Abstract
A study was conducted to develop good quality medium fat liquid dairy whitener from buffalo milk employing ultrafiltration (UF) process. The buffalo skim milk was UF concentrated to 4.05 to 4.18 (23.63 ± 0.30 % TS) fold and standardized to 10 % fat (on Dry Matter Basis) (i.e. formulation) and homogenized at 175.76 kg/cm2. The addition of 0.4 % mixture of monosodium and disodium phosphate (2:1 w/w) improved the heat stability of homogenized formulation to an optimum of 66 min. The bland flavour of homogenized formulation with added 0.4 % mixture of monosodium phosphate and disodium phosphate (2:1 w/w) and 18 % sugar (on DMB) (i.e. medium fat liquid dairy whitener) was improved significantly (P < 0.01) with the addition of 0.2 % potassium chloride, but heat stability of medium fat liquid dairy whitener got reduced substantially (i.e. 19 min). With subsequent heat treatment to 85 °C for 5 min, heat stability of medium fat liquid dairy whitener got improved to reasonable level of 27 min. Whitening ability in terms of L* value of medium fat liquid dairy whitener in both tea and coffee was significantly (P < 0.01) better when homogenized at 175.76 kg/cm2 vis-à-vis 140.61 kg/cm2. Standardized medium fat liquid dairy whitener had significantly (P < 0.01) greater protein content (i.e. approximately 2.43 times) compared to market dairy whitener samples. At 2 % solids level, standardized medium fat liquid dairy whitener in tea/coffee fetched significantly (P < 0.01) better sensory attributes and instrumental whitening ability compared to market sample at 3 % solids level. There could be clear 33 % solids quantity saving in case of developed product compared to market dairy whitener sample.
Keywords: Dairy whitener, Ultrafiltration, Heat stability, Whitening ability, Buffalo milk, Sensory evaluation
Introduction
Dairy whitener are increasingly being used in food/beverage products for their ease of handling, improved shelf-life and specific requirements like use in restaurants, railways, airways and waterways. Dairy whiteners should have the ability to withstand the high temperature (80–90 °C) and low pH (4.6–5.2) of coffee solution (Khatkar and Gupta 2013). An ideal dairy whitener should also possess good whitening ability, feathering resistance and emulsion stability and have the ability to lighten coffee and to neutralize coffee acids for producing smoother, milder or mellower drinks (Oldfield and Singh 2005).
In order to satisfy the requirements of coffee/tea stability dairy whiteners must firstly possess ‘Instant’ solubility properties i.e. satisfy the dispersibility and solubility criteria normally required when fat-containing whiteners are added to water. The second requirement is that, on dissolving in coffee, they shouldn’t coagulate or give rise to a sludge-like precipitate or sediment (Khatkar and Gupta 2013). Further, the most essential property of any whitener is its organoleptic quality and whitening ability. In general, these properties are imparted by milk fat and protein content. Milk fat not only improves flavour, mouthfeel and viscosity, but also enhances whitening power and overall acceptability. The whitening power comes mainly from a well emulsified and finely dispersed fat and protein in colloidal state (Khatkar et al. 2012b). Moreover, milk protein also contributes to other important quality characteristics of dairy whitener like feathering, mouthfeel and other organoleptic characteristics (Kelly et al. 1999).
Ultrafiltration (UF) process is one of the mean of increasing protein concentration in milk. In UF of milk, non protein nitrogen and soluble components such as lactose, salts and some vitamins pass through the membrane, whereas milk fat, proteins and insoluble salts are retained by the membrane (Mehaia 1997). The growing use of UF in the dairy industry, especially in the area of value addition, promises to dramatically change the technology of concentrated and dried milk products (Khatkar and Gupta 2013). The heat stability of milk proteins is very essential in UF based concentrated and dried milk products. Because of the altered compositional properties, particularly higher protein and different mineral profile, UF concentrated milk may conceivably exhibit potentially different heat stability characteristics and there is a need to optimize the heat stability of ultrafiltered milk retentate for the preparation of dairy whitener.
Therefore, there is need to develop a technology for the manufacture of milk protein based dairy whitener rather than milk powder based using UF process. Furthermore, there is also a demand for lower fat dairy whiteners in the market. There are consumers, who are health conscious and do not prefer much fat in the product. There are others, who require a relatively cheaper product. Further, the characteristic features of milk industry are that almost 90 % of the total volumes of buffalo milk (contribute 13 % of total world milk production) are solely produced in India and Pakistan with strong annual growth every year (IDF 2008). There is need to develop suitable technologies for the manufacture of new products from buffalo milk to meet the requirement of national and international markets. Presently, there is great scope for the manufacture of medium fat dairy whitener particularly from buffalo milk employing UF process and its export. Keeping all these points in mind, the present investigation has been undertaken for the preparation of good quality medium fat liquid dairy whitener employing ultrafiltration process.
Material and methods
Materials
Pasteurized buffalo skim milk, cream and sugar were procured from the Experimental Dairy Plant of the National Dairy Research Institute, Karnal. Stabilizing salts namely monosodium phosphate, disodium phosphate, other additive like potassium chloride, chemicals and reagents of analytical grade were procured from S.D. Fine Chem. Ltd., Mumbai.
Preparation of liquid dairy whitener
Buffalo skim milk (9.93 ± 0.58) was heat treated to 85 °C/5 min and ultrafiltered at 50 °C to about 4-fold concentration in a pilot UF plant (Tech-Sep, France, tubular module, having ZrO2 membrane, membrane surface area, 1.68 m2 and membrane molecular weight cut off, 50,000 Da). The plant was fitted with a balance tank (capacity, 200 l). During UF operation, the inlet and outlet pressures on the retentate side were maintained at 4.6 kg/cm2 and 3.6 kg/cm2, respectively. The permeate side pressure was kept at 1 kg/cm2. The UF retentate was standardized to 10 % fat (on DMB) with buffalo milk cream having 65–70 % fat content (i.e. formulation) and homogenized. The heat stability of formulation was improved by the addition of stabilizing salts, while bland flavour was improved with sugar and KCl. Then the product was heat treated to 85 °C/5 min followed by cooling.
Analytical methods
The total solids content and ash content of skim milk, UF retentate and medium fat liquid dairy whitener were determined gravimetrically as per the method of BIS (2001a). The fat percentage of buffalo skim milk was determined by Gerber method by using skim milk butyrometer as per the method of BIS (2001a) and that of milk cream, UF retentate and medium fat liquid dairy whitener was determined as per the method for condensed milk described in BIS (2001b). The protein content of skim milk, UF retentate and medium fat liquid dairy whitener was determined as method described by Manefee and Overman (1940). The pH of milk, UF retentate and medium fat liquid dairy whitener was determined by using pH meter PHAN LABINDIA (Labtek Engg. Pvt. Ltd., India). The titratable acidity of skim milk was determined as per BIS (2001a) and that of UF retentate and medium fat liquid dairy whitener was determined as per the method for condensed milk described in BIS (2001b). Lactose content of samples was determined as per method described by Lawrence (1968). Amount of calcium in skim milk, UF retentate and medium fat liquid dairy whitener was determined as method described by Davis and White (1962). The heat coagulation time (HCT) of UF retentate and medium fat liquid dairy whitener was determined as per the procedure described by Tayal and Sindhu (1983). For assessment of homogenization efficiency, determination of fat globule size in the homogenized liquid dairy whitener was carried out by using microscopic method as described here. The microscope was adjusted to a magnification of 1000× by using a 100× oil immersion objective and calibrated with the help of stage and ocular micrometers. A 1:50 dilution of the sample was prepared by adding 1 ml of the concentrate sample to 49 ml of the diluents (40 % glycerol solution). A diluents made up 30–50 % of glycerin in water does not affect clumping of fat, does not obstruct light rays and show Brownian movement sufficient to facilitate counting. Sample was filled in the cavity of a slide. A 24 × 30 mm No.1 cover glass was then placed over the cavity and lightly pressed into place. The slide was then focused under microscope after 15–20 min of the preparation of slide, during which the fat globules rise. The sizes of fat globules were recorded in the interval of 0–0.5, 0.5–1.0, 1.0–1.5, 1.5–2.0, etc. divisions. Five fields were selected at random and all fat globules in the field were classified.
Average diameter was calculated using the formula:
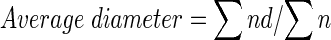 |
Where,
- n
Number of fat globules
- d
Diameter of fat globules
Flavour evaluation
Formulation of liquid dairy whitener were transferred in coded 50 ml glass beaker and served to a panel of trained six judges (Scientist), selected from the Dairy Technology Division of the Institute, within an hour for flavour evaluation on a 9-point Hedonic scale. The judges were also asked to note their observations on the score cards.
Tea and coffee preparation
Tea and coffee concoction were prepared with the required quantity of developed medium fat liquid dairy whitener and with the well branded (not disclosed due to integrity) market dairy whitener sample (i.e. added at 3 % solids level throughout the study) as per the method described by Khatkar and Gupta (2013).
Sensory evaluation
After preparation, tea and coffee samples were transferred to insulated thermos-bottles and served approximately 35–40 ml in 50 ml coded beaker, to a panel of six trained judges (scientist) selected from the Dairy Technology Division of Institute, within an hour at 60–70 °C for sensory evaluation. The judges were also asked to note their observations on a 9-point Hedonic scale.
Whitening ability
The developed medium fat liquid dairy whitener vis-à-vis market dairy whitener sample was evaluated within an half h for their whitening ability in tea/coffee as per method described by Khatkar and Gupta (2013) by using Tristimulus spectrophotometer Hunter Lab model Colour Flex® (Hunter Associates Laboratory Inc., VA, U.S.A.) along with the software (version 4.10).
Statistical analysis
The data obtained during the study were subjected to analysis of variance (i.e. one way analysis of variance and two way analysis of variance without interaction) as described by Singh (1996) under the guidance of a statistician. Wherever required, the overall mean, standard deviation of the compositional data and critical difference (with CDLSD equation) using MS-Excel software (version 2007, Microsoft, USA) was also calculated.
Result and discussion
UF behavior of buffalo skim milk
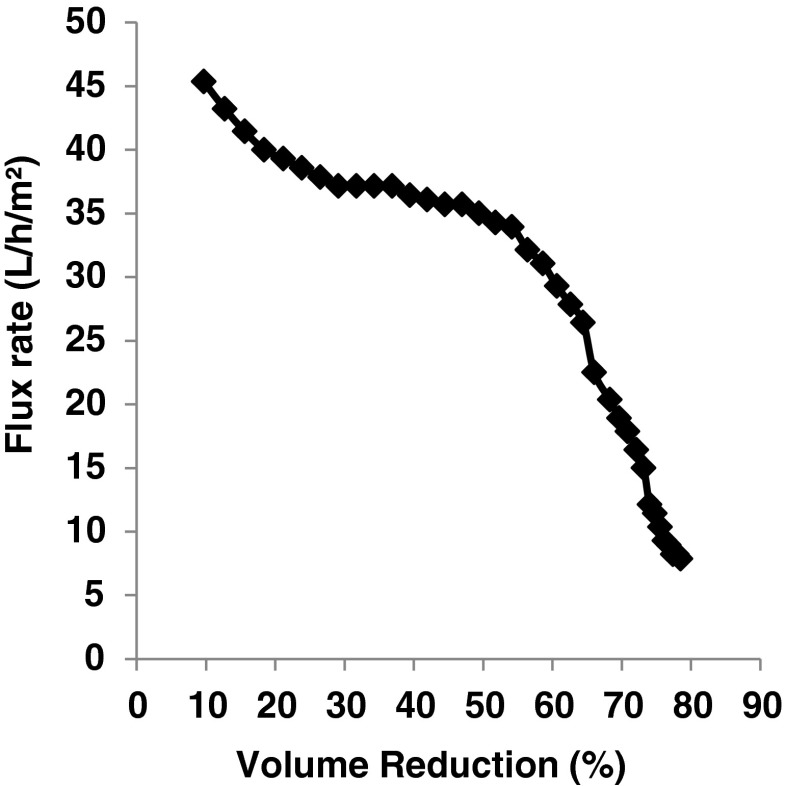
During UF concentration, the permeate flux rate was observed to drop from an initial 45.35 L/h/m2 to 7.85 L/h/m2 after 78.52 % volume reduction (Fig. 1). It was observed that after approximately 55 % volume reduction of buffalo skim milk, permeate flux rate was observed to drop steeply and so the efficiency of UF got decreased. Earlier, Patel et al. (1992) reported similar behavior of permeate flux rate during UF of buffalo skim milk. During about 4-fold (i.e. 4.05–4.18 fold) UF concentration of buffalo skim milk, the total solids, fat, protein, ash and calcium content of buffalo skim milk were observed to increase from 9.93 ± 0.58 to 23.63 ± 0.30 %, 0.02 ± 0.01 to 0.04 ± 0.01 %, 4.20 ± 0.29 to 17.31 ± 0.26 %, 0.73 ± 0.06 to 1.99 ± 0.09 %, and 0.23 ± 0.03 to 0.70 ± 0.14 %, respectively, while lactose decreased from 4.95 ± 0.25 to 4.20 ± 0.18 %. The decrease in lactose content was obviously due to the use of UF process that facilitates the retention of almost all true protein, fat and colloidal salts in the retentate, but lactose, water soluble minerals and non protein nitrogen are fractionated between the retentate and permeate. Earlier, Patel and Mistry (1997) reported similar behavior during UF of buffalo skim milk.
Fig. 1.
Change in permeate flux rate during ultrafiltration of buffalo skim milk
Effect of homogenization pressure on the quality of dairy whitener’s formulation
The standardized formulation was homogenized at different pressures i.e. 140.61 kg/cm2 and 175.76 kg/cm2 for 1st stage and 35.15 kg/cm2 for 2nd stage and evaluated for fat globule size and it was observed that average fat globule size of the formulation decreased significantly (P < 0.01) to 3.51 ± 0.97 μ and 1.97 ± 0.41 μ with 1st stage homogenization pressures of 140.61 kg/cm2 and 175.76 kg/cm2, respectively. Similar behavior of varying homogenization pressures on fat globule size were also reported by Kieczewska et al. (2003); Thiebaud et al. (2003) and Khatkar et al. (2012a). Smaller fat globule sizes being better for dairy whitener, further studies were carried out at homogenization pressure of 175.76 kg/cm2 and 35.15 kg/cm2 at 1st and 2nd stage, respectively.
Heat stability
Homogenized formulation was evaluated for heat stability. The heat stability of dairy products, especially of dairy whitener having high protein content (or casein content), is of great importance, when the products are subjected to high heat treatment. Any process or product variable, which causes an alteration in protein and mineral equilibrium is likely to influence heat stability. Hence, the UF concentrated buffalo milk may exhibit potentially different heat stability characteristics (Singh 2004). Heat stability of the formulation was observed to be only 23 min at 6.92 pH. An attempt was, therefore, made to improve the heat stability.
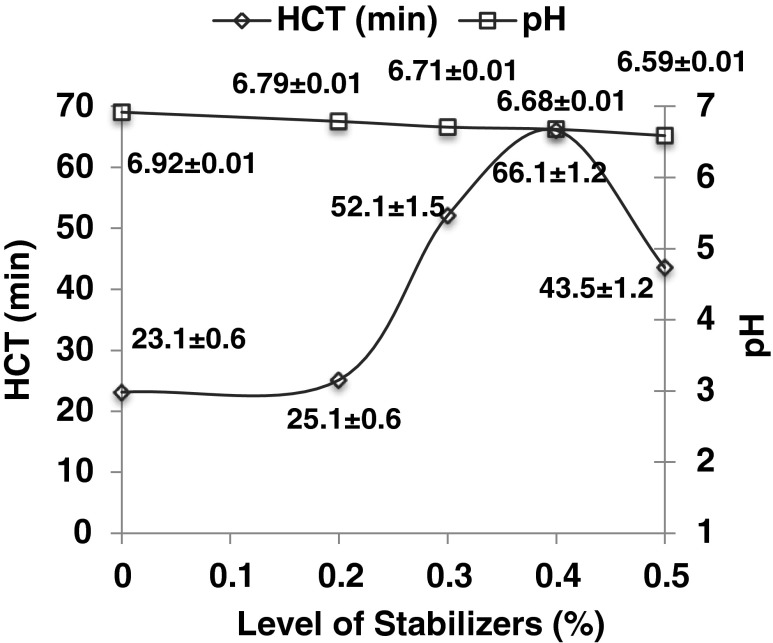
Different levels (0.2 %, 0.3 %, 0.4 %, and 0.5 %) of the mixture of monosodium phosphate and disodium phosphate (2:1 w/w) were added to formulation (Fig. 2). The heat coagulation time (HCT) of the formulation was significantly (P < 0.01) increased from 23 min to 26, 52, 66 and 43 min, respectively, while pH was significantly (P < 0.01) decreased from 6.92 to 6.79, 6.71, 6.68 and 6.59, respectively, at 0.2 %, 0.3 %, 0.4 % and 0.5 % level of mixture of stabilizers, respectively. The fluctuation in the values reflects that the stability of formulation is controlled by the salt balance and pH. Hence, optimum level of stabilizer addition helps in the maintaining proper salt balance and pH in the product. Similar observations were reported earlier by Sweetsur and Muir (1980) for buffalo skim milk UF retentate and Khatkar et al. (2012a) for full fat liquid dairy whitener formulation. Homogenized formulation showed optimum heat stability i.e. 66 min at 6.68 pH after addition of 0.4 % mixture of monosodium phosphate and disodium phosphate (2:1 w/w).
Fig. 2.
Effect of different levels of mixture of monosodium and disodium phosphate (2:1 w/w) on heat coagulation time (HCT) and pH of formulation (n = 3)
Flavour attribute
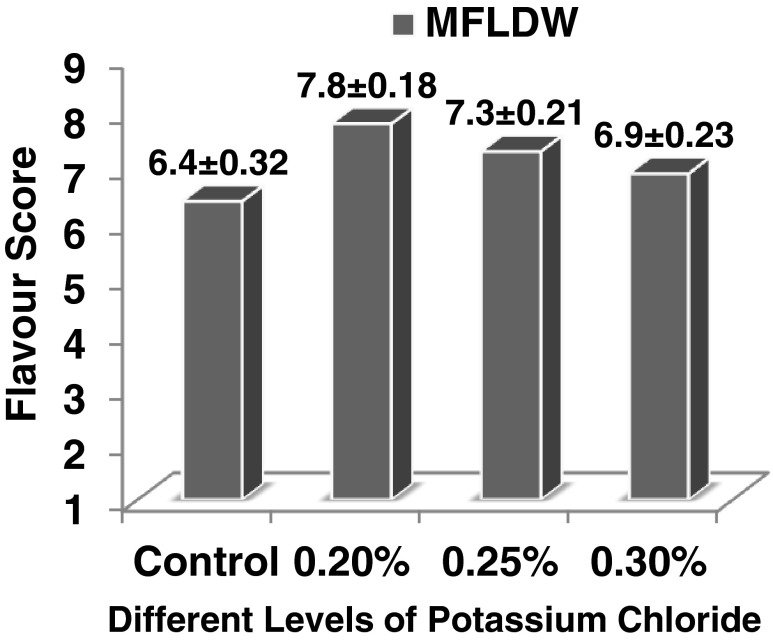
Homogenized formulation with added 0.4 % mixture of monosodium phosphate and disodium phosphate (2:1 w/w) and 18 % sugar (on DMB) (i.e. medium fat liquid dairy whitener) scored poorly 6.4 on 9-point Hedonic scale for flavour. The product lacked the typical flavour mostly because during UF, loss of minerals and reduction in lactose content occurs. The improvement in bland flavour of medium fat liquid dairy whitener was studied by addition of potassium chloride (KCl) at 0.2 %, 0.25 % and 0.3 % levels (Fig. 3). The flavour scores were significantly (P < 0.01) better after addition of KCl in medium fat liquid dairy whitener. But addition of 0.2 % potassium chloride improved the flavour score (7.8) of liquid dairy whitener significantly (P < 0.01) than at other levels (C.D. 0.49). Similar observations were made earlier by Kelly et al. (1999); Khatkar et al. (2012a) and Khatkar and Gupta (2013) for dairy whitener formulation.
Fig. 3.
Effect of addition of different levels of potassium chloride on sensory flavor scores of medium fat liquid dairy whitener (MFLDW) (n = 3)
But with the addition of 18 % sugar (on DMB) and 0.2 % KCl, the HCT of medium fat liquid dairy whitener decreased substantially to 19 min, while pH decreased to 6.60. After heat treatment at 85 °C for 5 min, the HCT of the medium fat liquid dairy whitener increased to a reasonable level of 27 min, while pH decreased to 6.42. Similar observations were reported earlier by Khatkar et al. (2012a) for buffalo skim milk UF retentate based full fat liquid dairy whitener formulation.
Whitening ability of liquid dairy whitener
The whitening ability in terms of L*, a*, b* values of medium fat liquid dairy whitener in tea and coffee as affected by different homogenization pressures is given in Table 1. At homogenization pressure of 175.76 kg/cm2, L* value of medium fat liquid dairy whitener was significantly (P < 0.01) greater than at 140.61 kg/cm2 in both tea and coffee. Similar observations were reported earlier by Khatkar et al. (2012a) for buffalo skim milk UF retentate based full fat liquid dairy whitener formulation. There was significantly (P < 0.01) lower a* value of medium fat liquid dairy whitener in tea at homogenization pressure of 175.76 kg/cm2 compared to 140.61 kg/cm2 which was desirable, while b* value was significantly (P < 0.01) greater in tea at homogenization pressures of 175.76 kg/cm2. There was no statistically significant difference for a* and b* value of liquid dairy whitener in coffee at varying homogenization pressures.
Table 1.
Effect of homogenization pressures on whitening ability of medium fat liquid dairy whitener (MFLDW) in tea and coffee at 3 % solid level (n = 9)
| Product | Homogenization pressure (kg/cm2) | L* | a* | b* | |
|---|---|---|---|---|---|
| 1st stage | 2nd stage | ||||
| MFLDW in tea | 140.61 | 35.15 | 48.03 ± 0.06 | 11.21 ± 0.07 | 25.09 ± 0.08 |
| 175.76 | 35.15 | 48.31 ± 0.03** | 11.11 ± 0.03** | 25.27 ± 0.03** | |
| MFLDW in coffee | 140.61 | 35.15 | 54.42 ± 0.34 | 4.92 ± 0.27 | 20.50 ± 0.63 |
| 175.76 | 35.15 | 55.22 ± 0.29** | 4.73 ± 0.2ns | 20.85 ± 0.29ns | |
**Significant at P < 0.01
ns not significant
Bassett et al. (1978), Aneja (1974) and Oldfield et al. (2000) explained that the surface area of a given amount of fat is inversely proportional to the radius of the globule. Thus, the light-reflecting surface of a 10 μ globule is increased 10 fold when it is scattered to produce 1000 globules with diameter of 1 μ. Homogenization reduces the fat globule size creating a greater surface area and more light reflection with resultant greater whitening effect. Thus, it was concluded that 175.76 kg/cm2 pressure resulted in significantly (P < 0.01) lesser fat globule size and significantly (P < 0.01) greater whitening effect in tea/coffee for liquid dairy whitener.
Thus, the standardized formulation and process for the preparation of medium fat liquid dairy whitener consisted of about 4-fold UF concentration of buffalo skim milk, its standardization to 10 % fat (on DMB of final product), addition of 0.4 % mixture of monosodium phosphate and disodium phosphate (2:1 w/w), addition of 18 % sugar (on DMB of final products) and 0.2 % KCl, homogenization at 175.76 kg/cm2 for 1st stage and 35.15 kg/cm2 2nd stage and heat treatment of 85 °C for 5 min. The standardized medium fat liquid dairy whitener was prepared with these standardized conditions and processing parameters and further evaluated for their quality attributes.
Quality evaluation of medium fat liquid dairy whitener (MFLDW)
Standardized MFLDW was evaluated for chemical composition, sensory evaluation and instrumental whitening ability vis-à-vis market dairy whitener sample.
Chemical composition
The chemical composition of standardized MFLDW vis-à-vis market dairy whitener sample is given in Table 2. Medium fat dairy whitener is not identified in the BIS: 12299 (1998) standards, but it was prepared to meet specific consumers’ demand of cheap and low fat product. The protein content of MFLDW was significantly (P < 0.01) greater (approximately 2.43 times) compared to those of market samples A and B. The higher protein content in MFLDW was obviously due to the use of UF milk retentate as a base for their preparation.
Table 2.
Comparison of chemical composition of MFLDW vis-à-vis market dairy whitener samples
| Constituents | MFLDW | Market sample (A) | Market sample (B) | |
|---|---|---|---|---|
| (%) | (% DMB) | (%) | (%) | |
| Total solids | 32.89 | 100 | – | – |
| Fat | 3.32 | 10.09 | 10 | 20 |
| Protein | 16.39 | 49.82** | 25.5 | 20 |
| Total carbohydrate | 10.82 | 32.89 | 56 | 55 |
| Ash | 2.36 | 7.17 | 5 | 5 |
| Calcium | 0.667 | 2.03 | 0.843 | – |
Average of three trials; – not mentioned on the package; market sample A (medium fat) and B (full fat) are powdered dairy whitener samples
**Significant at P < 0.01
Sensory evaluation in tea and coffee
Sensory attributes of MFLDW vis-à-vis market sample were evaluated in tea and coffee at 3 % solids level (Table 3). Standardized MFLDW in tea and coffee fetched significantly (P < 0.01) greater mouthfeel, whiteness and overall acceptability scores compared to market sample. Flavour scores of both tea and coffee with MFLDW were observed significantly (P < 0.01) poorer compared to that of market sample mostly due to masking effect of MFLDW on tea and coffee flavor. In fact, MFLDW exhibited ‘over whiteness’ in both tea and coffee and were generally ‘liked slightly’ by judges. There was no statistically significant difference in sweetness and appearance scores. These results revealed that at 3 % solids level, MFLDW in both tea and coffee exhibited poorer flavour scores and also exhibited ‘over whiteness’ in tea/coffee compared to market sample.
Table 3.
Sensory evaluation and whitening ability of standardized medium fat liquid dairy whitener (MFLDW) vis-à-vis market dairy whitener sample (MS) in tea and coffee
| Flavor | Mouthfeel | Whiteness | Appearance | Sweetness | Overall acceptability | L*-value | a*-value | b*-value | |
|---|---|---|---|---|---|---|---|---|---|
| At 3 % solids level in tea | |||||||||
| MS @ 3 % | 7.83 ± 0.26 | 7.25 ± 0.27 | 7.17 ± 0.26 | 7.83 ± 0.41a | 7.92 ± 0.20a | 7.60 ± 0.09 | 41.12 ± 0.05 | 12.18 ± 0.04 | 24.23 ± 0.10 |
| MFLDW @ 3 % | 7.25 ± 0.27** | 7.83 ± 0.25** | 8.08 ± 0.20** | 8.17 ± 0.26a | 8.17 ± 0.26a | 7.90 ± 0.17** | 48.11 ± 0.01** | 11.17 ± 0.02** | 25.19 ± 0.02** |
| At 3 % solids level in coffee | |||||||||
| MS @ 3 % | 7.75 ± 0.27 | 7.08 ± 0.20 | 7.33 ± 0.26 | 8.08 ± 0.20a | 7.92 ± 0.20a | 7.63 ± 0.05 | 45.14 ± 0.17 | 7.21 ± 0.33 | 24.07 ± 0.29 |
| MFLDW @3 % | 7.33 ± 0.26** | 7.83 ± 0.26** | 8.00 ± 0.32** | 8.08 ± 0.20a | 7.92 ± 0.20a | 7.83 ± 0.10** | 55.61 ± 0.18** | 5.55 ± 0.42** | 20.64 ± 0.32** |
| Effect of addition of different solids level in tea | |||||||||
| MFLDW@3 % | 7.30 ± 0.64a | 7.63 ± 0.61a | 7.83 ± 0.53a | 7.83 ± 0.63a | 7.50 ± 0.45a | 7.62 ± 0.50a | 48.18 ± 0.02a | 11.16 ± 0.03a | 25.49 ± 0.10a |
| MFLDW@2.5 % | 7.45 ± 0.69a | 7.38 ± 0.68a | 7.35 ± 0.58b | 7.50 ± 0.53a | 7.46 ± 0.59a | 7.43 ± 0.59a | 46.54 ± 0.03b | 11.55 ± 0.18a | 25.27 ± 0.02b |
| MFLDW@2 % | 7.60 ± 0.25a | 7.09 ± 0.40a | 7.00 ± 0.67b | 7.29 ± 0.61a | 7.17 ± 0.70a | 7.23 ± 0.44a | 44.08 ± 0.04c | 12.27 ± 0.27b | 25.13 ± 0.03c |
| CD-value | – | – | 0.43** | – | – | – | 0.11** | 0.77** | 0.10** |
| Effect of addition of different solids level in coffee | |||||||||
| MFLDW@3 % | 7.25 ± 0.42a | 8.25 ± 0.42a | 8.25 ± 0.69a | 8.17 ± 0.26a | 8.00a | 7.98 ± 0.18a,b,c | 55.65 ± 0.15a | 4.76 ± 0.41a | 21.18 ± 0.56a |
| MFLDW@2.5 % | 8.00 ± 0.32b | 8.25 ± 0.27a | 8.25 ± 0.42a | 8.17 ± 0.26a | 8.00a | 8.13 ± 0.08b | 53.73 ± 0.16b | 5.07 ± 0.40a | 21.54 ± 0.22a |
| MFLDW@2 % | 7.92 ± 0.20b | 7.75 ± 0.27b | 8.00 ± 0.32a | 8.17 ± 0.26a | 7.92 ± 0.20a | 7.95 ± 0.05c | 50.55 ± 0.07c | 5.66 ± 0.21a | 21.69 ± 0.23a |
| CD-value | 0.39** | 0.33** | – | – | – | 0.15* | 0.60** | – | – |
| At optimized 2 % solids level in tea | |||||||||
| MS @ 3 % | 7.08 ± 0.86a | 7.03 ± 0.71a | 6.68 ± 0.51 | 7.42 ± 0.66a | 7.67 ± 0.51a | 7.18 ± 0.50a | 41.21 ± 0.21 | 12.13 ± 0.50a | 23.94 ± 0.42a |
| MFLDW @ 2 % | 7.23 ± 0.62a | 7.17 ± 0.75a | 7.43 ± 0.55** | 7.58 ± 0.49a | 7.67 ± 0.52a | 7.42 ± 0.52a | 44.07 ± 0.11** | 12.29 ± 0.32a | 25.78 ± 0.29a |
| At optimized 2 % solids level in coffee | |||||||||
| MS @ 3 % | 6.75 ± 0.76 | 6.92 ± 0.38 | 7.46 ± 0.33a | 7.92 ± 0.20a | 7.96 ± 0.10a | 7.40 ± 0.20 | 48.93 ± 0.08 | 6.23 ± 0.27a | 24.56 ± 0.11 |
| MFLDW@ 2 % | 7.92 ± 0.20** | 7.58 ± 0.38** | 7.92 ± 0.20a | 8.00a | 7.75 ± 0.27a | 7.83 ± 0.10** | 49.60 ± 0.17* | 5.89 ± 0.06a | 22.74 ± 0.03** |
Means with the same superscript in a column are not significantly (P < 0.05) different from each other
*Significant at P < 0.05
**Significant at P < 0.01
Whitening ability in tea and coffee
The whitening ability of a dairy whitener is the most important quality parameter that determines the amount of dairy whitener to be added in tea/coffee (Aneja 1974). MFLDW was evaluated vis-à-vis market sample for their whitening ability in both tea and coffee (Table 3). Whitening ability in terms of L* values of MFLDW in tea and coffee was observed to be significantly (P < 0.01) greater, rather imparting ‘over whiteness’, compared to market sample. Further, MFLDW exhibited significantly (P < 0.01) poorer a* value (redness) in both tea and coffee, but exhibited significantly (P < 0.01) greater b* value (yellowness) only in tea than market sample. While in coffee b* value of MFLDW was observed significantly (P < 0.01) lower compared to market sample mostly due to higher value of L* value.
From the sensory as well as instrumental whiteness it was concluded that at 3 % solids level, MFLDW in both tea and coffee exhibited ‘over whiteness’ and masked natural tea/coffee flavour. Hence, a need was felt to reduce the quantity of solids level of MFLDW in tea and coffee both. Similar observations were reported earlier by Khatkar et al. (2012b) for buffalo milk based full fat liquid dairy whitener and by Khatkar and Gupta (2013) for cow milk based powdered dairy whitener.
Optimization of MFLDW’s quantity in both tea and coffee- Sensory attributes as affected by different solids level of MFLDW in tea/coffee
Standardized MFLDW was evaluated in tea and coffee for sensory attributes at different solids level (3 %, 2.5 % and 2.0 %) (Table 3). Whiteness scores of MFLDW in tea decreased significantly (P < 0.01) from 3 % solids level to 2.5 % and 2 % solids level, while there was no statistical significant difference in flavour scores, though there was apparent flavour improvement, with the decrease in the solids level. Though, at 2 % solids level, MFLDW in tea fetched lower mouthfeel, appearance and overall acceptability scores compared to 3 % solids level, 2 % solids level removed masking effect of MFLDW on tea flavor, thereby improving flavor score, reduced ‘over whiteness’ and saved 33 % product. Thus, the overall acceptability of MFLDW in tea at 2 % solids level was observed to be ‘liked moderately’ to ‘very much’ by judges.
In coffee, flavour scores of MFLDW increased significantly (P < 0.01) and mouthfeel scores decreased significantly (P < 0.01), while there was no statistical significant difference between whiteness, appearance and overall acceptability scores, but with in desirable range, with the decrease in solids level. Even at 2 % solids level, MFLDW was ‘liked moderately’ to ‘liked very much’ by judges. Addition of 2 % solids of MFLDW in coffee removed masking effect of dairy whitener on coffee flavour and imparted rich mouthfeel and appearance and also reduced ‘over whiteness’ of coffee sample. The optimized solids level of MFLDW to be added to both tea and coffee was observed to be 2 % by judges not only on the bases of flavour and mouthfeel but also on the bases of natural whiteness.
Whitening ability as affected by different solids level of MFLDW in tea/coffee
MFLDW in tea and coffee (Table 3) was evaluated for whitening ability at different solids level (3 %, 2.5 % and 2.0 %). Whitening ability in terms of L* value of MFLDW in tea and coffee was significantly (P < 0.01) decreased with decrease in milk solids level. MFLDW at 2 % solids level showed natural tea and coffee colour and reduced the ‘over whiteness’ of tea/coffee samples. Individually, in tea there was significantly (P < 0.01) decreased in b* value with the decrease in milk solids level, but a* value showed significantly (P < 0.01) decrease only between 3 % solids level and 2 % solids level. While in coffee, there was no statistical variations in a* and b* values which indicated redness and yellowness, respectively, which revealed that there was no much effect on natural coffee colour with respect to change in solids level of MFLDW expect over whiteness. MFLDW at 2 % solids level in tea and coffee resulted in natural tea colour and right whiteness. Judges also observed attractive whiteness and natural tea and coffee colour at 2 % solids level of MFLDW.
From the sensory as well as instrumental whiteness, it was concluded that at 2 % solids level, MFLDW in both tea and coffee exhibited good whiteness and fetched good flavour and mouthfeel scores. Hence, the optimized solids level of MFLDW to be added to both tea and coffee were observed to be 2 %. Similar observations were reported earlier by Khatkar et al. (2012b) and Khatkar and Gupta (2013) for dairy whitener.
Sensory evaluation of MFLDW at optimized 2 % solids level in tea and coffee
Tea and coffee samples were prepared with the optimized 2 % solids level of MFLDW and subjected to sensory evaluation vis-à-vis market sample (added at 3 % solids level) (Table 3). MFLDW in tea fetched significantly (P < 0.01) greater whiteness scores compared to market sample. The flavour, mouthfeel, appearance and overall acceptability scoring of MFLDW in tea was ‘liked moderately’ to ‘very much’ as compared to ‘liked moderately’ of market sample. Flavour, mouthfeel and overall acceptability scores of coffee with MFLDW were observed significantly (P < 0.01) greater compared to with of market sample. The overall acceptability of MFLDW in coffee was ‘liked very much’ compared to ‘liked moderately’ to ‘liked very much’ of market sample. There was no statistically significant difference in appearance and sweetness scores of both samples. From the sensory evaluation, it was observed that even at lesser quantity at 2 % solids level, MFLDW imparted better sensory attributes in tea and coffee compared to market sample at 3 % solids level. Thus, there could be clear 33 % solids quantity saving in case of developed product compared to market sample.
Whitening ability of MFLDW at optimized 2 % solids level in tea and coffee
Whitening ability in terms of L* value of MFLDW in tea (Table 3) was observed significantly (P < 0.01) greater compared to those of market sample (added at 3 % solids level). There was no statistical variations in a* value and b* value of both the samples in tea. Higher a* value and b* value exhibited by MFLDW in tea was desirable as it indicated redness and yellowness and imparted natural colour to tea, compared to market sample. While in coffee, whitening ability in terms of L* value of MFLDW (Table 3) was significantly (P < 0.05) greater compared to those of market sample. MFLDW in coffee exhibited significantly (P < 0.01) lesser b* value. Lower a* and b* value, as it indicated redness and yellowness, respectively, which provided desirable colour to coffee sample, compared to light colour of market sample. Similar pattern of L*, a* and b* values in coffee were reported earlier by Jimenez-Flores and Kosikowski (1986) for non fat dairy coffee whitener. Judges also observed natural tea and coffee colour and good whiteness of tea and coffee samples with MFLDW at 2 % solids level.
This revealed that even at lesser quantity (at 2 % solids level), MFLDW exhibited greater whitening ability in both tea and coffee than market sample (at 3 % solids level). Similar observations were also reported earlier by Khatkar et al. (2012b) for buffalo milk based full fat liquid dairy whitener. Hence, the possibility of saving 33 % MFLDW compared to market sample, thereby decreasing the cost of dairy whitener formulation per cup of tea/coffee, was confirmed. Eventually, this study will grant a ready to transfer technology to the dairy processors that would be able to prepare value added good quality milk protein-based dairy whitener employing UF process.
Conclusion
The developed medium fat liquid dairy whitener exhibited good heat stability with the addition of 0.4 % mixture of monosodium and disodium phosphate (2:1 w/w) and also showed significant (P < 0.01) improvement in the bland flavour of formulation with the addition of 0.2 % potassium chloride. But with the addition of 18 % sugar (on DMB) and 0.2 % potassium chloride, the heat stability of medium fat liquid dairy whitener was reduced substantially to 19 min. Further, heat treatment with 85 °C/5 min, the heat stability of formulation was improved to a reasonable level of 27 min. Developed MFLDW also showed significantly (P < 0.01) better whitening ability when homogenized at 175.76 kg/cm2 and 35.15 kg/cm2 in first stage and second stage, respectively, in both tea and coffee without leaving any feathering/suspended particles. From the sensory evaluation and instrumental whitening ability, it was observed that even at lesser quantity at 2 % solids level, MFLDW imparted better sensory attributes and good/natural whitening ability in tea and coffee compared to market sample at 3 % solids level. Thus, there could be clear 33 % solids quantity saving in case of developed product compared to market sample.
Acknowledgments
The first author is thankful to the Director, National Dairy Research Institute, Karnal for providing three and half year financial assistance in the form of Institutional Senior Research Fellowship for carrying out this work.
Contributor Information
Sunil Kumar Khatkar, Email: absuneelkhatkar@gmail.com.
Vijay Kumar Gupta, Email: vkg@ndri.res.in.
Anju Boora Khatkar, Email: abkhatkar@gmail.com.
References
- Aneja RP (1974) Whitening properties of milk constituents in coffee creamers. In: 19th International Dairy Congress, 1E: 748–749
- Bassett HJ, Shelly DS, Anderson ME. Processing coffee whiteners (Cream substitutes from hydrogenated vegetable oil) Am Dairy Rev. 1978;40(2):35–37. [Google Scholar]
- BIS (2001a) Chemical examination of milk. In: Hand book of food analysis: (Part XI) Dairy products. Bureau of Indian Standards (3rd reprint). Manak Bhavan, New Delhi, pp 21–45
- BIS (2001b) Condensed milk and dried milk. In: Hand book of food analysis: (Part XI) Dairy products. Bureau of Indian Standards (3rd reprint). Manak Bhavan, New Delhi, pp 120–126
- BIS:12299 (1998) Dairy whitener-Specification (first revision). Manak Bhavan, New Delhi-110002
- Davies DT, White JCD. Determination of calcium and magnesium in milk and diffusate. J Dairy Res. 1962;29:285. doi: 10.1017/S0022029900011109. [DOI] [Google Scholar]
- IDF (2008) International Dairy Federation, “The world dairy situation 2008”, Bulletin of the IDF, no. 432/2008, International Dairy Federation, Brussels, Kingdom of Belgium, 2008
- Jiménez-Flores R, Kosikowski FV. Nonfat dairy coffee whitener made from ultrafiltered skimmilk retentates. J Food Sci. 1986;51(1):235–236. doi: 10.1111/j.1365-2621.1986.tb10881.x. [DOI] [Google Scholar]
- Kelly P, Oldfield D, O’Kennedy B. The thermostability of spray dried imitation coffee whiteners. Int J Dairy Technol. 1999;52:107–113. doi: 10.1111/j.1471-0307.1999.tb02082.x. [DOI] [Google Scholar]
- Khatkar SK, Gupta VK. Physico-chemical & functional quality attributes of dairy whitener prepared from ultrafiltration process. J Food Proc Preser. 2013 [Google Scholar]
- Khatkar SK, Gupta VK, Kumar S. Effect of homogenization, stabilizing and flavoring salts on the quality of liquid dairy whitener from buffalo milk employing ultrafiltration process. Indian J Dairy Sci. 2012;65(2):115–121. [Google Scholar]
- Khatkar SK, Gupta VK, Khatkar AB. Studies on quality attributes of liquid dairy whitener prepared from ultrafiltration process in tea and coffee. Indian J Dairy Sci. 2012;65(4):265–273. [Google Scholar]
- Kieczewska K, Kruk A, Czerniewicz M, Warminska M, Haponiuk E. The effect of high-pressure homogenization on changes in milk colloidal and emulsifying systems. Pol J Food Nutr Sci. 2003;12(Suppl.1):43–44. [Google Scholar]
- Lawrence AJ. The determination of lactose milk products. Austr J Dairy Technol. 1968;23:103–104. [Google Scholar]
- Maneffee SC, Overman OD. A semimicro-Kjeldahl method for the determination of total nitrogen in milk. J Dairy Sci. 1940;23:1177–1185. doi: 10.3168/jds.S0022-0302(40)92829-6. [DOI] [Google Scholar]
- Mehaia MA. Studies on the rennet coagulation of skim camel milk concentrated by ultrafiltration. J King Saud Univ. 1997;9(1):11–123. [Google Scholar]
- Oldfield D, Singh H. Functional properties of milk powder. In: Onwulata C, editor. Encapsulated and powdered foods. New York: CRC Press; 2005. pp. 365–386. [Google Scholar]
- Oldfield DJ, Teehan CM, Kelly PM. The effect of preheat treatment and other process parameters on the coffee stability of instant whole milk powder. Intl Dairy J. 2000;10(9):659–667. doi: 10.1016/S0958-6946(00)00088-1. [DOI] [Google Scholar]
- Patel RS, Mistry VV. Physicochemical and structural properties of ultrafiltered buffalo milk and milk powder. J Dairy Sci. 1997;80:812–817. doi: 10.3168/jds.S0022-0302(97)76002-8. [DOI] [Google Scholar]
- Patel RS, Gupta VK, Singh S, Reuter H. Ultrafiltration behaviour of buffalo milk and cow milk. Indian J Dairy Sci. 1992;45(6):322–325. [Google Scholar]
- Singh RP. Sensory evaluation of food. In: Singh RP, editor. Computer applications in food technology: Use of spreadsheets in graphical, statistical, and process analyses. San Diego: Academic Press; 1996. pp. 160–176. [Google Scholar]
- Singh H. Heat stability of milk. Intl J Dairy Technol. 2004;57(2–3):111–119. doi: 10.1111/j.1471-0307.2004.00143.x. [DOI] [Google Scholar]
- Sweetsur AWM, Muir DD. Effect of concentration by ultrafiltration on the heat stability of skim milk. J Dairy Res. 1980;47:327–335. doi: 10.1017/S002202990002121X. [DOI] [Google Scholar]
- Tayal M, Sindhu JS. Heat stability and salt balance of buffalo milk as affected by concentrate and addition of casein. J Food Proc Preser. 1983;7:151–160. doi: 10.1111/j.1745-4549.1983.tb00673.x. [DOI] [Google Scholar]
- Thiebaud M, Dumay E, Picart L, Guiraud JP, Cheftel JC. High–pressure homogenization of raw bovine milk. Effects on fat globule size distribution and microbial inactivation. Intl Dairy J. 2003;13:427–439. doi: 10.1016/S0958-6946(03)00051-7. [DOI] [Google Scholar]