Abstract
Physico-chemical changes in ripe tomato (Lycopersicon esculentum Mill.) were analyzed on day 0 and 2 weeks after ultraviolet-C (UV-C) light treatment or modified atmosphere (MA) storage and combined UV-C + MA storage at 10 °C. Modified atmosphere packaging (MAP) film was used to create MA conditions. The tomatoes were evaluated for surface colour, mass loss, firmness, respiration rate, total soluble solids and antioxidant capacity. The tomatoes treated with UV-C and MA storage underwent least changes in their physico-chemical properties, indicating that combination of UV-C and MA storage was successful in retaining the attributes of the fresh product. The increase in antioxidant capacity of the tomatoes during UV-C treatment suggested that UV treatment during post harvest handling may be successfully combined with MA storage, resulting in a product with better nutritive value.
Keywords: Cherry tomato, Modified atmosphere packaging/storage, Ultraviolet, Peak fresh bags®, Antioxidants, Respiration rate
Introduction
Tomato (Lycopersicon esculentum Mill.) is one of the most widely grown crops in the world, with global tomato production exceeding 141 million t yr-1 (FAOSTAT, 2009). Apart from been eaten fresh, they are available and used in a variety of processed forms. The highly perishable nature of fresh tomatoes has encouraged researchers to develop innovative techniques to prolong their shelf life, such as the use of modified atmosphere packaging (MAP).
Modified Atmosphere packaging involves increased concentration of CO2 and reduced concentration of O2 to levels which help extend the shelf life and maintain the quality of the produce (Zagory and Kader 1988; Kuenwoo et al. 2000; Rojas-Graü et al. 2009). Increased shelf life by alteration of gas compositions have been reported for various commodities (Wang 1993). Hong and Gross (2001) studied the changes in quality of fresh cut tomatoes under various MAP conditions, including films and temperatures.
Hormetic effects can be induced by both ionizing and non-ionizing radiations. Ultraviolet (UV) treatment can alter the chemistry of plants and in some cases enhance the nutraceutical potential of plant foods (Cisneros-Zevallos 2003). UV treatment has also shown to prolong the shelf life of fruits and vegetables (Ben-Yehshua et al. 1992). Delay in ripening, reduction in colour change, production of anti-fungal compounds, maintenance of the firmness and texture due to UV treatment in fresh produce have been widely reported (Mercier et al. 1993; Arul et al. 2001; Gonzalez-Aguilar et al. 2001; Charles et al. 2003; Maharaj et al. 1999; 1993; Barka 2001; Barka et al. 2000a; b). Optimal, beneficial UV dosages for different fruit and vegetables ranges from 0.5 kJ m-2 in strawberries (Fragaria × ananassa Duchesne), to 9.0 kJ m-2 in oranges (Citrus × sinensis (L.) Osbeck) (Shama and Alderson 2005).
Apart from maintaining the quality of the product, UV treatment of fruit and vegetables has been observed to increase their antioxidant capacities. An increase in anthocyanin levels in apples (Malus domestica Borkh.) and strawberries, vitamin D2 content in edible mushrooms [Agaricus bisporus (J.E.Lange) Imbach], and phenolics in grapes berries (Vitis vinifera L.), have been reported (Dong et al. 1995; Mau et al. 1998; Cantos et al. 2000).
Given the increasing consumer demand for high nutrient and nutraceutical-containing products, a business estimated to be worth $65 billion (Lachance 2002); the increase in anti-oxidant capacity of fruits and vegetables through UV treatment is greatly desirable. This would result in the production of a value added commodity during post harvest handling, greatly benefiting the processing companies and hence the growers.
In this study, MA storage and UV treatments were factorially combined and their effects on physiochemical properties such as surface colour, firmness, total soluble solids, mass loss, respiration rate and antioxidant capacity of cherry tomatoes was investigated.
Materials and methods
Tomato fruits
Mature red cherry tomatoes (product of Mexico) of uniform size and colour purchased from a local market were used. All fruit were gently washed with distilled water and air dried.
Treatment/storage conditions
The fruits were divided into four treatment sets: Control, UV, MA storage and UV + MA storage. Each treatment was performed on 10 fruit and replicated three times. The UV-C treatment with a dosage of 3.7 kJ m-2 (Maharaj et al. 1999) was given to treatment sets UV-C and UV-C + MAP using a low pressure mercury discharge lamp emitting quasimonochromatic UV radiation at 254 nm (FOTODYNE- BIOCAN Scientific, ON, Canada). The amount of radiation was measured by a portable digital radiometer (MILTEC, Model ML1400A, Canada).
Fruit from control and UV treatment sets were placed into perforated plastic (Ziploc®) bags, while fruit from MAP and UV-C + MAP treatment sets were transferred to MAP film bags (PEAKfresh ® bags, Australia, 5063) and sealed (Multivac®,D-8941 Wolfertschenwenden, Germany). According to the manufacturer, the packaging film has the following transmission rates: O2-6500-7500 cc/m2/24 h, CO2- 23000–24000 cc/m2/24 h, Ethylene- 3900 cc/m2/24 h. The surface area of the MAP film bags (180–240 cm2; depending on the mass of the fruits in the bag) used to maintain MA conditions were calculated with an empirical formula (Raghavan et al. 1982).
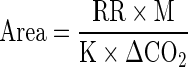 |
1 |
Where,
- Area
is the surface area of the PEAKfresh® bag (m2)
- RR
is the respiration rate (mL CO2 kg-1 h-1)
- M
is the mass of the produce (kg)
- K
is the permeability of the membrane to CO2 (mL d-1 m-2 atm-1)
- ΔCO2
is the desired CO2 partial pressure difference across the membrane (atm).
The bagged fruits were stored for 14 days at 10 ± 0.5 °C, which is the commercial cold storage temperature recommended for ripe tomatoes (Díaz de León-Sánchez et al. 2009). The gas composition of MAP and UV-C + MAP treated sample bags was determined using a gas Chromatograph (GC) (SRI-8610A Gas Chromatograph, SRI international, Menlo Park, California, USA) by analyzing the gas samples collected from the headspace of the bags after 14 days storage.
Evalutation of physiochemical properties
Five randomly selected fruits from each of three treatment replicates were used for evaluations which included colour change, firmness, total soluble solids, mass loss, respiration rate, and antioxidant capacity. The physico-chemical attributes were assessed prior to any treatment (Day 0) and after respective treatments and storage (Day 14).
Surface colour change
Colour measurements were carried out on the surface of the fresh fruits using a tristimulus colorimeter (Minolta Co. Ltd., Japan). Colour values, expressed as L* (lightness), a* (redness/greenness) and b* (yellowness/blueness) were determined for all samples. Three readings were taken per fruit and mean values were calculated per fruit and for each treatment combination (5 fruit x 3 replicates x 3 per fruit). Reference colour values for the fresh samples (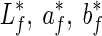 ) and colour values from stored samples (
) and colour values from stored samples (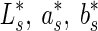 ) were employed in calculating the colour change (ΔE), as defined by (McGuire 1992):
) were employed in calculating the colour change (ΔE), as defined by (McGuire 1992):
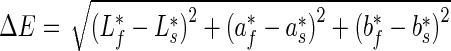 |
2 |
Firmness
Firmness of a fruit or a vegetable is one of the physical attributes which considerably affects its marketability. Firmness of tomato generally decreases with an increase in the duration of storage (Moneruzzaman et al. 2008). Firmness of the tomato samples were measured using a penetrometer (FT 327 QA suppliers, Norfolk, VA). Firmness was expressed in terms of Newtons.
Total Soluble Solids (TSS)
TSS is an index of the concentration of soluble solids in fruit, and is predominantly influenced by the sugars present in samples (Saltveit 2005). The TSS of the samples was measured using a handheld refractometer (Reichert Inc. NY, USA). A drop of clear tomato juice obtained by squeezing the fruit in a muslin cloth was placed in the reading chamber and the TSS was read in units of percent.
Mass loss
Mass loss of the samples after 14 days’ storage was calculated as a percentage of the initial mass (Day 0) (Javanmardi and Kubota 2006).
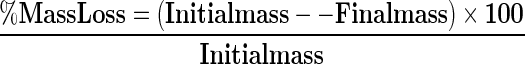 |
3 |
Respiration Rate (RR)
Respiration is an oxidative process which occurs in living cells, where energy is released in a series of metabolic steps leading to the breakdown of complex materials, such as sugars, into carbon dioxide and water (Forcier et al. 1987). Pre-storage and post-storage tomato samples of known mass were placed in airtight jars equipped with a rubber septum. Air samples were drawn through the septum with a syringe initially and after 6 h at 21 °C and their gas composition analyzed by gas chromatography. Respiration rate was determined by calculating the change in percentage of carbon dioxide and oxygen in the gas sample (mg kg-1 h-1) over the 6 h incubation period (Forcier et al. 1987).
Measuring antioxidant capacity of the samples
The main antioxidants in tomatoes are carotenoids, ascorbic acid and phenolic compounds (Giovanelli et al. 1999). Lycopene is the major carotenoid accounting for more than 80 % of the total carotenoids present in a fully ripe tomato fruit (Nguyen and Schwartz 1999). Today, estimation of antioxidant capacity has become an important parameter to evaluate the nutritional quality of food.
The antioxidant capacity of the tomato samples was measured using the Ferric Reducing Ability of Plasma (FRAP) assay (Benzie and Strain 1996), which determines the antioxidant capacity of the sample through the reduction of yellow-coloured ferric tripyridyltriazine complex to a blue-coloured ferrous complex upon reaction with antioxidant molecules. The capacity is measured spectrophotometrically (Beckman DU 640, Beckman Instruments, Fullerton, CA) by the change in absorbance at 593 nm. Freeze dried, seed-free powdered tomato tissue was used for the antioxidant analysis. Ascorbic acid was used as the standard to quantify the antioxidant concentration of the tomato tissue samples and the results were expressed in μg ascorbic acid equivalents g -1 DM.
Statistical analysis
Analysis of variance (ANOVA) test using General Linear Model (GLM) method has been performed to investigate treatments effects (P ≤ 0.05) using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). Means were compared using Duncan’s comparison tests (P ≤ 0.05). Correlations among fruit quality parameters were measured using Pearson’s correlation coefficients (P ≤ 0.05).
Results and discussion
Mass loss
Mass loss of the samples stored in perforated plastic bags (control and UV) was significantly greater (P ≤ 0.05) than samples stored in sealed bags (MA and UV + MA) (Fig. 1a). Mass loss of the samples was unaffected by UV-C treatment (P > 0.05). There was no significant difference in mass loss between MA and UV + MA or control and UV treated samples. Also, the interaction between UV-C and MA was found to be statistically non- significant (P > 0.05).
Fig. 1.
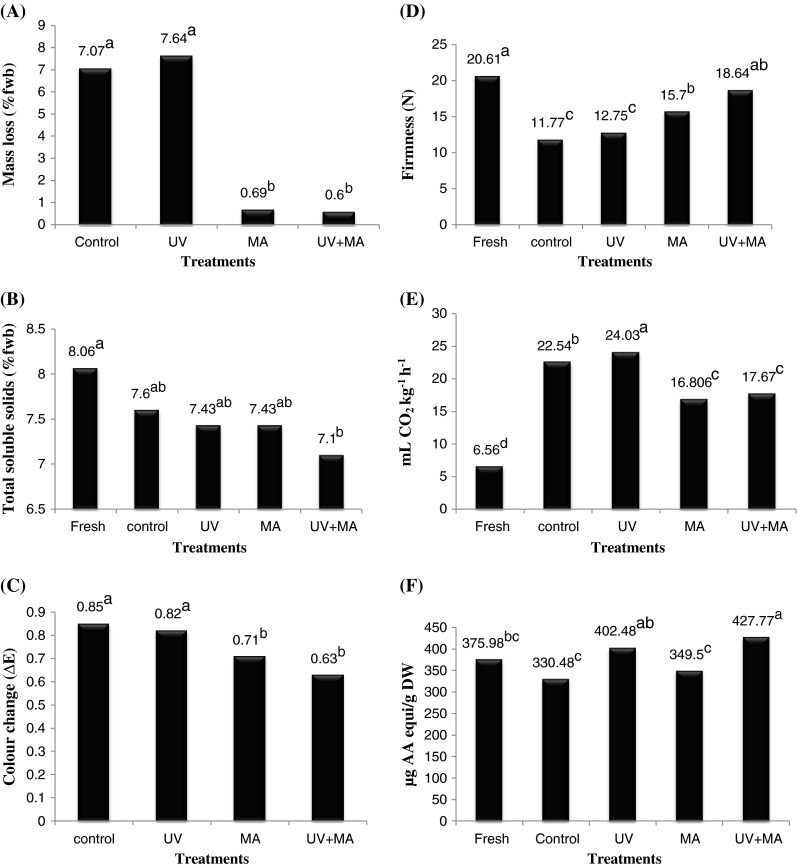
Changes in quality parameters of Cherry tomatoes (percent fresh weight basis (%fwb)) after 14 days storage at 10 °C in standard perforated or modified atmosphere (MA) bags, with or without pre-packaging and pre-storage Ultra violet –C (UV-C) treatment (a Values with different letters are significantly different (P ≤ 0.05); n = 3). a Percent mass loss of cherry tomatoes. b Total soluble solids. c Colour change (ΔE) in cherry tomatoes. d Firmness of cherry tomato fruit (Newtons (N)). e Respiration rates (mL CO2 kg-1 h-1) of different treatment samples. f Antioxidant capacity (micrograms ascorbic acid equivalents per gram dry weight (μg AA equi g-1 DW) of different treatments
Total Soluble Solids (TSS)
There were no significant treatment effects (P > 0.05) on % TSS except between fresh and UV-C + MA (Fig. 1b). Fresh samples (before storage) tended to show a greater % TSS than stored tomatoes, regardless of the treatment imposed. The lack of significant change in % TSS with a short storage period treatment was consistent with a similar study (10 days of storage at 12 °C) reported by Kagan-Zur and Mizrahi (1993). TSS reduction with storage is generally related to sugar-acid metabolism. A higher level of CO2 in modified atmosphere packaging was reported to restrict this reduction in TSS of cherry tomatoes with storage (Akbudak et al. 2006). In this study neither UV-C nor MA treatment caused significant reduction in loss of % TSS with storage. But UV-C treatment combined with MA (UV-C + MA) showed some significant reduction in the loss of % TSS compared to the fresh. Also the interaction between the two treatments (UV-C and MA) was found to be statistically significant (P ≤0.05); the reason for this was not determined.
Surface colour change
Colour change was greatest in control and least in UV-C + MA treatments (Fig. 1c). Colour change of a fruit is a factor predominantly based on ripeness of the fruit, senescence, storage duration and conditions (Moneruzzaman et al. 2008). In our study there was no significant difference in colour change found in between control and UV treated samples (P > 0.05) nor the interaction between MA and UV had any significant effect, indicating that UV treatment has no effect on colour change in this case as we used ripe tomatoes. MA storage samples (MA and UV + MA) showed significantly lower colour change compared to control and UV treatment samples. Formation of lycopene which is known to be the main constituent of red pigments in tomato is dependent upon the presence of O2 (Hobson and Davies 1971). Higher CO2 and lower O2 inhibits further ripening by lycopene formation (Yang et al. 1987). MA storage, which in our study maintained higher CO2 (11.3 %) and lower O2 (3.1 %) concentrations (mean values) plausibly inhibited further ripening of already ripe tomatoes.
Firmness
Highest firmness was retained in UV + MA and least in control treatment among the stored samples (Fig. 1d). There was no significant difference found among the modified atmosphere storage treatments (MA and UV + MA) nor the interaction between MA and UV had any significant effect, which in turn were significantly different from UV and control treated samples. Firmness of the samples was negatively correlated with mass loss and respiration rate (Table 1). In this study MA storage (MA and UV + MA) which maintains the higher CO2 concentration retained better firmness than the non-MA (UV and Control). Fruit samples with greater moisture content will be more firm than wrinkled samples of lesser moisture content. Storage under MA conditions maintained greater firmness than under non-MA conditions (Fig. 1d), as the mass loss was reduced more than ten-folds by MA storage compared to perforated non-MA storage. This is indicative that moisture content of the samples along with storage conditions plays a vital role in firmness.
Table 1.
Correlation among different evaluated parameters (Pearson Correlation Coefficient test)
| Factors | ML | CH | F | TSS | AA | RR |
|---|---|---|---|---|---|---|
| ML | – | 0.32 NS | −0.79 *** | 0.34 NS | −0.27 NS | 0.91 *** |
| CH | 0.32 NS | – | −0.55 NS | 0.34 NS | −0.88 *** | 0.55 NS |
| F | −0.79 *** | −0.55 NS | – | 0.08 NS | 0.31 NS | −0.85 *** |
| TSS | 0.34 NS | 0.34 NS | 0.08 NS | – | −0.48 NS | −0.4 NS |
| AA | −0.27 NS | −0.88 *** | 0.31 NS | −0.48 NS | – | −0.13 NS |
| RR | 0.91 *** | 0.55 NS | −0.85 *** | −0.4 NS | −0.13 NS | – |
(Pearson's r2); NS – Not significant; * Significant at the 0.05 probability level; **Significant at the 0.01 probability level; ***Significant at the 0.001 probability level. Mass loss (ML), colour change (CH), firmness (F), total soluble solids (TSS), antioxidants (AA), respiration rate (RR) (n = 3)
In tomato, with the onset of ripening, pectin degrading enzymes starts to accumulate and contribute to softening the cell walls (Shama and Alderson 2005). Tomato samples treated with UV-C are found to inhibit microbial growth, retard decaying and delayed senescence (Allende and Artés 2003). It has been reported that there is a reduction in the cell wall degrading enzymes like polygalacturonase, pectin methyl esterase, cellulose, xylanase, β-D-galactosidase and protease in UV-treated ripening tomatoes (Barka et al. 2000b). However, in this study there was no affect of UV treatment on the firmness of the samples (compared to control) as we used ripe tomato fruits. Firmness of the UV treated samples was significantly lower than UV + MA indicating that firmness was affected by modified atmosphere storage conditions.
Respiration rate
Respiration rate of the produce predominantly depends on the stage of ripening, temperature and gas composition of the surrounding air. As all the samples were kept at the same temperature and ripening stage, the respiration rate would have been affected by the gas composition and the effect of UV treatment on the fruit. UV treatment accelerates several biological processes like respiration rate in wide range of fruits and vegetables including tomatoes (El-Ghaouth and Wilson 1995; Maharaj et al. 1999; Erkan et al. 2001; Allende and Artés 2003). In this study, UV-C treated samples showed highest respiration rate, which was significantly higher than all the treatments including control which were stored in the same packaging material as UV-C treated samples (perorated plastic bags). The MA treated samples (MA and UV-C + MA) were not significantly different, but UV-C + MA (17.67 mL CO2 kg-1 h-1) showed slightly higher respiration rate than MA (16.806 mL CO2 kg-1 h-1) possibly because of the effect of UV-C treatment in UV-C + MA. Also, the interaction between UV-C and MA was found to be statistically significant (P ≤ 0.05). Respiration rate of all the treatments were significantly higher than that of fresh fruits (6.563 mL CO2 kg-1 h-1). Respiration rate of samples stored in MA storage (MA and UV-C + MA) was significantly lower than non MA treated samples (Control and UV-C) as MA storage had higher % CO2 (11.3 %) and lower O2 (3.1 %) compared to non MA storage. Low O2 and higher CO2 concentrations suppress the respiration rate and also the ethylene production in tomatoes as O2 is involved in the conversion of 1-amino-cycloprane-1-carboxylic acid to ethylene (Klieber et al. 1996; Alejandra et al. 2009). Ethylene gas (a promoter of senescence) is known to show rise in respiration rate in many fruits and vegetables like potato (Pharr and Kattan 1970), tomato (Streif and Bangerth 1976). The samples stored in MA storage showed low respiration rate and delay in senescence as the ethylene gas produced inside the bags might have been removed (based on the claim of the manufacturer) because of the use of the MAP films.
Respiration rate was positively and negatively correlated with mass loss and firmness respectively (Table 1). Ethylene gas other than raising the rate of respiration, negatively affects the firmness of tomatoes (Allende and Artés 2003). MA storage samples (MA and UV-C + MA) not only retained the moisture content of the sample, but also might have removed the ethylene gas which affects the firmness of the samples.
Antioxidant capacity of the samples
Among all the treatments UV-C + MA showed greater antioxidant capacity (427.77 μg AA eq. mL-1) than fresh (375.98 μg AA eq. mL-1) and control fruit (330.48 μg AA eq. mL-1). UV-C treatment has been shown to enhance accumulation of phenolics in tomato fruits. Barka et al. (2000a) observed a significant induction of lipid peroxidation markers in ripening tomatoes during the first 5 days after UV treatment (3.7 kJ m-2) suggesting that the cell membrane is the primary target for UV treatment. After 5 days, the level of lipid peroxidation markers dropped lower than in control fruit, suggesting the induction of a defense or repair mechanism. This suggests that UV treatment results in rapid accumulation of photo oxidation products and that the plants react by stimulating their defense mechanisms against oxidation (Shama and Alderson 2005).
Antioxidant capacity decreases with time under normal conditions and some non-enzymatic antioxidants like ascorbic acid levels go down much quicker than others (Varit and Yasuo 2003). UV-C + MA which were not significantly different from UV-C showed significant difference with fresh, control and MA treatment indicating the increase in antioxidants is affected by UV-C treatment.
In tomato samples treated with UV-C, there was a decline in ascorbate oxidase which degrades ascorbic acid (Barka 2001). UV-C + MA samples though not significantly higher than UV-C treatment showed the highest antioxidants, which could be because of UV-C treatment which enhanced antioxidants combined with modified atmosphere storage which helps in retaining the antioxidants with better storage conditions like lower O2 concentration. Higher availability of O2 throughout the storage of the produce is believed to dramatically degrade the ascorbic acid by oxidation (Alejandra et al. 2009). As MA storage (MA and UV-C + MA) maintains lower O2 than non-MA storage, may help in better retaining the ascorbic acid in the samples. Also, the interaction between UV-C and MA was found to be statistically significant (P ≤ 0.05). Antioxidants are negatively affected by the respiration rate of the samples. Abdulnabi et al. (1997) study showed that concentration of ascorbic acid content of tomatoes decreased with increase in rate of respiration because of the antioxidant function of ascorbic acid. In our study there was a negative trend between antioxidants and respiration though it was not significant (Table 1).
Conclusion
The modified atmosphere (MA) storage conditions were successfully achieved with the usage of modified atmosphere packaging (MAP) film bags. Tomatoes subjected to MA storage conditions at a temperature of 10 °C for a period of 2 weeks, showed a reduction in % mass loss, colour change, respiration rate, and retained the firmness. UV-C treatment enhanced the antioxidant capacity of the tomatoes, but had no beneficial effect in reducing the % mass loss, colour change, respiration rate, or in maintaining the firmness of the fruits. The best quality tomatoes, in terms of minimum changes in their physico-chemical attributes (mass loss, respiration rate, colour change, TSS, firmness) and enhanced antioxidant capacity were achieved with UV + MA storage conditions, suggesting that UV treatment can be successfully combined with modified atmosphere storage, resulting in a produce with increased shelf life and nutritive quality. Future studies could involve the sensory evaluation of the UV + MA storage treated fruits to assess the overall quality and consumer acceptance of the fruits.
Acknowledgements
The authors are grateful to the Natural Sciences and Engineer Research Council of Canada (NSERC) for financial support and Dr. Atef Nassar for his valuable help.
References
- Abdulnabi AA, Ehmed AH, Hussein GD, Peter AB. Determination of antioxidant vitamins in tomatoes. Food Chem. 1997;60:207–212. doi: 10.1016/S0308-8146(96)00321-4. [DOI] [Google Scholar]
- Akbudak B, Akbudak N, Seniz V, Eris A. The effect of harpin treatment on storage of cherry tomato. Acta Hort. 2006;712:237–243. [Google Scholar]
- Alejandra M, Rojas G, Gemma O, Robert SF, Martin-Belloso O. The use of packaging techniques to maintain freshness in fresh-cut fruits and vegetables. Int J Food Sci Technol. 2009;44:875–889. doi: 10.1111/j.1365-2621.2009.01911.x. [DOI] [Google Scholar]
- Allende A, Artés F. Combined ultraviolet-C and modified atmosphere packaging treatments for reducing microbial growth of fresh processed lettuce. LWT- Food Sci Technol. 2003;36:779–786. doi: 10.1016/S0023-6438(03)00100-2. [DOI] [Google Scholar]
- Arul J, Mercier J, Charles M, Baka M, Maharaj R. Phytochemical treatment for control of post-harvest diseases in horticulture crops. In: Vincent C, Panneton B, Fleurat-Lessard F, editors. Physical control methods in plant protection. Paris: INRA; 2001. pp. 146–161. [Google Scholar]
- Barka EA. Protective enzymes against oxygen species during ripening of tomato (Lycopersicon esculentum) fruits in response to low amounts of UV-C. Australian J Plant Physiol. 2001;28:785–791. [Google Scholar]
- Barka EA, Kalantari S, Makhlouf J, Arul J. Effects of UV-C irradiation on lipid peroxidation markers during ripening of tomato (Lycopersicon esculentum L.) fruits. Australian J Plant Physiol. 2000;27:147–152. [Google Scholar]
- Barka EA, Kalantari S, Makhlouf J, Arul J. Impact of UV-C irradiation on the cell wall-degrading enzymes during ripening of tomato (Lycopersicon esculentum L.) fruit. J Agr Food Chem. 2000;48:667–671. doi: 10.1021/jf9906174. [DOI] [PubMed] [Google Scholar]
- Ben-Yehshua S, Rodov V, Kim J, Carmeli S. Preformed and induced antifungal materials of citrus fruits in relation to the enhancement of decay resistance by heat and ultraviolet treatments. J Agr Food Chem. 1992;40:1217–1221. doi: 10.1021/jf00019a029. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Cantos E, Garcia-Viguera C, Pascual-Teresa S, Tomas-Berberan FA. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J Agr Food Chem. 2000;48:4606–4612. doi: 10.1021/jf0002948. [DOI] [PubMed] [Google Scholar]
- Charles MT, Corcuff R, Roussel D, Arul J. Effect of maturity and storage on Rishitin accumulation disease resistance to Botrytis cinerea in UV-C treated tomato fruit. Acta Hort. 2003;599:573–576. [Google Scholar]
- Cisneros-Zevallos L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J Food Sci. 2003;68:1560–1565. doi: 10.1111/j.1365-2621.2003.tb12291.x. [DOI] [Google Scholar]
- Díaz de León-Sánchez F, Pelayo-Zaldívar C, Rivera-Cabrera F, Ponce-Valadez M, Ávila-Alejandre XJ, Fernández F, Escalona-Buendía H, Pérez-Flores L. Effect of refrigerated storage on aroma and alcohol dehydrogenase activity in tomato fruit. Postharvest Biol Tec. 2009;54:93–100. doi: 10.1016/j.postharvbio.2009.07.003. [DOI] [Google Scholar]
- Dong YH, Mitra D, Kootstra A, Lister C, Lancaster J. Postharvest stimulation of skin colour in Royal gala apple. J Am Soc Hortic Sci. 1995;120:95–100. [Google Scholar]
- El-Ghaouth A, Wilson CL. Biologically based technologies for the control of postharvest diseases. Postharvest news and Information. 1995;6:5–11. [Google Scholar]
- Erkan M, Wang CY, Krizek DT. UV-C radiation reduces microbial populations and deterioration in Cucurbita pepo fruit tissue. Environ Exp Bot. 2001;45:1–9. doi: 10.1016/S0098-8472(00)00073-3. [DOI] [PubMed] [Google Scholar]
- Forcier F, Raghavan V, Gariepy Y. Electronic sensor for the determination of fruit and vegetable respiration. Int J Refrig. 1987;10:353–356. doi: 10.1016/0140-7007(87)90122-8. [DOI] [Google Scholar]
- Giovanelli G, Lavelli V, Peri C, Nobili S. Variation in antioxidant compounds of tomato during vine and post-harvest ripening. J Sci Food Agr. 1999;79:1583–1588. doi: 10.1002/(SICI)1097-0010(199909)79:12<1583::AID-JSFA405>3.0.CO;2-J. [DOI] [Google Scholar]
- Gonzalez-Aguilar GA, Wang CY, Buta JG, Krizek DT. Use of UV-C irradiation to prevent decay and maintain postharvest quality of ripe `Tommy Atkins' mangoes. Int J Food Sci Technol. 2001;36:767–773. doi: 10.1046/j.1365-2621.2001.00522.x. [DOI] [Google Scholar]
- Hobson GE, Davies JN. The tomato. In: Hulme AC, editor. The biochemistry of fruits and their products. London: Academic; 1971. pp. 437–482. [Google Scholar]
- Hong JH, Gross K. Maintaining quality of fresh-cut tomato slices through modified atmosphere packaging and low temperature storage. Food Eng Physical Propt. 2001;66:960–965. [Google Scholar]
- Javanmardi J, Kubota C. Variation of lycopene, antioxidants activity, total soluble solids and weight loss of tomato during postharvest storage. Postharvest Biol Tec. 2006;41:151–155. doi: 10.1016/j.postharvbio.2006.03.008. [DOI] [Google Scholar]
- Kagan-Zur V, Mizrahi Y. Long shelf-life small sized (cocktail) tomatoes may be picked in bunches. Scientia Hort. 1993;56:31–41. doi: 10.1016/0304-4238(93)90099-C. [DOI] [Google Scholar]
- Klieber A, Ratanachinakorn B, Simons DH. Effects of low oxygen and high carbon dioxide on tomato cultivar ‘Bermuda’ fruit physiology and composition. Scientia Hort. 1996;65:251–261. doi: 10.1016/0304-4238(96)00881-3. [DOI] [Google Scholar]
- Kuenwoo P, Homin K, Dongman K, Hyungwoo P. Effect of the packaging films and storage temperatures on modified atmosphere storage of ripe tomato. Postharvest News Info. 2000;11:1082. [Google Scholar]
- Lachance PA. Nutraceuticals, for real nutraceuticals and functional foods. Food Technol. 2002;56:20–21. [Google Scholar]
- Maharaj R, Arul J, Nadeau P (1993) Photochemical therapy in the preservation of fresh tomatoes by delaying senescence. Institute of Food Technologists Annual Meeting, Chicago, USA. Book of abstracts 294
- Maharaj R, Arul J, Nadeau P. Effect of photochemical treatment in the preservation of fresh tomato (Lycopersicon esculentum cv. Capello) by delaying senescence. Postharvest Biol Technol. 1999;15:13–23. doi: 10.1016/S0925-5214(98)00064-7. [DOI] [Google Scholar]
- Mau J, Chen P, Yang J. Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. J Agr Food Chem. 1998;46:5269–5272. doi: 10.1021/jf980602q. [DOI] [Google Scholar]
- McGuire RG. Reporting of objective colour measurements. Hort Sci. 1992;27:1254–1255. [Google Scholar]
- Mercier J, Arul J, Julien C. Effect of UV-C on phytoalexin accumulation and resistance to Botrytis cenerea in stored carrots. J Phytopathol. 1993;139:17–25. doi: 10.1111/j.1439-0434.1993.tb01397.x. [DOI] [Google Scholar]
- Moneruzzaman KM, Hossain ABMS, Sani W, Saifuddin M. Effect of stages of maturity and ripening conditions on the physical characteristics of tomato. Am J Biochem Biotech. 2008;4:329–335. doi: 10.3844/ajbbsp.2008.329.335. [DOI] [Google Scholar]
- Nguyen ML, Schwartz SJ. Lycopene: chemical and biological properties. Food Technol. 1999;53:38–45. [Google Scholar]
- Pharr DM, Kattan AA. Effects of air flow rate, storage temperature, and harvest maturity on respiration and ripening of tomato fruits. Plant Phys. 1970;48:53–55. doi: 10.1104/pp.48.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan GSV, Tessier S, Chayet M, Phan CT, Lanson A. Storage of vegetables in a membrane system. Trans. ASAE (American Society of Agricultural and Biological Engineers) 1982;25:433–436. doi: 10.13031/2013.33549. [DOI] [Google Scholar]
- Rojas-Graü MA, Oms-Oliu G, Soliva-Fortuny R, Martín-Belloso O. The use of packaging techniques to maintain freshness in fresh-cut fruits and vegetables: a review. Int J Food Sci Technol. 2009;44:875–889. doi: 10.1111/j.1365-2621.2009.01911.x. [DOI] [Google Scholar]
- Saltveit ME. Fruit ripening fruit quality. In: Heuvelink E, editor. Tomatoes. Wallingford: CAB International; 2005. pp. 145–170. [Google Scholar]
- Shama G, Alderson P. UV hormesis in fruits: a concept ripe for commercialization. Trends Food Sci Technol. 2005;16:128–136. doi: 10.1016/j.tifs.2004.10.001. [DOI] [Google Scholar]
- Streif J, Bangerth F. The effect of different partial pressures of oxygen and ethylene on the ripening of tomato fruits. Sci Hortic. 1976;5:227–237. doi: 10.1016/0304-4238(76)90086-8. [DOI] [Google Scholar]
- Varit S, Yasuo T. Changes in respiratory and antioxidative parameters in cucumber fruit stored under high and low oxygen concentrations. J Japanese Soc Hortic Sci. 2003;72:525–532. doi: 10.2503/jjshs.72.525. [DOI] [Google Scholar]
- Wang C. Approaches to reduce chilling injury of fruits and vegetables. Hortic Rev. 1993;15:63–95. [Google Scholar]
- Yang CC, Brennan P, Chinnan MS, Shewfelt RL. Characterisation of tomatoes ripening process as influenced by individual seal-packaging and temperature. J Food Qual. 1987;10:21–33. doi: 10.1111/j.1745-4557.1987.tb00286.x. [DOI] [Google Scholar]
- Zagory D, Kader A. Modified atmosphere packaging of fresh produce. Food Technol. 1988;42:70–74. [Google Scholar]


