Abstract
Shelf life of two products namely chikki and oilseed butter were evaluated. Sunflower was substituted for groundnut at three levels (0, 50 and 100 %). Products were stored up to 2 months in ambient conditions (25–30 °C; RH 40–60 %). Chikki was packed in Low density polyethylene (LDPE) and laminated pouches and oil seed butter was stored in glass and plastic jars. Products were evaluated for sensory characteristics, absence of rancidity; per cent free fatty acid and peroxide value. Stored chikki was evaluated for microbial load. Products were acceptable for sensory attributes even at the end of storage period. Product chikki stored in laminated pouches had higher per cent free fatty acid and peroxide value compared to that stored in Low density polyethylene (LDPE) pouches. Oilseed butter stored in glass jar had higher per cent free fatty acid when compared to that stored in plastic jar. Stored chikki had higher microbial load in the Low density polyethylene (LDPE) when compared to that stored in laminated pouches. Products made with groundnut alone (control) were preferred over those made in combination with sunflower and groundnut (1:1) or sunflower alone. However all products were highly acceptable at the end of storage period.
Keywords: Sunflower kernels, Chikki, Oilseed butter, Storage stability
Introduction
Sunflower is one of the major oilseeds crops ranking fourth with a worldwide production of about 37.07 million metric tons in 2012 (FAO-STAT 2013). Sunflower (Helianthus annuus L.) noted as a rich source of edible oil and is an introduced crop in India (Miller et al. 1992). Per 100 g the seed is made-up to enclose protein 20.78 g, total lipid (fat) 51.46 g, ash 3.02 g, carbohydrate 20 g and fiber 8.6 g with total energy of 2,445 kj, high levels of potassium (710 mg/100 g) and magnesium (390 mg/100 g) and is especially rich in polyunsaturated fatty acids (approximately 31.0 %) in comparison with other oilseeds (USDA 2008; McCance and Widdowson 2002). Sunflower seed contains an appreciable amount of vitamin E—37.8 mg/100 (Murillo et al. 1999; McCance and Widdowson 2002). Tocols (tocopherols and tocotrienols) that possess vitamin E activities are recognized as the most important tissue antioxidants, having a role in preventing or controlling non-specific reactions from various oxidizing species produced in normal metabolism (Škrbića and Filipčevb 2008). Sunflower seed more appropriately the sunflower kernel is now gaining popularity and becoming readily available for use in bakery products. Sunflower kernels are considered as ‘healthy’ product. Sunflower kernels have a pleasant flavor and a nutty texture. Sunflower kernels add a unique mild taste and a crunch to baked goods (Hofland 1990). Many researchers have also advocated the incorporation of either defatted flour or roasted sunflower grits for protein enrichment of bakery products (Leelavathi et al. 1991; Bajaj et al. 1991; Gupta et al. 1996; Gatta and Piergiovanni 1996; Škrbića and Mačvanin 2011; Salem et al. 2012). Roasted peanuts, almonds, cashew nuts etc. are few popular snacks foods but are costly; sunflower seed kernels may be cheaper substitutes. The lipid profile of the sunflower seed is unique; however rancidity due to high poly unsaturated fatty acids content is a problem (Yoshida et al. 2006). It is important to assess changes during storage when sunflower is incorporated in prepared food products. The proper selection of sunflower cultivar is important for specific product development. There are two types of sunflower seeds, oil type and confectionery type (Fernández-Martínez et al. 2009). Globally confectionery types of seeds are preferred for product development. The confection type seeds are specially designed for table purpose but have not been made popular in the country. In Karnataka, only oil type sunflower cultivars are grown. Recently attempts to grow sunflower kernels for preparing products are being made (Shadakshari, Y. G. Geneticist, AICRP Sunflower, GKVK, UAS, Bengaluru, Personnel communication 2010). Several cultivars such as KBSH 1, KBSH 41, KBSH 42, KBSH 44, KBSH 53, Surajmukhi etc., (Pallavi et al. 2011) are available. Screening some of varieties showed that KBSH 44 was having a good hullability (Muttagi, GKVK, UAS, Bengalu, unpublished work, 2009). Therefore this variety was used in the study. Sunflower has occupied a large area of cultivation and has probably replaced the cultivation of staple. However sunflower is only used as an oil crop and is not being used as a food crop. This has a very serious impact on food security. There is paucity of literature on the use of sunflower as food (Fernández-Cuestaa et al. 2012). Therefore looking at the impact of using the sunflower as a food crop to augment food security this study was planned. Much research on sunflower has been done on the agricultural aspects of sunflower, but studies of the acceptability and stability of sunflower products are limited. Thus keeping in view health benefits, nutrients, taste and economics of sunflower kernels, the present investigation, to evaluate added value products prepared from sunflower seed kernels for their acceptability and stability at ambient conditions was conducted. Two types of products, one made from whole kernels and another from pulverized seed were evaluated.
Materials and methods
Sunflower cultivar KBSH-44 was procured in bulk from AICRP on Sunflower, GKVK, UAS, Bengaluru. The seeds were mechanically dehulled and were stored at 4 °C and used for making the products.
Products
Two products were standardized chikki (a toffee prepared with jaggery—a crude cane sugar) and oilseed butter. Sunflower was substituted for groundnut at three levels (0, 50 and 100 %). To prepare chikki weighed amount of jaggery was heated in a pan with little amount of water to a temperature of approximately 115 to 118 °C. At this temperature weighed amount of roasted sunflower seed kernels were transferred to the pan and mixed well and cooked for 30 s to attain a semisolid consistency. Hot mixture was poured to an oiled wooden board and was spread and rolled to a thickness of 5 mm. The rolled spread was then cut in to square slabs and removed from the board and cooled to room temperature.
To prepare oilseed butter weighed amount of sunflower seed kernels were roasted in a pan. The kernels were finely ground in a mixing blender and poured in to a mixing bowl. Weighed amount of melted ghee, sugar and salt were added and mixed well. The mixture was beaten till it became creamy in texture by using a rotary beater. Butter was transferred to a glass or plastic container and stored.
Storage condition
The products were stored up to 2 months at ambient condition (25–30 °C and RH 40–60 %). Two types of packaging materials low density polyethylene (LDPE, 350 gauge) and laminated pouch were used. Sensory evaluation was performed and chemical parameters like per cent free fatty acid and peroxide value were determined. The storage stability was evaluated by evaluating the products when they were fresh and at the end of 2 months. The parameters for evaluation were sensory characteristics, acid value, peroxide value and microbial load.
Sensory evaluation
All the developed products were evaluated by a panel of semi trained judges (n = 20) for fresh, 30 days and 60 days. The products were evaluated for appearance, texture, aroma, taste, overall acceptability and absence of rancidity on a five point hedonic scale. Where 5 = excellent, 4 = good, 3 = neither good nor bad, 2 = poor, 1 = very poor (Amerine et al. 1965).
Peroxide value and free fatty acid and
The oil was extracted from the products in a mixture of chloroform and methanol (2:1 V/V) (Bligh and Dyer 1959). Peroxide value and percent free acid of oil was determined according to AOAC procedure (AOAC 1980). Percent free fatty acids (FFAs) were calculated using oleic acid as a factor.
Microbial load
Initial and final microbial counts were determined. The microbial contamination was estimated by analyzing microbial load in chikki by using Nutrient Agar for bacteria and Martins Rose Bengal Agar for mould count and Davis yeast extract agar for yeasts following the dilution pour plate method. 10−2, 10−3, 10−4, were the dilutions used for analyzing bacteria and 10−1, 10−2, 10−3 for moulds and 10−3, 10−4, 10−5 for yeasts (Anon 1957; Martin 1950).
Statistical analysis
Mean and standard deviations was worked out for all the parameters in the study. Two-way analysis of variance was employed in testing the significant difference between varieties, between methods and storage periods.
Results and discussion
Although sunflower is grown extensively in Karnataka, use of its kernel is very limited. It can be used as a substitute for groundnuts. Therefore two products which are commonly made with groundnut were standardized using sunflower kernels in combination with groundnut (1:1). These were compared with products made only with groundnut. It was reported that sensorily acceptable and nutritionally improved bread can be made with as much as 16 % of sunflower seeds on flour basis (Škrbića and Filipčevb 2008). It was also found that high protein cookies of superior acceptability can be made with the addition of 20 % sunflower seed and 30 % of wheat germ (Bajaj et al. 1991). Compared to these reports higher per cent of sunflower seeds was incorporated. Storage stability was anticipated as major factor in the acceptability of products due to high PUFA (Poly Unsaturated Fatty Acids) content. Sunflower is rich in polyunsaturates and low in saturates. Because of high level of PUFA, sunflower oil is susceptible to oxidation during roasting (Yoshida et al. 2001, 2002). The most important factor affecting the storage of sunflower seeds is their moisture content (Abreu et al. 2013). Seeds with high moisture content are susceptible to quality deterioration at high temperature because of hydrolysis of the oil and phospholipids and an increase in acidity (Dios 1996). In the present study, the initial moisture content of sunflower kernels and groundnuts were 4.48 % and 3.47 % respectively. Lipid oxidation is one of the major reasons that foods deteriorate and is caused by the reaction of fats and oils with molecular oxygen leading to off flavours that are generally called rancidity. Exposure to light, pro-oxidants and elevated temperature will accelerate the reaction. Rancidity is associated with characteristic off-flavour and odour of the oil (Hamilton 1994). The results are discussed under following headings.
Sensory characteristics
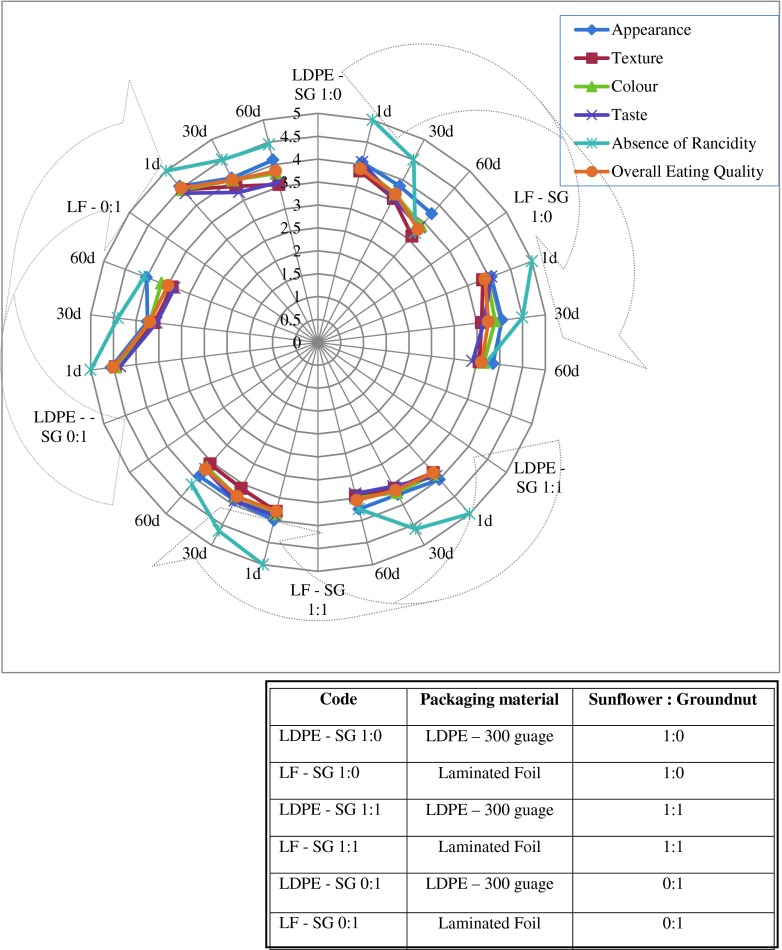
Effect of storage on sensory characteristics and rancidity for product chikki stored in LDPE and laminated pouches are shown in Fig. 1. Chikki is an indigenous product similar to butterscotch made from Indian crude brown sugar called “jaggery” and groundnuts. It was observed that almost all the scores for sensory parameters dropped marginally throughout the duration of storage. However, they remained close to the acceptable level even at the end of storage period. By the end of the storage periods there was an increase in rancidity as revealed from the lower scores for the variable “absence of rancidity”. It may be noted that, even for this attribute the score was within the acceptable limits at the end of storage period. There were no statistically significant differences in the scores for the different durations of storage (Table 1). Chetana and Sunkireddy (2011) evaluated the sensory characteristics of peanut chikki, Crisp texture was observed up to 45 days under accelerated conditions and up to 60 days under ambient conditions, after which the chikkis lost their crisp and crunchy texture. Chikki became soggy and lost its characteristic crunchy texture. Sensory scores of all desirable attributes decreased slightly at both ambient and accelerated conditions at the end of 30 days when compared to the initial values (Table 2) but were still acceptable. Slight off taste and rancidity was evident in chikki without added antioxidant at the end of 30 days of storage at accelerated temperature, compared to that with antioxidant thus increasing the shelf life of the product. In the present study the product remained viable for a longer period.
Fig. 1.
Effect of storage duration and type of packaging on sensory score of chikki developed with sunflower seeds and groundnut
Table 1.
Statistical significance of effect of storage and type of packaging on sensory score of products developed with sunflower seeds and groundnut
| Product | Appearance | Texture | Colour | Taste | Absence of rancidity | Overall acceptability | Appearance | Texture | Colour | Taste | Absence of rancidity | Overall acceptability | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chikki | Oil seed butter | ||||||||||||
| SG (1:1) | Packaging | LDPE and laminated pouch | Glass and plastic jars | ||||||||||
| F-value | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| SEm± | 0.09 | 0.11 | 0.1 | 0.12 | 0.11 | 0.1 | 0.1 | 0.11 | 0.1 | 0.11 | 0.13 | 0.1 | |
| CD | – | – | – | – | – | – | – | – | – | – | – | – | |
| Duration | Initial (0 days) and 60 days | Initial (0 days) and 60 days | |||||||||||
| F-value | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| SEm± | 0.12 | 0.14 | 0.13 | 0.14 | 0.14 | 0.13 | 0.13 | 0.13 | 0.13 | 0.14 | 0.16 | 0.12 | |
| CD | – | – | – | – | – | – | – | – | – | – | – | – | |
| SG (1:1) | Packaging | LDPE and laminated pouch | Glass and plastic jars | ||||||||||
| F-value | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| SEm± | 0.08 | 0.1 | 0.09 | 0.1 | 0.09 | 0.1 | 0.11 | 0.1 | 0.09 | 0.1 | 0.1 | 0.09 | |
| CD | – | – | – | – | – | – | – | – | – | – | – | – | |
| Duration | Initial (0 days) and 60 days | Initial (0 days) and 60 days | |||||||||||
| F-value | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | ns | |
| SEm± | 0.09 | 0.13 | 0.11 | 0.12 | 0.11 | 0.12 | 0.13 | 0.13 | 0.12 | 0.12 | 0.13 | 0.11 | |
| CD | – | – | – | – | – | – | – | – | – | – | 0.35 | – | |
| SG (0:1) | Packaging | LDPE and laminated pouch | Glass and plastic jars | ||||||||||
| F-value | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| SEm± | 0.073 | 0.09 | 0.09 | 0.11 | 0.08 | 0.09 | 0.09 | 0.1 | 0.09 | 0.11 | 0.1 | 0.08 | |
| CD | – | – | – | – | – | – | – | – | – | – | – | – | |
| Duration initial (0 days) and 60 days | Initial (0 days) and 60 days | ||||||||||||
| F-value | ns | ns | ns | ns | ns | * | ns | ns | ns | ns | * | * | |
| SEm± | 0.09 | 0.08 | 0.11 | 0.13 | 0.09 | 0.1 | 0.11 | 0.12 | 0.12 | 0.13 | 0.13 | 0.1 | |
| CD | – | – | – | – | – | 0.3 | – | – | – | – | 0.4 | 0.3 | |
SG: Sunflower: Groundnut
*Significant at 5 % level nsNon-significant at 5 % level
Table 2.
Effect of storage duration and type of packaging on free fatty acid and peroxide value of products developed with sunflower seeds and groundnut
| Packaging materiala | Duration (days) | Per cent free fatty acid | Peroxide value (meq/kg) | |||
|---|---|---|---|---|---|---|
| Chikki | Oil seed butter | Chikki | Oil seed butter | |||
| Sunflower | LDPE1/plastic jar2 | 0 (fresh) | 0.61 | 0.86 | 5.90 | 8.58 |
| 60 | 1.57 | 1.90 | 18.10 | 23.41 | ||
| Laminated foil1/glass jar2 | 0 (fresh) | 0.61 | 0.86 | 5.90 | 8.58 | |
| 60 | 1.28 | 1.86 | 17.64 | 22.87 | ||
| Packaging | ||||||
| F-value | * | * | * | * | ||
| SEm± | 0.0126 | 0.0092 | 0.045 | 0.026 | ||
| CD | 0.037 | 0.0272 | 0.132 | 0.0812 | ||
| Duration | ||||||
| F-value | * | * | * | * | ||
| SEm± | 0.012 | 0.009 | 0.045 | 0.026 | ||
| CD | 0.0371 | 0.027 | 0.1323 | 0.081 | ||
| Sunflower + groundnut (1:1) | LDPE1/plastic jar2 | 0 (fresh) | 0.63 | 0.81 | 7.39 | 7.84 |
| 60 | 1.09 | 1.73 | 16.04 | 22.68 | ||
| Laminated foil1/glass jar2 | 0 (fresh) | 0.63 | 0.81 | 7.39 | 7.84 | |
| 60 | 0.93 | 1.70 | 15.12 | 21.11 | ||
| Packaging | ||||||
| F-value | * | ns | ns | * | ||
| SEm± | 0.016 | 0.0118 | 0.25 | 0.1118 | ||
| CD | 0.047 | – | – | 0.32 | ||
| Duration | ||||||
| F-value | * | NS | NS | * | ||
| SEm± | 0.0161 | 0.0118 | 0.25 | 0.1118 | ||
| CD | 0.047 | – | – | 0.32 | ||
| Groundnut | LDPE1/plastic jar2 | 0 (fresh) | 0.62 | 0.79 | 6.30 | 7.37 |
| 60 | 0.85 | 1.4 | 14.16 | 18.43 | ||
| Laminated foil1/glass jar2 | 0 (fresh) | 0.62 | 0.79 | 6.30 | 7.37 | |
| 60 | 0.73 | 1.36 | 13.21 | 18.26 | ||
| Packaging | ||||||
| F-value | * | * | * | ns | ||
| SEm± | 0.0083 | 0.0131 | 0.0276 | 0.0442 | ||
| CD | 0.024 | 0.039 | 0.081 | – | ||
| Duration | ||||||
| F-value | * | * | * | ns | ||
| SEm± | 0.0083 | 0.0131 | 0.028 | 0.0442 | ||
| CD | 0.024 | 0.039 | 0.081 | – | ||
*Significant at 5 % level nsNon-significant at 5 % level
aPackaging material: 1. LDPE and laminated foil—packaging material for Chikki. 2. Glass jar and plastic jar—packaging material for oilseed butter
In the present study the level of protein and micro element contents slightly increased with sunflower seeds alone and the incorporation in to the products (Data not shown). Many researchers also found increase in protein, fibre and micro elements when sunflower seeds are incorporated in their products (Škrbića and Filipčevb 2008; Škrbić and Cvejanov 2011; Srilatha and Krishnakumari 2003). Addition of sunflower seeds alone and along with barley helped to raise its protein, micro element contents and improved sensory properties of the cookies (Škrbić and Cvejanov 2011). Therefore the products made from sunflower and barley can support nutritional security. Anjum et al. (2012) in their review on therapeutic potential of sunflower seeds, have also supported that sunflower seeds are an unique nutritional and nutraceutical package.
Between the different types of packaging materials there were no statistical differences. It can be concluded that both laminated pouches and LDPE pouches were suitable for packaging this type of product. It has been demonstrated by other workers that type of packaging influences the sensory characteristics of stored products. Pajin et al. (2006) reported the quality and shelf-life of product dragee (similar to chikki) obtained by coating a confectionery sunflower kernel with sugar syrup. The product was packed in oriented polypropylene/oriented polypropylene; oriented polypropylene/metalized oriented polypropylene, polyester/polyethylene and kept at room temperature in daylight for 5 months. At the beginning of the experiment, the dragee product was in the category of excellent sensory quality in terms of its colour, smell, taste, mastication and structure. During the storage, these properties changed and the product lost its stability. Gupta et al. (2010) studied packaging and storage aspects of coconut burfi to extend its shelf life. The products were packed in flexible pouches of multilayer films consisting of polyester/polyethylene; and polyester/aluminum foil/polypropylene under the following conditions: normal packing; 99 % vacuum packaging; and in-package heat processing, and were stored at 27 °C temperature and 65 % relative humidity and periodically withdrawn to monitor changes in chemical, microbiological and sensory qualities. A maximum of 75 days shelf life was obtained for heat-processed samples as against <15 and 45 days for air and vacuum packed samples respectively in both packaging materials. Satyanarayana Rao et al. (1990) developed two traditional Indian sweet meats, Sugar holige and Modaka, which were packed in polypropylene and then paper foil aluminium polyethylene laminate pouches and stored at various temperatures. These two items were found to be acceptable for a period of 2 months at 37 °C and 4 months at ambient temperatures, without use of any preservatives. In the present study the products were acceptable up to 60 days of storage period without use of any preservatives and vacuum packaging.
Mean scores of sensory characteristics including rancidity of oilseed butter stored in glass jar and plastic jar are shown in Fig. 2. Oilseed butter stored in glass jar recorded slightly higher scores for some of the sensory characters. The butter stored in glass and plastic jar recorded acceptable scores for the first and second month of storage. Oilseed butter in glass container was found to be somewhat superior compared to that stored in plastic container; however, this difference was not statistically significant. Sunflower butter was found to have a good overall nutritional value with a protein quality approximately equal to that of peanuts (Dreher et al. 1983). Thus, sunflower seeds can be used to create oil seed butter with a different nutritional and taste characteristics.
Fig. 2.
Effect of storage duration and type of packaging on sensory score of oilseed butter developed with sunflower seeds and groundnut
Duration of storage had expectedly a greater influence on the rancidity scores of the product. Rancidity was perceived as the most important trait in the deterioration of the product. Roasting conditions had a significant impact on nutritional and sensory quality, color and spreadability of sunflower butter. Dreher et al. (1983) reported that taste panelists generally rated sunflower butter lower than peanut butter as more intensive roasting resulted in poor product. However in the present study the product with 50 % level of incorporation of sunflower seed kernels was statistically significantly superior to peanut butter. The different levels of incorporation did not affect the scores for appearance, texture and color. The lower scores seen in stored samples of oilseed butter were also attributed to the separation of oil. At the end of storage period separation of oil to the extent of 6.15 % v/v was observed in oilseed butter made with combination of sunflower and groundnut (1:1). This may be due to poor emulsification due to an imbalance of saturated and unsaturated fats. Gills and Resurreccion (2000) reported that shelf-life of unstabilized peanut butter (UPB) stored at 21, 30, and 45 °C was 75 days. Peanut butter with 2.5 % palm oil had a shelf-life of 113 days. Totlani and Chinnan (2007) also found that stabilizer concentrations of 1.0, 1.5 and 2.0 % levels were found to be adequate in stabilizing peanut butter samples at 35 °C for 3 months. Therefore by using stabilizers such as palm oil and hydrogenated vegetable oil, the shelf life of oil seed butter can be improved (Lima and Guraya 2005; Aryana et al. 2003). In the present study no stabilizers were used hence the separation of oil in the product. Sattar et al. (1990) reported the effect of packages on oxidative deterioration of dry nuts and it increased in all samples during storage but was lowest in samples stored in amber colored bottles, black polyethylene (PE) or silver colored polyethylene. In the present study transparent glass bottle and plastic bottles are used hence the deterioration of the oilseed butter.
Per cent free fatty acid and peroxide value (PV) of stored products
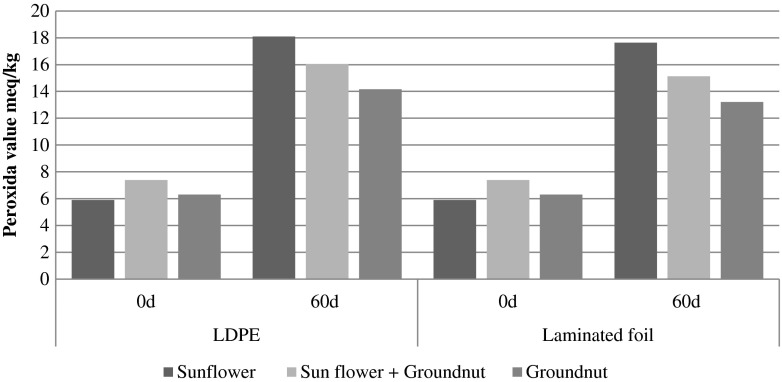
Free fatty acid content reflects the degree of hydrolysis and peroxide value represents the amount of oxygen associated with rancidity (Bax et al. 2004). The PV expressed as meq O2/Kg oil, accepted for commercial oil is 10 meq O2/Kg. Storage resulted in a marked increase in the free fatty acid content in all samples of chikki as well as oilseed butter (Figs. 3 and 4). Chikki had slightly lower values for free fatty acid compared to oil seed butter. Samples containing groundnut had lower free fatty acid content at the end of storage period. The higher mono unsaturated fatty acid content in groundnut exerted a protection against a higher raise in the free fatty acid levels during storage (Budin and Breene 1993). The percent free fatty acid values in chikki were 0.61 to 0.63 % in fresh samples, upon storage these values recorded an increase (0.85 to 1.57 % and 0.73 to 1.28 % in LDPE and laminated foil pouches respectively). The per cent free fatty acid values in oilseed butter were 0.79 to 0.86 % in fresh samples, upon storage these values increased (1.40 to 1.90 % and 1.36 to 1.86 % in plastic and glass jars respectively).
Fig. 3.
Graphical representation of effect of storage and type of packaging on free fatty acid value of chikki developed with sunflower seeds and groundnut
Fig. 4.
Graphical representation of effect of storage and type of packaging on free fatty acid value of oilseed butter developed with sunflower seeds and groundnut
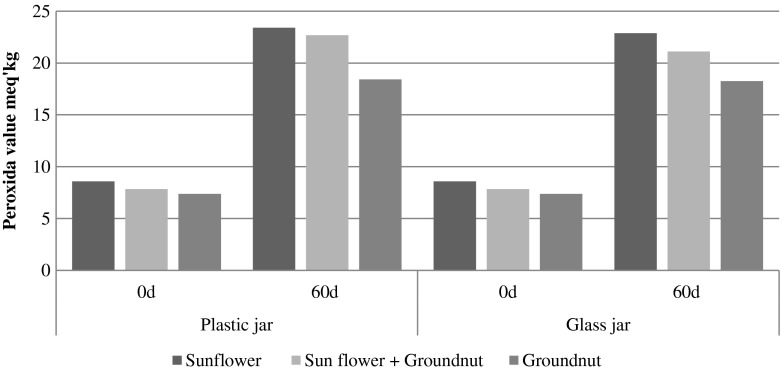
The peroxide values in chikki were 5.9 to 7.39 meq/kg in fresh samples, after storage they were between 14.16 and 18.10 meq/kg and 13.21 to 17.64 meq/kg in LDPE and laminated foil pouches respectively (Fig. 5). Similar findings were reported by Chetana and Sunkireddy (2011), the peroxide value of oil in peanut chikki increased gradually at 37 °C on storage. At the end of 60 days at 37 °C, rancidity developed in samples without antioxidant but not in that with added antioxidant. The peroxide values in oilseed butter were 7.37 to 8.58 meq/kg in fresh samples, upon storage these rose to 18.26–23.41 meq/kg and 18.26–22.87 meq/kg in plastic and glass jars respectively (Fig. 6). It can be seen that during storage there are changes in free fatty acid content and peroxide value, because of changes in fats of kernel. Changes in free fatty acid content and peroxide value of the product based on sunflower kernel depend on initial moisture content of the product and storage conditions (temperature, humidity) and the packaging material used (Fritsch et al. 2006). In the present study the moisture content of sunflower seeds were found to be higher compared to that of groundnut seeds. It is clear that the transparent packaging material used did not give satisfactory results in keeping the product for longer period of storage (60 days). Pajin et al. (2006) reported that peroxide value in the dragee product increased from 0.5 to 9.0 mmol O/kg during the 5 months of storage. The free fatty acid content also increased from 1.3 % in fresh dragee product to 2.5 % after 5 months of storage. They concluded that packaging material metalized polyester/polyethylene, labeled met PET/PE was most suitable for storage of dragee product. The packaging material metalized polyester/polyethylene, labeled metPET/PE, has the lowest oxygen permeability (8.0 mL m−2 /dan Δp 1 bar) and because of this it had the strongest influence in the prevention of hydrolytic and oxidative changes in the final product. LDPE and laminated foil, glass and plastic jars are common packaging materials used for packaging these types of materials as stored in the Indian stores. Taking in to account the different parameters evaluated. It may be said that the keeping quality of sunflower chikki was on par with groundnut chikki, while the shelflife of sunflower chikki shorter than groundnut chikki. The values between 20 and 40 meq/kg results in rancid taste (Akubugwo and Ugbogu 2007). Lee et al. (2004) suggested that the greater the roasting time, the greater would be the increase in peroxide value. In sunflower kernels linoleic acid and linolenic acids predominate, which have more double bond in the molecule. These oxidize more rapidly than oleic acid, which has one double bond (Anjum et al. 2006). In the present study the groundnut products were more stable compared to sunflower seed products. This might be due to high oleic acid present in groundnut seeds. Budin and Breene (1993) also reported dry roasted high oleic acid kernels were more stable than dry roasted high linoliec acid kernels. Özge et al. (2009) also reported roasting may destroy some bioactive compounds, but it can also form antioxidant compounds through the Maillard reaction. Preventive measures especially the use of antioxidants such as Vitamin E, ascorbic acid may be tried in future to combat the increase in free fatty acid and peroxide value.
Fig. 5.
Graphical representation of effect of storage and type of packaging on peroxide value of chikki developed with sunflower seeds and groundnut
Fig. 6.
Graphical representation of effect of storage and type of packaging on peroxide value of oilseed butter developed with sunflower seeds and groundnut
Microbial load of stored chikki
Microbial load i.e. bacteria, fungi and yeast count was determined for the three variations of chikki and results are depicted in Table 3. The microbial load of stored chikki, both in LDPE and laminated pouches substantially increased throughout the storage period. The chikki stored in LDPE pouches had higher microbial counts as compared to that stored in laminated pouches. When analyzed statistically there was a significant difference up to the end of second month of storage between two packaging materials. The microbial counts were found lower in groundnut chikki compared to those made with sunflower and groundnut in the ratio of 1:1, and sunflower alone. Robertson et al. (1984) reported that at 13.4 % moisture content, FFA (Free fatty acids) increased significantly during storage and were positively correlated with the invasion of seed by Aspergillus. In present study, similar effect of rising free fatty acid and the increased fungi count was observed. Miller et al. (1986) reported that confectionery type sunflower seed showed a greater sensitivity to moisture content and fungal attack as reflected by the higher free fatty acid values, compared to the high-oil seed type. Higher free fatty acid values indicate lypolytic activity which may be endogenic, caused by germination, or exogenic caused by mould growth. Generally the maximum moisture content considered safe for the storage of sunflower seeds is 9 to 10 % (Abreu et al. 2013), which results in a relative humidity of 75 % in the atmosphere surrounding the seeds. RH of 75 % and lower inhibit the growth of moulds and other microorganisms. The changes in quality of groundnut burfi packed in polypropylene (PP, 75 μ) and metalized polyester (12 μ) low density/linear low density (MP, 75 μ) were monitored during storage in order to assess the shelf life (Khan et al. 2008). The sample without sorbic acid spoiled within 30 days of storage due to mold growth and fermented odor. Peroxide values were higher for the product packed in PP than for the one packed in MP pouches. The rate of degradation was higher for samples packed in PP than those packed in MP pouches. Similar effect of packaging material was also observed in present study. The product chikki stored in LDPE pouches showed significantly higher microbial counts as compared to that stored in laminated pouches.
Table 3.
Effect of storage and type of packaging on microbial load of products developed with sunflower seeds and groundnut
| Sample | Packaging material | Duration (days) | TBC (×104 CFU) | Fungi (×102 CFU) | Yeast (×103 CFU) |
|---|---|---|---|---|---|
| Sunflower | LDPE | 0 (fresh) 60 |
2.6 6.3 |
4.4 7.2 |
4.3 7.6 |
| Laminated foil | 0 (fresh) 60 |
2.6 5.4 |
4.4 6.5 |
4.3 6.8 |
|
| Packaging | |||||
| F-value | * | * | * | ||
| SEm± | 0.04 | 0.05 | 0.05 | ||
| CD | 0.12 | 0.14 | 0.14 | ||
| Duration | |||||
| F-value | * | * | * | ||
| SEm± | 0.04 | 0.05 | 0.05 | ||
| CD | 0.12 | 0.14 | 0.14 | ||
| Sunflower + groundnut (1:1) | LDPE | 0 (fresh) 60 |
2.1 5.4 |
3.6 5.8 |
4.2 6.7 |
| Laminated foil | 0 (fresh) 60 |
2.1 4.3 |
3.6 5.5 |
4.2 6.2 |
|
| Packaging | |||||
| F-value | * | * | * | ||
| SEm± | 0.04 | 0.05 | 0.05 | ||
| CD | 0.12 | 0.16 | 0.15 | ||
| Duration | |||||
| F-value | * | * | * | ||
| SEm± | 0.04 | 0.05 | 0.05 | ||
| CD | 0.12 | 0.16 | 0.15 | ||
| Groundnut | LDPE | 0 (fresh) 60 |
1.8 3.8 |
3.7 5.2 |
3.7 5.7 |
| Laminated foil | 0 (fresh) 60 |
1.8 3.2 |
3.7 4.4 |
3.7 5.0 |
|
| Packaging | |||||
| F-value | * | * | * | ||
| SEm± | 0.03 | 0.05 | 0.04 | ||
| CD | 0.08 | 0.15 | 0.12 | ||
| Duration | |||||
| F-value | * | * | * | ||
| SEm± | 0.02 | 0.05 | 0.04 | ||
| CD | 0.08 | 0.15 | 0.12 | ||
*Significant at 5 % level
Conclusion
Parameters such as sensory characteristics, per cent free fatty acid, peroxide value and microbial load were used to measure the acceptability of the fresh as well as stored products namely chikki and oilseed butter. Taking these into considerations, it may be concluded that, the products made in combination with sunflower and groundnut (1:1) or sunflower alone. However all products were highly acceptable. The product chikki was acceptable at the end of storage period i.e., for 2 months. Better acceptability of the product chikki was found in laminated pouch with regard to free fatty acid, peroxide value, sensory characteristics and microbial load and suggested that both packaging materials can be used to store the product chikki up to 2 months. The acceptability of oilseed butter was found better in glass jar compared to that stored in plastic jar and it can be noted that oilseed butter can be stored in glass jar for 2 months without greatly alleviating its per cent free fatty acid, peroxide value and sensory characteristics.
References
- Abreu LAS, Carvalho MLM, Pinto CAG, Kataoka VY, Silva TTA. Deterioration of sunflower seeds during storage. J Seed Sci. 2013;35(2):240–247. doi: 10.1590/S2317-15372013000200015. [DOI] [Google Scholar]
- Akubugwo IE, Ugbogu AE. Physicochemical studies on oils from five selected Nigerian plant seeds. Pak J Nutr. 2007;6:75–78. doi: 10.3923/pjn.2007.323.326. [DOI] [Google Scholar]
- Amerine MD, Pangborn RM, Roesster EB. Principles of sensory evaluation of foods. London: Academic; 1965. [Google Scholar]
- Anjum F, Anwar F, Jamil A, Iqbal M. Microwave roasting effects on the physico-chemical composition and oxidative stability of sunflower seed oil. JAOCS. 2006;83(9):777–784. [Google Scholar]
- Anjum FM, Nadeem M, Khan MI, Hussain S. Nutritional and therapeutic potential of sunflower seeds: a review. Br Food J. 2012;114(4):544–552. doi: 10.1108/00070701211219559. [DOI] [Google Scholar]
- Mannual of microbiological methods. New York: McGraw Hill; 1957. p. 127. [Google Scholar]
- Official methods of analysis. 13. Washington DC: Association of Official Analytical Chemists; 1980. [Google Scholar]
- Aryana KJ, Resurreccion AVA, Chinnan MS, Beuchat LR. Functionality of palm oil as a stabilizer in peanut butter. J Food Sci. 2003;68(4):1301–1307. doi: 10.1111/j.1365-2621.2003.tb09643.x. [DOI] [Google Scholar]
- Bajaj M, Kaur A, Sidhu JS. Studies on the development of cookies utilizing sunflower kernels and wheat germ. Plant Foods Hum Nutr. 1991;41:381–387. doi: 10.1007/BF02310631. [DOI] [PubMed] [Google Scholar]
- Bax MM, Gely MC, Santalla EM. Prediction of crude sunflower oil deterioration after seed drying and storage processes. JAOCS. 2004;81(5):511–515. [Google Scholar]
- Bligh EG, Dyer WJ. Estimation of total lipids. Can J Biochem Physiol. 1959;37:911. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Budin JT, Breene WM. Factors affecting the shelf stability of sunflower nuts. JAOCS. 1993;70(5):493–496. [Google Scholar]
- Chetana R, Sunkireddy YR. Preparation and quality evaluation of peanut chikki incorporated with flaxseeds. J Food Sci Technol. 2011;48(6):745. doi: 10.1007/s13197-010-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dios CA. Secado De Diversos Granos. In: Organización de las Naciones Unidaspara la Agricultura y la Alimentación, editor. Secado de Granos y Secadoras. Chile: Oficina Regional para América Latina y el Caribe; 1996. pp. 243–255. [Google Scholar]
- Dreher ML, Schantz RM, Holm ET, Frazier RA. Sunflower butter: nutritional evaluation and consumer acceptance. J Food Sci. 1983;48(1):237–239. doi: 10.1111/j.1365-2621.1983.tb14832.x. [DOI] [Google Scholar]
- FAO-STAT (2013) Food Bulletin. Web. http://faostat.fao.org/site/567/default.aspx Accessed 28 August 2013
- Fernández-Cuestaa A, Nabloussib A, Fernández-Martíneza JM, Velascoa L. Tocopherols and phytosterols in sunflower seeds for the human food market. Grasas y Aceites. 2012;63(3):321–327. doi: 10.3989/gya.010112. [DOI] [Google Scholar]
- Fernández-Martínez JM, Pérez-Vich B, Velasco L. Sunflower. In: Vollmann J, Rajcan I, editors. Oil crops. New York: Springer; 2009. pp. 155–232. [Google Scholar]
- Fritsch CW, Hofland CN, Vickers ZM. Shelf life of sunflower kernels. J Food Sci. 2006;62(2):425–428. doi: 10.1111/j.1365-2621.1997.tb04018.x. [DOI] [Google Scholar]
- Gatta C, Piergiovanni AR. Technological and nutritional aspects in hyperproteic bread prepared with the addition of sunflower meal. Food Chem. 1996;57:493–496. doi: 10.1016/0308-8146(95)00200-6. [DOI] [Google Scholar]
- Gills A, Resurreccion AVA. Sensory and physical properties of peanut butter treated with palm oil and hydrogenated vegetable oil to prevent oil separation. J Food Sci. 2000;65(1):173–180. doi: 10.1111/j.1365-2621.2000.tb15975.x. [DOI] [Google Scholar]
- Gupta H, Mehta U, Singha J. Characteristics of cookies and muffins supplemented with full fat sunflower grits. J Food Sci Technol. 1996;33(6):513–515. [Google Scholar]
- Gupta V, Vijayalakshmi NS, Ashwini B, et al. Shelf life enhancement of coconut burfi—an indian traditional sweet. J Food Qual. 2010;33(3):329–349. doi: 10.1111/j.1745-4557.2010.00312.x. [DOI] [Google Scholar]
- Hamilton RJ. The chemistry of rancidity in foods. In: Allen JC, Hamilton RJ, editors. Rancidity in foods. Glasgow: Blackie Academic and Professional; 1994. pp. 1–21. [Google Scholar]
- Hofland C (1990) Sunflower kernels in bakery foods. Research department technical, bulletin XII(5)
- Khan MA, Semwal AD, Sharma JK, Yadav DN, Srihari KA. Studies on the development and storage stability of groundnut (arachis hypogea) burfi. J Food Qual. 2008;31(5):612–626. doi: 10.1111/j.1745-4557.2008.00224.x. [DOI] [Google Scholar]
- Lee YC, Kim IH, Chang J, Rhee YK, Oh HI, Park HK. Chemical composition and oxidative stability of safflower oil prepared with expeller from safflower seeds roasted at different temperatures. J Food Sci. 2004;69:33–38. doi: 10.1111/j.1365-2621.2004.tb17852.x. [DOI] [Google Scholar]
- Leelavathi K, Rao HP, Sastry SMC. Studies on the utilization of sunflower kernels in bakery products. J Food Sci Technol. 1991;28(5):280–284. [Google Scholar]
- Lima IM, Guraya HS. Optimization analysis of sunflower butter. J Food Sci. 2005;70(6):S365–S370. doi: 10.1111/j.1365-2621.2005.tb11457.x. [DOI] [Google Scholar]
- Martin JP. Use of acid rose bengal agar Streptomycin in plate method for estimating soil fungi. Soil Sci. 1950;9:215–216. doi: 10.1097/00010694-195003000-00006. [DOI] [Google Scholar]
- McCance, Widdowson . The composition of foods, 6th summary edn. Cambridge: Royal Society of Chemistry; 2002. Food Standards Agency and Institute of Food Research. [Google Scholar]
- Miller N, Pretorius HE, Van Der Riet WB. The effect of storage conditions on mould growth and oil quality of confectionery and high-oil sunflower seeds. J Food Sci. 1986;19:101–103. [Google Scholar]
- Miller JF, Fick GN, Edeno TP. Improvement of oil content and quality in sunflower (Helianthus annus, L.). Agr. Abstracts. Madison: American Society of Agronomy; 1992. [Google Scholar]
- Murillo M, Benzo Z, Marcano E, Gomez C, Garaboto A, Marin C. Determination of copper, iron and nickel in edible oils using emulsified solutions by ICP-AES. J Anal At Spectrom. 1999;14:815–820. doi: 10.1039/a808159j. [DOI] [Google Scholar]
- Özge ÇA, Gökmen V, Pellegrini N, Fogliano V. Direct evaluation of the total antioxidant capacity of raw and roasted pulses, nuts and seeds. Eur Food Res Technol. 2009;229(6):961–969. doi: 10.1007/s00217-009-1131-z. [DOI] [Google Scholar]
- Pajin B, Lazic V, Jovanovic O, Gvozdenovic J. Shelf life of a dragge product based on sunflower kernel depending on packaging materials used. Int J Food Sci Technol. 2006;41(6):717–721. doi: 10.1111/j.1365-2621.2005.01147.x. [DOI] [Google Scholar]
- Pallavi HM, Gowda R, Vishwanath K, Shadakshari YG, Bhanuprakash K. Identification of ssr markers for hybridity and seed genetic puritytesting in sunflower (Helianthus annuus L.) Seed Sci Technol. 2011;39:259–264. doi: 10.15258/sst.2011.39.1.28. [DOI] [Google Scholar]
- Robertson JA, Chapman GW, Wilson RL, Russel RB. Effect of moisture content of oil type sunflower seed on fungal growth and seed quality during storage. JAOCS. 1984;61(4):768–771. [Google Scholar]
- Salem EM, Hamed NA, Awlya OFA. Implementation of the sunflower seeds in enhancing the nutritional values of cake. J Appl Sci Res. 2012;8(5):2626–2631. [Google Scholar]
- Sattar A, Mohammad J, Saleem A, Jan M, Ahmad A. Effect of fluorescent light, gamma radiation and packages on oxidative deterioration of dry nuts. Sarhad J Agric. 1990;6(3):235–240. [Google Scholar]
- Satyanarayana Rao TS, Kaverappa NM, Jayaraman KS. Development of shelf stable ready-to-eat Indian sweet meats based on sugar and coconut. J Food Sci Technol. 1990;27(6):359–361. [Google Scholar]
- Škrbić B, Cvejanov J. The enrichment of wheat cookies with high-oleic sunflower seed and hull-less barley flour: Impact on nutritional composition, content of heavy elements and physical properties. Food Chem. 2011;124(4):1416–1422. doi: 10.1016/j.foodchem.2010.07.101. [DOI] [Google Scholar]
- Škrbića B, Filipčevb B. Nutritional and sensory evaluation of wheat breads supplemented with oleic-rich sunflower seed. Food Chem. 2008;108(1):119–129. doi: 10.1016/j.foodchem.2007.10.052. [DOI] [Google Scholar]
- Škrbića B, Mačvanin N. Nutritional and sensorial aspects of wheat bread enriched with high-oleic sunflower seed. Acta Aliment. 2011;40(2):194–204. doi: 10.1556/AAlim.40.2011.2.3. [DOI] [Google Scholar]
- Srilatha K, Krishnakumari K. Proximate composition and protein quality evaluation of recipes containing sunflower cake. Plant Foods Hum Nutr. 2003;58:1–11. doi: 10.1023/B:QUAL.0000041139.37434.5c. [DOI] [Google Scholar]
- Totlani VM, Chinnan MS. Effect of stabilizer levels and storage conditions on texture and viscosity of peanut butter. Peanut Sci. 2007;34(1):1–9. doi: 10.3146/0095-3679(2007)34[1:EOSLAS]2.0.CO;2. [DOI] [Google Scholar]
- USDA (2008) Web. http://www.nal.usda.gov/fnic/foodcomp/cgibin/list_nut_edit.pl Accessed 25 July 2009
- Yoshida H, Hirakawa Y, Abe S. Roasting influence on molecular species of triglycerols in sunflower seeds (Helianthus annus L.) Food Res Int. 2001;34:613–619. doi: 10.1016/S0963-9969(01)00079-5. [DOI] [Google Scholar]
- Yoshida H, Hirakawa Y, Abe S. Influence of microwave roasting on positional distribution of fatty acids of triglycerols and phospholipids in sunflower seeds (Helianthus annus L.) Eur J Lipid Sci Technol. 2002;103:201–207. doi: 10.1002/1438-9312(200104)103:4<201::AID-EJLT201>3.0.CO;2-6. [DOI] [Google Scholar]
- Yoshida H, Tomiyama Y, Hirakawa Y, Mizushina Y. Microwave roasting effects on the oxidative stability of oils and molecular species of triacylglycerols in the kernels of pumpkin (Cucurbita spp.) seeds. J Food Compos Anal. 2006;19:330–339. doi: 10.1016/j.jfca.2004.10.004. [DOI] [Google Scholar]