young-Hee K. Effects of ethylene and Ca2+ on activity of phenylalanine ammonia lyase in glucan treated Daucus carota. J Plant Biol. 1994;37:263–269. [Google Scholar]
- Ismail B, Haffar I, Baalbaki R, Henry J. Physico-chemical characteristics and sensory quality of two date varieties under commercial and industrial storage conditions. LWT. 2008;41:896–904. doi: 10.1016/j.lwt.2007.06.009. [DOI] [Google Scholar]
- Jones KC, Alcock RE, Johnoson DL, Nothcott GL, Semple KT, Woolgar PJ. Organic chemicals in contaminated land: analysis, significances and research priorities. Land Contam Reclamat. 1996;3:189–197. [Google Scholar]
- Kalra SK, Jawanda JS. Softening of doka dates by salt and acetic acid treatments. Punjab Hortic J. 1974;14:116–121. [Google Scholar]
- Knuth ML, Heinis LJ. Dissipation and persistence of chlorpyrifos within littoral enclosures. J Agric Food Chem. 1992;40:1257–1263. doi: 10.1021/jf00019a036. [DOI] [Google Scholar]
- Kosanić M, Ranković B, Vukojević J. Antioxidant properties some lichen species. J Food Sci Technol. 2011;48(5):584–590. doi: 10.1007/s13197-010-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouloumbos VN, Tsipi DF, Hiskia AE, Nicolic D, van Breeman RB. Identification photocatalytic degradation products of diazinon on TiO2 aqueous suspensions using GC/MS/MS and LC/MS with quadrupole time of-flight mass spectrometry. J Am Mass Spectrom. 2003;14:803–817. doi: 10.1016/S1044-0305(03)00333-7. [DOI] [PubMed] [Google Scholar]
- Krol WJ, Arsenault TL, Pylypiw HM, Mattina MJI. Reduction of pesticide residues on produce by rinsing. J Agric Food Chem. 2000;48:4666–4670. doi: 10.1021/jf0002894. [DOI] [PubMed] [Google Scholar]
- Kulkarni SG, Vijayanand P, Shubha L. Effect of processing of dates into date juice concentrate and appraisal of its quality characteristics. J Food Sci Technol. 2010;47(2):157–161. doi: 10.1007/s13197-010-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushi LH, Meyer KA, Jacobs DR., Jr Cereals, legumes, and chronic disease risk reduction: evidence from epidemiological studies. Am J Clin Nutr. 1999;70:451S–458S. doi: 10.1093/ajcn/70.3.451s. [DOI] [PubMed] [Google Scholar]
- Laurila E, Ahvenainen R. Minimal processing of fresh fruits and vegetables. In: Jongen W, editor. Fruit and vegetable processing. Cambridge, UK/Boca Raton, FL: Woodhead Publishing Limited/CRC Press LLC; 2002. [Google Scholar]
- Lee SR, Mourer CR, Shibamoto T. Analysis before and after cooking processes of a trace chlorpyrifos spiked in polished rice. J Agric Food Chem. 1991;39:906–908. doi: 10.1021/jf00005a020. [DOI] [Google Scholar]
- Liu YH, Liu Y, Chen ZS, Lian J, Huang X, Chung YC. Purification and characterization of a novel organophosphorus pesticide hydrolase from penicillium lilacimum PB303. Enzym Microb Tech. 2004;34:297–303. doi: 10.1016/j.enzmictec.2003.11.009. [DOI] [Google Scholar]
- Mansouri A, Embarek G, Kokkalou E, Kefalas P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera) Food Chem. 2005;89:411–420. doi: 10.1016/j.foodchem.2004.02.051. [DOI] [Google Scholar]
- Ormad MP, Miguel N, Claver A, Matesanz JM, Ovelleiro JL. Pesticides removal in the process of drinking water production. Chemosphere. 2008;71:97–106. doi: 10.1016/j.chemosphere.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Osman KA. Lindane, chlorpyrifos and paraquat induced oxidative stress in female rats. Alex J Agric Res. 1999;44:345–355. [Google Scholar]
- Osman KA, Al-Rehiayani S. Risk assessment of pesticide to human and the environment. Saudi J Biol Sci. 2003;10:81–106. [Google Scholar]
- Osman KA, Al-Rehiayani S, Al-Doghairi MA, Salama AK. Bioremediation of oxamyl in sandy soil using animal manures. Int Biodeterior Biodegrad. 2009;63:341–346. doi: 10.1016/j.ibiod.2008.10.008. [DOI] [Google Scholar]
- Pugliese P, Moltó JC, Damiania P, Marín R, Cossignania L, Mañes J. Gas chromatographic evaluation of pesticide residue contents in nectarines after non-toxic washing treatments. J Chromatogr A. 2004;1050:185–191. [PubMed] [Google Scholar]
- Rigas F, Papadopoulou K, Dritsa V, Doulia D. Bioremediation of a soil contaminated by lindane utilizing the fungus Ganoderma australe via response surface methodology. J Hazard Mater. 2007;140:325–332. doi: 10.1016/j.jhazmat.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Ritenour MA, Ahrens MJ, Saltveit ME. Effects of temperature on ethylene-induced ammonia lyase activity and russet spotting in harvested iceberg lettuce. J Am Soc Hortic Sci. 1995;120:84–87. [Google Scholar]
- Saleem SA, Baloch AK, Baloch MK, Baloch WA, Ghaffoor A. Accelerated ripening of Dhakki dates by artificial means: ripening by acetic acid and sodium chloride. J Food Eng. 2005;70:61–66. doi: 10.1016/j.jfoodeng.2004.09.013. [DOI] [Google Scholar]
- Sasidharan I, Menon AN. Effects of temperature and solvent on antioxidant properties of curry leaf (Murraya koenigii L.) J Food Sci Technol. 2011;48(3):366–370. doi: 10.1007/s13197-010-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer CH, Dupras EF., Jr Factors affecting the stability of Dursban in polluted waters. J Econ Entomol. 1970;63:701–705. doi: 10.1093/jee/63.3.701. [DOI] [PubMed] [Google Scholar]
- Shamshiri MH, Rahemi M. Effect of ethephon, sodium chloride and acetic acid on quality of Mazafati date fruits. Iran J Agr Sci. 1999;29:777–785. [Google Scholar]
- Sherrard RM, Bearr JS, Murray-Gulde CL, Rodgers JH, Jr, Shah YT. Feasibility of constructed wetlands for removing chlorothalonil and chlorpyrifos from aqueous mixtures. Environ Pollut. 2004;127:385–394. doi: 10.1016/j.envpol.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Štanbaher D, Zupančič-Kralj L. Multiresidue method for determination of 90 pesticides in fresh fruits and vegetables using solid-phase extraction and gas chromatography–mass spectrometry. J Chromatogr A. 2003;1015:185–198. doi: 10.1016/S0021-9673(03)01211-1. [DOI] [PubMed] [Google Scholar]
- The agrochemical hand book. 2. Nottingam: RSC; 1988. [Google Scholar]
- Tomlin CDS. The e-Pesticide manual, version 2.2. Surrey, UK: The British Crop Protection Council; 2002. [Google Scholar]
- Vayalil PK. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. arecaceae) J Agr Food Chem. 2002;50:610–617. doi: 10.1021/jf010716t. [DOI] [PubMed] [Google Scholar]
- Wang QQ, Lemley AT. Oxidation of diazinon by anodic Fenton treatment. Water Res. 2002;36:3237–3244. doi: 10.1016/S0043-1354(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agr Food Chem. 1996;44:701–705. doi: 10.1021/jf950579y. [DOI] [Google Scholar]
- Wu C, Linden KG. Degradation and byproduct formation of parathion in aqueous solutions by UV and UV/H2O2 treatment. Water Res. 2008;42:4780–4790. doi: 10.1016/j.watres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-Y, Liu X-J, Hong X-Y. Effects of home preparation on pesticide residues in cabbage. Food Cont. 2007;18:1484–1487. doi: 10.1016/j.foodcont.2006.11.002. [DOI] [Google Scholar]


